Abstract
Mitochondria are the major source of energy for the normal functioning of brain cells. Increasing evidence suggests that the amyloid precursor protein (APP) and amyloid beta (Aβ) accumulate in mitochondrial membranes, cause mitochondrial structural and functional damage, and prevent neurons from functioning normally. Oligomeric Ab is reported to induce intracellular Ca2+ levels and to promote the excess accumulation of intracellular Ca2+ into mitochondria, to induce the mitochondrial permeability transition pore to open, and to damage mitochondrial structure. Based on recent gene expression studies of APP transgenic mice and AD postmortem brains, and APP/Aβ and mitochondrial structural studies, we propose that the overexpression of APP and the increased production of Aβ may cause structural changes of mitochondria, including a increase in the production of defective mitochondria, a decrease in mitochondrial trafficking, and the alteration of mitochondrial dynamics in neurons affected by AD. This article discusses some critical issues of APP/Aβ associated with mitochondria, mitochondrial structural and functional damage, and abnormal intracellular calcium regulation in neurons from AD patients. This article also discusses the link between Aβ and impaired mitochondrial dynamics in AD.
Introduction
Alzheimer’s disease (AD) is a late-onset, progressive, age-dependent neurodegenerative disorder, characterized clinically by the impairment of cognitive functions and changes in behavior and personality (Selkoe, 2001; Mattson, 2004; LaFerla et al., 2007; Reddy and Beal, 2008). AD is associated with intracellular neurofibrillary tangles and extracellular amyloid beta (Ab) plaques in the regions of the brain that are responsible for learning and memory. Recent molecular, cellular, gene expression and immunochemical studies of AD postmortem brains and brain specimens from AD transgenic mice revealed a loss of neuronal subpopulations, decreased presynaptic and postsynaptic immunoreactivity of terminals, a decrease in cholinergic fibers, the proliferation of reactive astrocytes and microglia, and abnormal mitochondrial structural and functional alterations (Mattson, 2004; LaFerla et al., 2007; Reddy 2006, 2007; Reddy and McWeeney, 2006; Reddy and Beal, 2005, Swerdlow, 2007). Among these important changes, mitochondrial oxidative damage and synaptic pathology have recently been reported as early events in AD progression (reviewed in Reddy and Beal, 2008; Pratico, 2008). However, it is still unclear the causal factors of mitochondrial oxidative damage and synaptic pathology in AD pathogenesis. Further, the precise link between Ab and the structure/function of mitochondria in the development and progression of AD - is not completely understood. This article outlines the role of the amyloid precursor protein (APP) and Ab in mitochondrial structural and functional alterations in neurons affected by AD. This article also discusses the latest developments in mitochondrial structural and functional abnormalities in relation to Ab in AD pathogenesis.
Transport of Ab to Mitochondrial Membranes
Recently, Aβ monomers and oligomers were found in mitochondrial membranes, in the neurons of postmortem brain specimens from AD patients and in neurons of brain specimens from AD mice and neuronal cells expressing mutant APP (Manczak et al., 2006; Crouch et al., 2005; Caspersen et al., 2006; Devi et al., 2006). However, the mechanism by which is Ab transported to mitochondrial membranes is not fully understood. Very recently, using immuno-electron microscopy, subcellular fractionation, and immunoblotting techniques, Hanson Peterson et al investigated the mitochondrial uptake of Ab in AD neurons. They found that Ab is transported into mitochondria via the translocase of the outer membrane machinery (Hanson Peterson et al., 2008). Subfractionation studies revealed that Ab is associated with the inner membrane of mitochondria, and immuno-electron microscopy studies showed Ab localized to the cristae of the inner membrane of mitochondria. Hanson Peters et al. also found a distribution pattern of Ab in the inner mitochondrial membrane in brain biopsies of the human cortex obtained from living subjects with normal-pressure hydrocephalus. Findings from the Hanson Peterson et al. study (2008), together those from earlier studies (Manczak et al., 2006; Crouch et al., 2005; Caspersen et al., 2006; Devi et al., 2006), suggest that Ab species transport to mitochondrial membranes (Fig. 1), cause mitochondrial dysfunction and oxidative damage, and ultimately damage AD neurons both structurally and functionally.
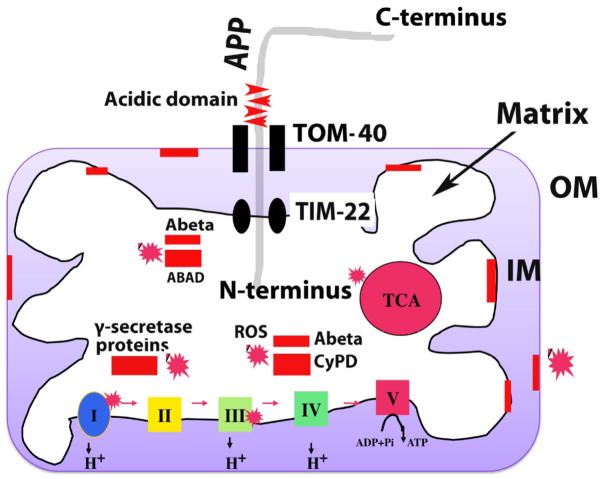
Figure 1.
