Abstract
A central question in the study of selective attention is whether top-down attentional control mechanisms are generalized or specialized for the type of information that is to be attended. The current study examined this question using a voluntary orienting task that cued observers to attend to either one of two locations or to one of two colors. Location (spatial) and color (nonspatial) conditions were presented either randomly intermixed within the same block of trials or in separate blocks. Functional magnetic resonance imaging revealed that directing attention to a location or to a color activated a network of overlapping dorsal frontal and parietal areas, previously implicated in attentional control. The pattern of observed overlap was not affected by the intermixed versus blocked presentation of location and color conditions. Although portions of the frontal-parietal network were more active in response to location cues than to color cues, a secondary analysis also revealed that medial dorsal frontal and parietal cortex were specifically engaged in shifting visual attention regardless of the cued dimension (location or color). Together, the present results support the conclusion that attentional control is the combination of a generalized network that works in concert with sub-regions of the fronto-parietal network that are highly specialized for directing attention based on the content of the to-be-attended information.
Keywords: attentional control, fMRI, selective attention, spatial attention, nonspatial attention, frontoparietal network
1. Introduction
At any given moment, multiple representations compete for limited processing resources and for control of behavior. Some models posit that attentional control processes play a critical role in resolving this competition by selectively enhancing representations of task-relevant information and/or by inhibiting representations of irrelevant information (e.g., Desimone and Duncan, 1995; LaBerge, 1995; Miller and Cohen, 2002). There is considerable disagreement, however, as to whether such control mechanisms operate across a wide range of sensory representations (Posner et al., 1982) or are somewhat specific to the type of information that is goal-relevant (Nobre et al., 2001). The purpose of the present study was to address this longstanding controversy.
A popular method for investigating the generality of attentional control processes has been through the use cued attention paradigms (Posner et al., 1980), in which a cue instructs subjects to voluntarily orient their attention to a particular stimulus feature (e.g., color, spatial location, etc.) of an upcoming target stimulus. To date, the vast majority of functional magnetic resonance imaging (fMRI) studies of voluntary orienting tend to support the view that attentional control mechanisms operate across a wide range of sensory representations. Most of these studies have examined voluntary orienting to visual information. Voluntary orienting to spatial (Corbetta et al., 2000, 2005; Giesbrecht et al., 2003; Hopfinger et al., 2000; Shulman et al., 2002b; Woldorff et al., 2004) and non-spatial (Giesbrecht et al., 2003; Luks & Simpson, 2004; Liu et al., 2003; Shulman et al., 2002a; Weissman et al., 2002; Wilson et al., 2005) visual information has been associated with a common network of neural areas that includes portions of dorsal frontal cortex, near the human homolog of the frontal eye fields, and dorsal parietal cortex, along the intraparietal sulcus. Interestingly, many studies have observed cue-triggered activity in visual areas that are specialized for processing the attended information before targets are presented (Giesbrecht et al., 2003, 2006; Hopfinger et al., 2000; Kastner et al., 1998, 1999; Ress et al., 2000; Woldorff et al., 2004), suggesting that attentional control circuitry in dorsal frontal and parietal cortex biases activity in the visual cortex to favor the processing of relevant stimuli over that of irrelevant stimuli. Nonetheless, the finding that very similar regions of the frontal-parietal attention network are activated by cues to attend to spatial and non-spatial stimulus features suggests that attentional control processes are fairly general and operate across a wide range of sensory representations.
Despite the evidence supporting a fairly generalized control network, several models posit that top-down selection of sensory representations depends upon the neural circuitry that enables working memory, which may differ for spatial and non-spatial sensory information (Desimone and Duncan, 1995; LaBerge, 1995). In these models, the posterior parietal cortex works together with the dorsolateral prefrontal cortex to process spatial information, while the anterior infero-temporal cortex and the ventrolateral prefrontal cortex process object features. Although there is some evidence that supports this view (Golman-Rakic, 1987; Levy and Goldman-Rakic, 2000; Banich et al., 2000), the dorsal-ventral distinction in attentional paradigms has not been specifically linked to cue-triggered control processes that guide the selection of task-relevant sensory representations (Corbetta and Shulman, 2002; Giesbrecht and Mangun, 2005). In addition, several studies in both monkeys (e.g., Rao et al., 1997) and humans (e.g., D’Esposito et al., 1998) have reported large overlap in the frontal brain areas involved in representing spatial and non-spatial types of information. Thus, the available data are still more consistent with a generalized control network than with a control network that includes specialized subregions for processing distinct types of sensory representations.