APP and Ab localization of mitochondria in neurons from patients with AD. APP is localized to mitochondria only in AD patients, and APP localization increases with disease severity of Alzheimer’s. As shown, the N-terminus of APP is localized to mitochondria and the C-terminus, facing out. Mitochondrially localized APP forms complexes with the translocase of the outer membrane (TOM) and of the inner membrane (TIM). However, the transmembrane-arrested form of APP (ACID domain) prevents the importation of the C-terminus of APP into mitochondria, and the mitochondrial pores are clogged by APP import and prevent (i.e., block) the transport of nuclear-encoded mitochondrial proteins to the mitochondria. Insufficient nuclear-encoded mitochondrial proteins transported to the mitochondria and cause abnormal mitochondrial electron activities, including increased free radical production, decreased mitochondrial enzyme activities, and low ATP production.
Ab was found in the outer and inner mitochondrial membranes and matrix. Ab associated localized to the outer membrane blocks the transport of nuclear-encoded mitochondrial proteins to the mitochondria and impairs ETC activities and ATP production in AD neurons. Ab localized to the inner membrane directly induced free radical production, increased free radical production, decreased mitochondrial enzyme activities including cytochrome oxidase activity, and interfered with ATP production. Ab localized to the mitochondrial matrix interacted with several matrix proteins, including ABAD and Cyclophilin D. The abnormal interaction between Ab and mitochondrial proteins caused mitochondrial damage and promoted neuronal damage in AD patients. Further, g-secretase complex proteins, PS1, APH and nicastrin were found in the matrix of mitochondria and may damage mitochondria by inducing free radical production and causing oxidative damage to mitochondria.
Amyloid Precursor Protein Associated with Mitochondria
APP is reported to be associated with mitochondria in neurons affected by AD. Using in vitro (human cortical neurons or HCN-1A neurons) and in vivo (Tg2576 mice) studies, Anandatheerthavarada and colleagues researched the association of endogenous and ectopically expressed full-length, wild-type, and mutant APP. They found both wild-type and mutant APP (with Swedish mutation) localized to the plasma membrane and the mitochondria of human HCN-1A neurons (Anandatheerthavarada et al., 2003). Other groups also reported full-length wild-type and/or mutant APP localized to the mitochondria of PC12 cells and HEK293 cells that were stably transfected with Swedish APP751 and APP695 (Kiel et al., 2004; Park et al., 2006). Several groups demonstrated the localization of mitochondrial APP in the NH2 -terminal inside the mitochondria and COOH-terminal of the protein faces the cytosolic side (Anandatheerthavarada et al., 2003; Park et al 2006).
Using postmortem brain specimens from patients with mild, moderate, and severe AD, and from age-matched healthy control subjects, Devi and colleagues studied the association of APP with mitochondria. They found full-length APP and C-terminal–truncated (lacking Aβ domain) APP species accumulated in the mitochondria from all three categories of AD brains, as compared to the control brains. The accumulation of APP in the mitochondrial membranes increased with severity of AD, suggesting that APP associated with mitochondria may be critically involved in the disease process of AD (Fig. 1).
APP, Ab, and Mitochondrial Abnormalities in AD
Mitochondrial dysfunction is a prominent feature of AD, but the underlying mechanism is still not completely understood. Mitochondrial dysfunction has been described in AD postmortem brains (Gibson et al., 1998; Maurer et al., 2000; Smith et al., 1996; Devi et al., 2006), platelets from AD patients (Parker et al., 1990), AD transgenic mice (Reddy et al., 2004; Lustbader et al., 2004; Li et al., 2004; Caspersen et al., 2005; Manczak et al., 2006; Eckert et al., 2008) and cell-lines that express mutant APP and/or cells treated with Ab (Kaminsky et al., 2008; Diana et al., 2008; Schmidt et al., 2008; Matsumoto et al., 2006 ).
Several lines of evidence suggest that mitochondrial abnormalities plays a large role in AD pathogenesis:
Early mitochondrial studies focused on describing mitochondrial dysfunction in fibroblasts, lymphoblasts, and postmortem brains from AD patients and control subjects and found that neurons, fibroblasts, and lymphoblasts from AD patients were deficient in cytochrome oxidase activity, pyruvate dehydrogenase, and a-ketodehydrogenase compared to those from control subjects (reviewed in Reddy and Beal, 2008). Several other studies found increased free radical production, lipid peroxidation, oxidative DNA damage, oxidative protein damage and decreased ATP production and cell viability in AD brains compared to age-matched control subjects (Gibson et al., 1998; Parker et al., 1990; Maurer et al., 2000; Smith et al., 1996; Devi et al., 2006; Wang et al., 2005; Sultana et al., 2007).
In mitochondrial DNA studies, the link between mitochondrial DNA changes and mitochondrial dysfunction was investigated in brain tissues from AD patients and control subjects (Lin et al., 2002; Coskun et al., 2004). Increased mitochondrial DNA changes were found in postmortem brain tissue from AD patients and aged-matched control subjects compared to DNA changes in young control subjects, suggesting that the accumulation of mitochondrial DNA in AD pathogenesis is age-related.
Several groups investigated mitochondrial gene expressions in postmortem AD brains, and brain specimens of AD transgenic mice, and found mitochondrial encoded genes were abnormally expressed in AD postmortem brains and in the brain specimens from AD transgenic mice (Chandrasekharan et al., 1994, 199; Simonian and Hyman, 1994; Reddy et al., 2004; Manczak et al., 2005). A recent time-course global gene expression study in Tg2576 mice and age-matched non-transgenic littermates revealed an up-regulation of mitochondrial genes in the Tg2576 mice, suggesting that mitochondrial metabolism is impaired by mutant APP/Ab and that the up-regulation of mitochondrial genes may be a compensatory response to mutant APP/Ab (Reddy et al., 2004). Further, Manczak et al found an abnormal expression of mitochondrial encoded genes in postmortem AD brains compared to the brains of control subjects (Manczak et al., 2004), suggesting impaired mitochondrial metabolism in AD.
Several groups recently found that Ab is localized to mitochondrial membranes and is responsible for generating free radicals and initiating mitochondrial dysfunction (Caspersen et al., 2005; Manczak et al., 2006; Devi et al., 2006; Hanson Peterson et al., 2008; Du et al., 2008 ). Other groups found presequence peptidase, a peptidase that degrades Ab species, in the mitochondria of AD neurons, further supporting the association of Ab with mitochondria and mitochondrial dysfunction in AD (Falkevall et al., 2006 ).
Recent studies of mitochondrial structure in brain tissue from AD patients and neuronal cells expressing mutant APP found that Ab fragments mitochondria and causes structural changes in neurons from AD patients (Hirai et al., 2001; Wang et al., 2008, Reddy and Manczak, 2008). However, it is still unclear how Ab causes mitochondrial structural and functional abnormalities in AD neurons.
Ab and Mitochondrial Structural Changes in AD
Structurally, mitochondria are compartmentalized with 2 lipid membranes: an inner membrane and an outer membrane. The inner membrane covers the mitochondrial matrix, which contains tricarboxylic acid and beta oxidation. The inner membrane houses the mitochondrial electron transport chain (ETC) and provides a highly efficient barrier to the flow of ions. The outer mitochondrial membrane is basically porous and allows the passage of low molecular-weight substances. Mitochondrial ATP is generated via oxidative phosphorylation. Through this succession of oxido-reduction reactions, electrons pass along the ETC complexes and generate an electrochemical gradient by fueling the extrusion of protons from the matrix, across the inner mitochondrial membrane complexes. ATP is then generated by the dissipation of this proton gradient through complex V. During the generation of ATP, ROS is generated as a physiological by-product of the ETC chain. During the transfer of electrons to molecular oxygen, 0.4% to 5% of ETC lose their way and participate in the formation of superoxide radicals (O2•-) (Reddy, 2008). The increase in O2•- generated from this loss of electrons ultimately activates the mitochondrial permeability transition pore, causes damage to the structure of mitochondria, and ultimately destroys the cell by apoptosis.
Several lines of evidence suggest that increased mitochondrially generated free radicals are responsible for changes in mitochondrial morphology, including mitochondrial fission and fusion imbalance (Fig. 2): 1. Barsoum et al. (2006) found that mitochondria undergo mitochondrial fission when treated with Ab in AD. 2. Bernard et al (2006) also found that when mammalian cells were treated with rotenone (pesticide), mitochondrial ROS production increased and cellular ATP production decreased, with these changes ultimately leading to mitochondrial fragmentation. 3. Yoon et al (2006) studied the link between mitochondrial fission and the high glucose-induced overproduction of ROS. They found that mitochondria are fragmented, with a concomitant increase in ROS after the exposure of cells to high glucose concentrations, suggesting that the dynamic change of mitochondrial morphology in high-glucose conditions contributes to ROS overproduction (Yoon et al., 2006). It is possible that in late-onset neurodegenerative diseases, such as AD, an increase in age-related ROS production may cause mitochondrial fragmentation, which may lead to mitochondrial fission and fusion imbalance, mitochondrial dysfunction, and neuronal damage.