Although the hypothesis that the frontal-parietal attentional control network is generalized for controlling visual attention is plausible, the possibility that certain regions of the frontal-parietal network are specialized for particular types of visual information cannot be fully excluded for the following three reasons. First, while many previous fMRI studies have examined the neural mechanisms underlying spatial and nonspatial attentional orienting, few have included conditions that permit a direct comparison between spatial and nonspatial control systems. It is possible that while spatial and non-spatial attention rely on similar neural control circuitry, they differentially recruit specific brain areas within this circuitry. In line with this possibility, using a within-subjects design to directly compare spatial and non-spatial attention, Giesbrecht et al. (2003) showed that although similar regions of frontal and parietal cortex were activated when attention was precued to attend to upcoming spatial or nonspatial information, certain regions within dorsal frontal and parietal cortex were more strongly activated when attention was directed to a spatial location. These findings indicate that some subregions of the fronto-parietal control network may be selectively involved in spatial attentional orienting.
A second reason that previous fMRI studies may not have observed (much) dimension-specific activity within the fronto-parietal network is that most prior fMRI studies randomly intermixed spatial and nonspatial trials within the same experimental blocks (e.g., Giesbrecht et al., 2003). Recent work has suggested that intermixing trials may lead subjects to adopt generic task strategies that permit preparation for all the different task possibilities (e.g., Kleinsorge et al., 2004; Slagter et al., 2006). Thus, the overlap in activated brain areas observed in some prior studies (e.g., Giesbrecht et al., 2003) may not provide a true characterization of the degree of specialization for spatial and nonspatial attentional control.
Third, although many models of attention posit that voluntary orienting can be fractionated into several processes, relatively few studies have investigated whether the neural bases of control of spatial and nonspatial attentional orienting varies for specific processes. For example, according to one influential model of visuospatial attention, voluntary orienting is mediated by three cognitive operations: disengagement of attention from its current focus, shifting attention to the new location, and engagement of attention at the new focus (Posner and Peterson, 1990). These operations have been associated with distinct brain circuits (Corbetta and Shulman, 2002). It is therefore possible that some orienting functions are generalized with respect to the type of visual information that is task-relevant, whereas others are not. This notion receives some support from a detailed comparison (Serences et al., 2005) of the atlas coordinates identified in different fMRI studies conducted in the laboratory of Yantis and colleagues (Liu et al., 2003; Serences et al., 2004; Yantis et al., 2002). This across-studies analysis revealed some differences in the brain areas that were transiently active during shifts of attention to locations, features, or objects, suggesting that suggest that functional compartments may exist within the fronto-parietal network, which are specialized for the control of shifts of attention within distinct perceptual domains (i.e., space, feature, object). However, few fMRI studies have included conditions that permit a direct comparison between spatial and nonspatial attentional orienting and their specific sub-functions. For example, in the Giesbrecht et al. (2003) study, activity elicited by spatial and nonspatial cues was directly compared, but no dissociation was made between trials in which attention was shifted versus maintained on the cued feature or location. In another fMRI study (Slagter et al., 2006) in which attention-shift-related preparatory activity was isolated, cue-triggered responses were collapsed across spatial and nonspatial attention-directing conditions. Thus, on the basis of these previous studies, it is unclear whether the various processes that enable attentional orienting are generalized with respect to the type of information that is to be attended.
The purpose of the present work was to address the above issues and gain more insight into the generality of spatial and nonspatial attentional control. To this end, participants performed an attentional cueing task while their brain activity was measured using fast-rate event-related fMRI1. In each trial of our task, participants were cued to prepare to discriminate the orientation of an upcoming target rectangle that would be displayed in the cued color (yellow or blue) or at the cued location (left or right) (see Figure 1). In many trials, a target display followed cue presentation, and the participant’s task was to indicate the orientation of the cued rectangle (vertical or horizontal) within that display. In some trials, however, no target display followed cue presentation. These cue-only trials allowed us to isolate cue-related preparatory activity without contamination of target-related activity. Subjects participated in two experimental sessions, one in which the color and location trials were randomly intermixed within blocks of trials (mixed task) and one in which color and location trials were presented in separate blocks of trials (blocked task).