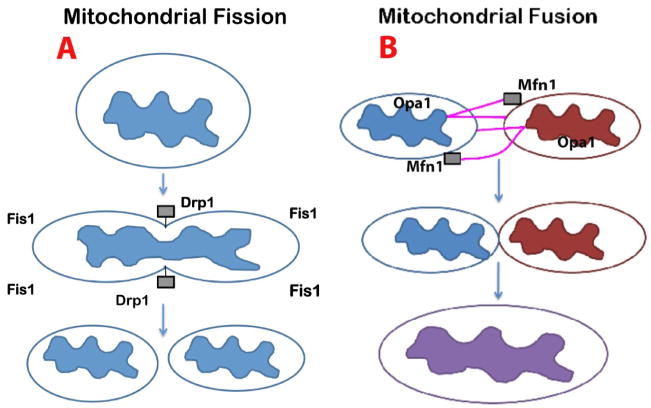
Figure 2.
Mitochondrial fission and fusion events in neurons. This figure shows the mitochondrial shape and structure are maintained by 2 opposing forces: mitochondrial fission (A) and mitochondrial fission (B). In a healthy neuron, fission and fusion mechanisms balance equally. Mitochondria alter their shape and size to move from cell body to the axons, dendrites, and synapses, and back to the cell body through mitochondrial trafficking. Fission and fusion are controlled by evolutionary conserved, large GTPases belonging to the family of dynamin. Fission is controlled and regulated by dynamin related protein (Drp1) and fission (Fis1); Fis1 is localized to the outer membrane of mitochondria. Most of the Drp1 protein is localized in the cytoplasm but a small part punctures the outer membrane, which promotes the fragmentation of mitochondria. Increased mitochondrial free radicals activate Fis1, which is also critical for mitochondrial fission. Mitochondrial fusion is controlled by 3 GTPase proteins: 2 outer-membrane localized proteins Mfn1 and Mfn2, and 1 inner-membrane localized protein Opa1. The C-terminal part of Mfn1 mediates oligomerization between Mfn molecules of adjacent mitochondria and facilitates the fusing of mitochondria. In AD neurons, mitochondrially generated free radicals activate Fis1 and promote increased mitochondrial fragmentation; this increased mitochondrial fragmentation produces defective mitochondria that ultimately damage neurons.
Based on the findings of our laboratory and others, we hypothesize that in AD, Ab is transported to mitochondria, which induces free radicals, causes mitochondrial structural and functional alterations, and ultimately damages neurons. To support this hypothesis, in a recent study using confocal and electron microscopy and M17 cells transfected with wild-type or mutant APP, Wang et al investigated the effect of APP and Ab on mitochondrial dynamics in neurons. Confocal and electron microscopic analysis demonstrated that about 40% of M17 cells overexpressing wild-type APP (APPwt M17 cells) and more than 80% of M17 cells overexpressing mutant APP (APPswe M17 cells) displayed alterations in mitochondrial morphology, particularly fragmented mitochondria. They also found increased levels of Fis1 is critical for mitochondrial fission in APPwt and APPswe M17 cells. The overexpression of APP and/or Ab -derived diffusible ligand treatment also led to mitochondrial fragmentation and reduced mitochondrial coverage in neuronal processes in differentiated primary hippocampal neurons.
In another study, Du et al (2008) investigated the connection between Ab and Cyclophilin D (a mitochondrial matrix protein) using AD postmortem brains, primary neuronal cultures from CypD knockout mice, APP transgenic mice, and double mutant mice (APP transgenic and Cyclophilin D deficient mice). Using cell biology, and electron and confocal microscopy techniques, they found that Aβ interacts with the mitochondrial matrix protein Cyclophilin D. To further investigate the interaction between Aβ and CypD, Du et al (2008) crossed Cyclophilin D knockout mice with APP transgenic mice and investigated Aβ pathology, Cyclophilin D expression, and the cognitive behavior of Cyclophilin D knockout mice, APP transgenic mice, and double-mutant mice (APP transgenic and Cyclophilin D knockout mice). They found that a deficiency in Cyclophilin D attenuated Aβ-induced mitochondrial oxidative stress, improved synaptic function, and ameliorated cognitive deficits in the double-mutant mice (Du et al., 2008). These findings suggest that a decreased interaction of Aβ with Cyclophilin D prevents the mitochondrial permeability transition pore from opening and preserves mitochondrial structure and function .
Based on findings about Aβ and mitochondria, we propose a model of mitochondrial dynamics in AD neurons (Fig. 3) in which increased APP and Aβ in association with mitochondria – mitochondrial trafficking and mitochondrial dynamics – are decreased in neurons from AD patients compared to non-demented healthy subjects. In other words, in this model of mitochondrial dynamics, increased mitochondrial APP and Aβ cause increase mitochondrial fragmentation and decrease the number of functionally active mitochondria, which ultimately imbalances mitochondrial dynamics in neurons from AD patients.
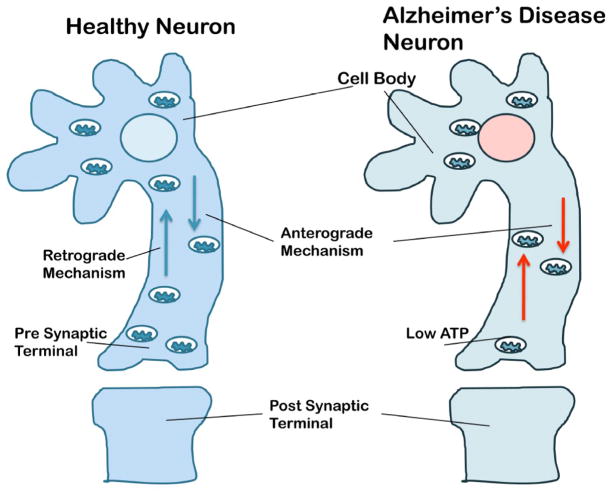
Figure 3.
Mitochondrial trafficking in neurons from healthy subjects and patients with AD. Mitochondria are synthesized in the cell body and travel along the axons and dendrites to supply energy to nerve terminals for normal neural communication; they travel back to the cell body via mitochondrial trafficking. Mitochondria are transported from the cell body to nerve terminals via an anterograde mechanism and from nerve terminals to the cell body via a retrograde mechanism. In healthy and functionally active neurons, anterograde and retrograde transport of mitochondria are equal and active. In AD neurons, both anterograde and retrograde transport of mitochondria are slow because of the presence of large number of defective and functionally inactive mitochondria. These defective mitochondria are not able to supply sufficient levels of energy at nerve terminals, which may impair neurotransmission, and ultimately result in synaptic damage, neurodegeneration, and cognitive decline in AD patients.
Ab Oligomers, Abnormal Intracellular Calcium Levels, and AD
Abnormal homeostasis of intracellular Ca2+ levels has been observed in aging and AD (Brown et al 2004, 2006; Naga et al 2008; Bezprozvanny and Mattson 2008; Giasomello et al., 2007; Norenberg and Rao, 2007; Mattson, 2007; Stavrovskaya and Kristal, 2005; Brzyska and Elbaum 2003; Huang et al., 2003; Chan et al., 2002; LaFerla, 2002, Pascale and Etcheberrigaray, 1999). Several lines of evidence support the involvement of calcium dyshomeostasis in AD. Mutant presenilins are known to increase the intracellular Ca2+ levels in AD neurons, and promote the entry of Ca2+ levels into mitochondria (Leissring et al., 2000; Chan et al., 2000; Begley et al., 1999; Guo et al., 1998; Furukawa et al., 1998), and Aβ has been reported to promote Ca2+ entry into neurons and neuronal mitochondria (Green and LaFerla, 2008; Sanz-Blasco et al., 2008; Mattson, 1994). It was unclear until recently which form of Ab – monomeric, oligomeric, or protofibrils – promotes the entry of Ca2+ into neurons and neuronal mitochondria. Oligomers of Aβ have been reported to be more toxic to neurons than other forms of Ab . However, the link between putative changes in intracellular Ca2+ and cell damage is still not completely understood. As discussed above, increased intramitochondrial Ca2+ may help damage the inner mitochondrial membrane and may lead to structural and functional abnormalities of mitochondria (Fig. 4).
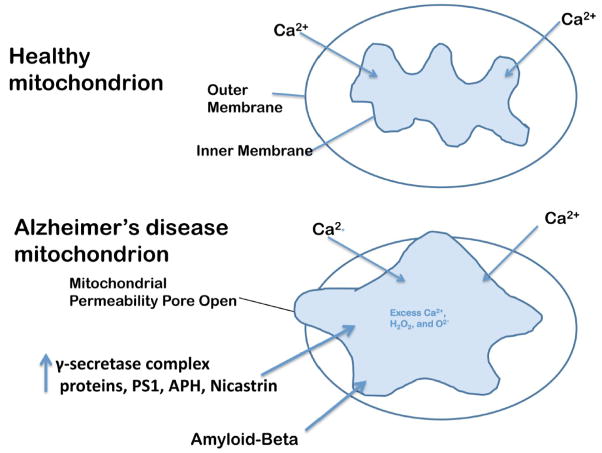
Figure 4.
Opening of the mitochondrial permeability transition pore. The increased entry of APP and Ab into mitochondria, and the increase in Ca2+ levels in the matrix of mitochondria may cause the inner mitochondrial pore to open. In general, the inner mitochondrial membrane provides a highly efficient barrier to ionic flow and protects mitochondria from toxic insults. However, in neurons from patients with AD, an age-dependent accumulation of APP and Ab1–42 oligomers and g-secretase complex proteins, PS1, APH and nicastrin may induce a massive entry of Ca2+ into neurons and may promote mitochondrial Ca2+ overload. Excess mitochondrial Ca2+ may promote the opening of the mitochondrial permeability transition pore and may destroy the neuron by apoptotic cell death.