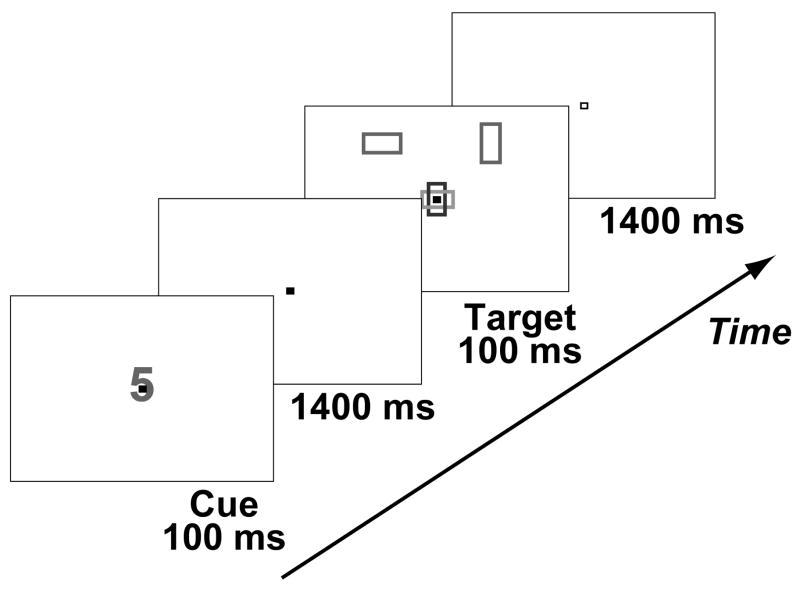
Figure 1.
Example of a cue-plus-target trial. The cue instructed participants to attend to a location (left, right) or color (blue, yellow) and to indicate the orientation (horizontal or vertical) of the rectangle that possessed the cued feature in the upcoming stimulus display. In the actual displays viewed by the participants, the back ground was dark grey, cues were light gray, both location targets were green, one color target was blue, and the other was yellow.
To address the issue of whether the overlap in the spatial and nonspatial attentional control networks is due to the intermixing of the tasks, we compared the overlap in color- and location-cue evoked activity in the blocked and mixed tasks. We predicted that if the intermixed presentation of the spatial and nonspatial orienting conditions resulted in the use of more generalized task strategies, there should be more dimension-specific activity in the blocked task, in which color and location conditions were presented in separate runs, than in the mixed task.
To address the issue of the specificity of the attention shift mechanism, we isolated cortical regions involved in attention shifting. To this end, each trial in the mixed task was categorized relative to the preceding trial. Each trial could be preceded by a trial in which the same dimension (i.e., location or color) and feature (left-right, blue-yellow) was cued (e.g., left - left; repeat trial), a different feature within the same dimension was cued (e.g., left – right; switch-within trial) or a feature within a different dimension was cued (e.g., left – blue; switch-across trial). Comparison of cue-related activity elicited in the different trial types permitted isolation of processes specific to shifting of spatial and nonspatial attention. We predicted that if spatial and nonspatial attention rely on different shift mechanisms, then distinct brain areas in frontal or parietal cortex should exhibit dimension-specific shift-related activity.
2. Results
2.1. Behavior
Mean behavioral performance measures for the mixed and blocked conditions are listed in Table 1. The intermixed versus blocked presentation of color and location cue conditions had no effect on response times (all Fs(1,13)<2.4, p>.15), error rates (all Fs(1,13)<1, p>.93) or omitted response rates (all Fs(1,13)<1.8, p>.20) (see Table 1). Thus, at least at the behavioral level, whether or not spatial and nonspatial attention conditions were intermixed in the same run did not appear to influence performance on discriminating the location or color targets.
Table 1.
Reaction times (in ms) and the percentage (%) of cue+target trials in which an error was made or a response omitted (misses), when color or location was task-relevant, displayed separately for the Blocked and the Mixed task. Standard error of mean is given in parentheses.
| Blocked Task | Mixed task | |||
|---|---|---|---|---|
| Color | Location | Color | Location | |
| RT | 703 (31) | 657 (33) | 671 (27) | 649 (27) |
| % Errors | 3.9 (0.7) | 3.6 (0.8) | 4.6 (1.0) | 3.6 (0.8) |
| % Misses | 5.8 (2.3) | 5.0 (1.7) | 4.3 (1.7) | 4.0 (1.5) |
Mean behavioral performance measures as a function of whether the trial was a repeat, switch-within, or switch-across trial are shown in Table 2. Response times were fastest for repeat trials in both blocked and mixed conditions (main effect of Switch; blocked task: F(1,13)=17.5, p=.001; mixed task: F(2,12)=19.3, p<.001). Planned comparisons revealed no differences in response time between switch-across and switch-within trials in the mixed task (F(1,13)=1.6, p>.23). Participants were faster when having to discriminate the orientation of the target rectangle when location was cued compared to when color was cued (main effect of Dimension (color, location): F(1,13)=12.3, p<.005 (blocked task); F(1,13)=6.6, p<.03 (mixed task)). Critically, however, the interaction between Switch and Dimension was not significant in either task (blocked task: F(1,13)<1, p>.78; mixed task: F(2,12) =1.2, p>.35). This precludes an interpretation of any putative differences in switch-related brain activity between color and location cue conditions in terms of differences in general task difficulty.