Recently, using photon-counting imaging of neurons expressing targeted aequorin, Nunez et al (2007) addressed the effects of Aβ assembly state on Ca2+ influx and mitochondrial Ca2+ uptake (Nunez et al. 2007). Sanz-Blasco et al (2008) investigated the connection between oligomers, and intracellular and intramitochondrial calcium levels using cerebellar granule cells and Ab oligomers (Sanz-Blasco et al 2008). They found that only Ab1–42 oligomers induced a massive entry of Ca2+ into neurons and promoted mitochondrial Ca2+ overload. Ab oligomers also induced mitochondrial permeability transition, cytochrome c, apoptosis, and cell death. Further, they found mitochondrial depolarization prevented mitochondrial Ca2+ overload, the release of cytochrome c, and cell death. In addition, Sanz-Blasco et al also observed that a series of non-steroidal anti-inflammatory drugs, including salicylate, sulindac sulfide, indomethacin, ibuprofen, and R-flurbiprofen, depolarized mitochondria and inhibited an overload of mitochondrial Ca2+, the release of cytochrome c, and cell death induced by Ab oligomers. Findings from this study suggest that Ab oligomers induce intracellular Ca2+ and promote the entry of Ca2+ into mitochondria, and cause mitochondrial structural and functional damage. Overall, these findings suggest that abnormal calcium homeostasis in relation to mitochondria and Ab oligomers play a key role in AD pathogenesis.
Ab , Mitochondrial Damage, and Synaptic Alterations in AD
Synaptic damage and the loss of synapses are critical factors responsible for cognitive decline in aged individuals and in patients with AD (Reddy and Beal, 2008). Using electron microscopy, several studies investigated the connection between synaptic loss and cognitive decline in AD (DeKosky et al., 1996; Bertoni-Freddari et al., 1990) and found a 25–30% decrease in synapses in the cortex and a 15–35% decrease in synapses per cortical neuron, suggesting that synaptic loss correlates with cognitive decline in AD patients more than does the number of Aβ plaques, the number of neurofibrillary tangles, or the loss of neurons. Several researchers investigating synaptic proteins in AD patients and healthy control subjects found fewer pre- and post-synaptic proteins in AD patients in comparison to control subjects, suggesting that pre- and post-synaptic proteins are critically involved in AD progression (Gylys et al., 2004; Reddy et al., 2005; Almeida et al., 2005).
Recently, using electron microscopy, a rapid Golgi method, and Bodian staining, Baloyannis et al (2007) investigated synaptic alterations including synapses and dendritic spines in the medial geniculate bodies and the inferior colliculi in subjects with early AD and control subjects. They found substantial neuronal loss and synaptic alterations in the medial geniculate bodies of the AD subjects, as well as in their inferior colliculi. Dendritic spines of the polyhedral and elongated cells of the medial geniculate bodies decreased. Mitochondrial alterations and fragmentation of Golgi apparatus were seen in 15% of the neurons of the medial geniculate bodies and in 5% of the neurons of the inferior colliculi. However, senile plaques and neurofibrillary tangles were not seen in either the medial geniculate bodies or the inferior colliculi. Based on these observations, Baloyannis et al concluded that neuronal loss, and the mitochondrial and synaptic alterations in the medial geniculate bodies and inferior colliculi are involved in the impairment of communication and symbolic sound perception in the early stages of AD.
Findings from electron microscopy, rapid Golgi, Bodian staining, and immunoblotting analyses of brain specimens from AD patients and control subjects suggest that the loss of synapses and synaptic proteins, and alterations in mitochondria and Golgi apparatus are critically involved in synaptic damage and cognitive decline in patients with AD.
Conclusions and Future Directions
Mitochondria are the major source of energy for the normal function of brain cells. Several lines of evidence suggest that Aβ and APP are localized to mitochondrial membranes, interact with mitochondrial proteins, increase ROS production, cause mitochondrial structural and functional damage, and prevent neurons from functioning normally. Further, Ab is transported into mitochondria via the translocase of the outer membrane machinery, and mitochondrial Ab are localized to the cristae of the inner mitochondrial membrane, and Ab are localized to matrix and outer membrane. Oligomeric Ab and g -secretase complex proteins are reported to induce intracellular Ca2+ levels and promote excess accumulation of mitochondrial Ca2+, induce the opening of mitochondrial permeability transition pore, in addition to interaction of mitochondrial proteins and damage mitochondrial intact structure. Based on recent initial APP/Aβ and mitochondrial structural studies, we propose that the overexpression of APP and the increased production of Aβ may cause structural changes in mitochondria, may increase the production of defective mitochondria, and may decrease mitochondrial trafficking and cause abnormal mitochondrial dynamics in neurons that are affected in AD. Further, the numbers of mitochondria per AD-affected neuron (cortical and hippocampal) and per AD-unaffected neuron (Purkinje) need to be quantified in order to determine the connection between the number of healthy and functionally active mitochondria and the synaptic activity in the mitochondria of affected and unaffected neurons in AD mice. The answers to these critical questions may improve our basic understanding of mitochondria, synaptic activity, and cognitive function in AD, and may help define a line of research for the development of treatments to AD patients.
Acknowledgments
The research for this article was supported by grants from National Institutes of Health (AG028072 and AG026051).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Devi L. Amyloid precursor protein and mitochondrial dysfunction in Alzheimer’s disease. Neuroscientist. 2007 Dec;13:626–638. doi: 10.1177/1073858407303536. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, Mauroudis I, Manolides SL, Manolides LS. Synaptic alterations in the medial geniculate bodies and the inferior colliculi in Alzheimer's disease: a Golgi and electron microscope study. Acta Otolaryngol. 2008;30:1–3. doi: 10.1080/00016480802579074. [DOI] [PubMed] [Google Scholar]
- Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T. Rossignol R.Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley JG, Duan W, Chan S, Duff K, Mattson MP. Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J Neurochem. 1999;72:1030–1039. doi: 10.1046/j.1471-4159.1999.0721030.x. [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Casoli T, Meier-Ruge W, Ulrich J. Morphological adaptive response of the synaptic junctional zones in the human dentate gyrus during aging and Alzheimer's disease. Brain Res. 1990;517:69–75. doi: 10.1016/0006-8993(90)91009-6. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Geddes JW, Sullivan PG. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J Bioenerg Biomembr. 2004;36:401–406. doi: 10.1023/B:JOBB.0000041775.10388.23. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Brzyska M, Elbaum D. Dysregulation of calcium in Alzheimer's disease. Acta Neurobiol Exp (Wars) 2003;63:171–183. doi: 10.55782/ane-2003-1465. [DOI] [PubMed] [Google Scholar]
- Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer'sdisease. FASEB J. 2005;19:2040–1. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- Chan SL, Culmsee C, Haughey N, Klapper W, Mattson MP. Presenilin-1 mutations sensitize neurons to DNA damage-induced death by a mechanism involving perturbed calcium homeostasis and activation of calpains and caspase-12. Neurobiol Dis. 2002;11:2–19. doi: 10.1006/nbdi.2002.0542. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K, Giordano T, Brady DR, Stoll J, Martin LJ, Rapoport SI. Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Brain Res Mol Brain Res. 1994;24:336–340. doi: 10.1016/0169-328x(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K, Hatanpää K, Brady DR, Rapoport SI. Evidence for physiological down-regulation of brain oxidative phosphorylation in Alzheimer's disease. Exp Neurol. 1996;42:80–88. doi: 10.1006/exnr.1996.0180. [DOI] [PubMed] [Google Scholar]
- Coskun PE, Beal MF, Wallace DC. Alzheimer's brains harbor somatic mtDNA control-region mutationsthat suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA. Copper-dependent inhibition of human cytochrome coxidase by a dimeric conformer of amyloid-beta1–42. J Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5:417–421. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana A, Simic G, Sinforiani E, Orrù N, Pichiri G, Bono G. Mitochondria morphology and DNA content upon sublethal exposure to beta-amyloid(1–42) peptide. Coll Antropol Suppl. 2008;1:51–58. [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentinm JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuatesmitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A, Hauptmann S, Scherping I, Rhein V, Muller-Spahn F, Götz J, Muller WE. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis. 2008;5:157–159. doi: 10.1159/000113689. [DOI] [PubMed] [Google Scholar]
- Falkevall A, Alikhani N, Bhushan S, Pavlov PF, Busch K, Johnson KA, Eneqvist T, Tjernberg L, Ankarcrona M, Glaser E. Degradation of the amyloid beta-protein by the novel mitochondrial peptidasome, PreP. J Biol Chem. 2006;281:29096–29104. doi: 10.1074/jbc.M602532200. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Guo Q, Schellenberg GD, Mattson MP. Presenilin-1 mutation alters NGF-induced neurite outgrowth, calcium homeostasis, and transcription factor (AP-1) activation in PC12 cells. J Neurosci Res. 1998;52:618–624. doi: 10.1002/(SICI)1097-4547(19980601)52:5<618::AID-JNR14>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007;14:1267–1274. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- Green KN, LaFerla FM. Linking calcium to Abeta and Alzheimer's disease. Neuron. 2008;59:190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Guo Q, Robinson N, Mattson MP. Secreted beta-amyloid precursor protein counteracts the proapoptotic action of mutant presenilin-1 by activation of NF-kappaB and stabilization of calcium homeostasis. J Biol Chem. 1998;273:12341–12351. doi: 10.1074/jbc.273.20.12341. [DOI] [PubMed] [Google Scholar]
- Gylys KH, Fein JA, Yang F, Wiley DJ, Miller CA, Cole GM. Synaptic changes in Alzheimer's disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol. 2004;165:1809–1817. doi: 10.1016/s0002-9440(10)63436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HM, Zhang H, Xu H, Gibson GE. Inhibition of the alpha-ketoglutarate dehydrogenase complex alters mitochondrial function and cellular calcium regulation. Biochim Biophys Acta. 2003;1637:119–126. doi: 10.1016/s0925-4439(02)00222-3. [DOI] [PubMed] [Google Scholar]
- Kaminsky YG, Kosenko EA. Effects of amyloid-beta peptides on hydrogen peroxide-metabolizing enzymes in rat brain in vivo. Free Radic Res. 2008;42:564–573. doi: 10.1080/10715760802159057. [DOI] [PubMed] [Google Scholar]
- Keil U, Bonert A, Marques CA, Scherping I, Weyermann J, Strosznajder JB, Muller-Spahn F, Haass C, Czech C, Pradier L, Muller WE, Eckert A. Amyloid beta-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J Biol Chem. 2004;279:50310–50320. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J Cell Biol. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Beal MF, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer's disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidativedamage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- Maurer I, Zierz S, Möller HJ. A selective defect of cytochrome coxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Akao Y, Yi H, Shamoto-Nagai M, Maruyama W, Naoi M. Overexpression of amyloid precursor protein induces susceptibility to oxidative stress in human neuroblastoma SH-SY5Y cells. J Neural Transm. 2006 Feb;113:125–135. doi: 10.1007/s00702-005-0318-0. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Secreted forms of beta-amyloid precursor protein modulate dendrite outgrowth and calcium responses to glutamate in cultured embryonic hippocampal neurons. J Neurobiol. 1994;25:439–450. doi: 10.1002/neu.480250409. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Naga KK, Sullivan PG, Geddes JW. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez L, Senovilla L, Sanz-Blasco S, Chamero P, Alonso MT, Villalobos C, García-Sancho J. Bioluminescence imaging of mitochondrial Ca2+ dynamics in soma and neurites of individual adult mouse sympathetic neurons. J Physiol. 2007;580:385–395. doi: 10.1113/jphysiol.2006.126524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Kim SS, Seong YM, Kim KH, Goo HG, Yoon EJ, Min do S, Kang S, Rhim H. Beta-amyloid precursor protein is a direct cleavage target of HtrA2 serine protease. Implications for the physiological function of HtrA2 in the mitochondria. J Biol Chem. 2006;281:34277–34287. doi: 10.1074/jbc.M603443200. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Pascale A, Etcheberrigaray R. Calcium alterations in Alzheimer's disease: pathophysiology, models and therapeutic opportunities. Pharmacol Res. 1999;39:81–88. doi: 10.1006/phrs.1998.0411. [DOI] [PubMed] [Google Scholar]
- Praticò D. Oxidative stress hypothesis in Alzheimer's disease: a reappraisal. Trends Pharmacol Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer's disease. J Neurochem. 2006;96:1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid & Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer's disease? Brain Res Brain Res Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Jr, Kaye J, Manczak M. Differential loss of synaptic proteins in Alzheimer's disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;27:103–117. doi: 10.3233/jad-2005-7203. [DOI] [PubMed] [Google Scholar]
- Reddy PH, McWeeney S. Mapping cellular transcriptosomes in autopsied Alzheimer's disease subjects and relevant animal models. Neurobiol Aging. 2006;27:1060–1077. doi: 10.1016/j.neurobiolaging.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: upregulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Hum Mol Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- Sanz-Blasco S, Valero RA, Rodríguez-Crespo I, Villalobos C, Núñez L. Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS ONE. 2008;3:e2718. doi: 10.1371/journal.pone.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Lepsverdize E, Chi SL, Das AM, Pizzo SV, Dityatev A, Schachner M. Amyloid precursor protein and amyloid beta-peptide bind to ATP synthase and regulate its activity at the surface of neural cells. Mol Psychiatry. 2008;13:953–969. doi: 10.1038/sj.mp.4002077. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Hyman BT. Functional alterations in Alzheimer's disease: selective loss of mitochondrialencoded cytochrome oxidase mRNA in the hippocampal formation. J Neuropathol Exp Neurol. 1994;53:508–512. doi: 10.1097/00005072-199409000-00010. [DOI] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Stavrovskaya IG, Kristal BS. The powerhouse takes control of the cell: is the mitochondrial permeability transition a viable therapeutic target against neuronal dysfunction and death? Free Radic Biol Med. 2005;38:687–967. doi: 10.1016/j.freeradbiomed.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Butterfield DA. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer's disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid Redox Signal. 2006;8:2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Treating neurodegeneration by modifying mitochondria: potential solutions to a complex problem. Antioxid Redox Signal. 2007;9:1591–603. doi: 10.1089/ars.2007.1676. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer's disease. J Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YS, Yoon DS, Lim IK, Yoon SH, Chung HY, Rojo M, Malka F, Jou MJ, Martinou JC, Yoon G. Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol. 2006;209:468–480. doi: 10.1002/jcp.20753. [DOI] [PubMed] [Google Scholar]