Table 2.
A) Performance (reaction times (RT), error and omitted response rates) in the blocked task: Switch W(ithin) and Repeat. Standard error of mean is given in parentheses. B) Performance (reaction times (RT), error and omitted response rates) in the mixed task: Switch W(ithin), Switch A(cross) and Repeat. Standard error of mean is given in parentheses.
| Table 2A | ||||
|---|---|---|---|---|
| Color Cue | Location Cue | |||
| Repeat | Switch W | Repeat | Switch W | |
| RT | 689 (30) | 718 (33) | 640 (33) | 673 (35) |
| % Errors | 3.4 (0.9) | 4.4 (1.0) | 4 (0.9) | 3.9 (1.2) |
| % Misses | 4.7 (1.8) | 6.9 (2.9) | 4.8 (1.8) | 5.2 (1.8) |
| Table 2B | ||||||
|---|---|---|---|---|---|---|
| Color Cue | Location Cue | |||||
| Repeat | Switch W | Switch A | Repeat | Switch W | Switch A | |
| RT | 630 (24) | 697 (31) | 684 (30) | 626 (28) | 661 (29) | 660 (25) |
| % Errors | 4.4 (1.4) | 5.7 (1.6) | 3.6 (1.0) | 3.3 (1.2) | 2.9 (0.9) | 4.5 (0.9) |
| % Misses | 3.3 (1.5) | 2.8 (1.8) | 6.7 (1.9) | 4 (1.3) | 3.3 (2.1) | 4.6 (1.6) |
Analysis of the error rates revealed no significant main effects or interactions in either blocked or mixed conditions (all Fs<1.7, all ps>.22). There were also no differences between omitted response rates (misses) on repeat and switch-within trials in the blocked task (all Fs<1.4, all ps>.25; Table 2a) However, omitted response rates were 1.6% lower on switch-within trials compared to switch-across and repeat trials in the mixed task (main effect Switch; F(2,26)=10.69; p<.001; Table 2b)
2.2 fMRI results
The aim of the present study was to investigate two unresolved issues that pertain to the generality of the fronto-parietal control network. First, we wanted to determine whether presenting spatial and nonspatial attention-directing conditions in separate blocks of trials would evoke more dimension-specific orienting activity relative to when spatial and non-spatial conditions were intermixed within the same block. Second, we wanted to determine the generality of the attentional shift mechanism, as would be revealed by directly comparing location and color-cue switch-related activity. Before describing the results from analyses examining these two issues, we first present the results of an analysis demonstrating that the current fast-rate event-related paradigm replicates the previous literature showing overlap between spatial and nonspatial attentional control systems (Giesbrecht et al., 2003).
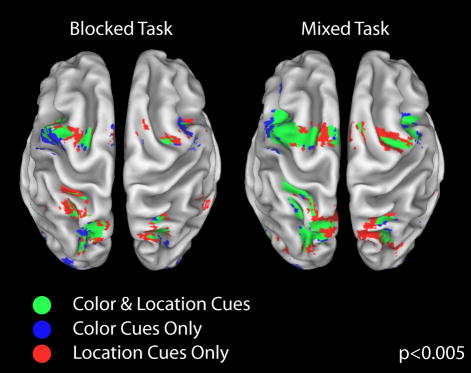
To assess whether the present design replicates previous studies showing overlap between spatial and nonspatial control regions, we first isolated cortical areas activated by color and location cues in both the blocked and mixed task. Shown in Figure 2 are regions activated by location cues (blue), regions activated by color cues (red), and regions activated by both types of cues (green). In line with previous studies (Giesbrecht et al., 2003), color and location cues generally activated similar regions of frontal and parietal cortex in the mixed task (Fig. 2, right panel), as well as in the blocked task (Fig. 2, left panel). The areas of overlap in both tasks included bilateral dorsal parietal cortex along the intraparietal sulcus (extending dorsomedially into the superior parietal lobule and anteriorly towards the postcentral sulcus), bilateral dorsal posterior frontal cortex at the intersection of the precentral and superior frontal sulci, and parts of medial frontal cortex. It is important to note that these activations include all processes elicited by the cues, i.e., attentional orienting processes as well as other attentional orienting-unspecific processes, such as cue identification, cue-symbol interpretation and motor preparation. As a next step therefore, we examined whether some of these cue-related brain areas were specifically activated by one type of cue (e.g., spatial) versus the other (e.g., color), by directly comparing color and location cue-only responses, separately for the blocked and mixed tasks.
Figure 2.
Cue-related activity. Group-averaged data for brain regions significantly activated by color and location attention-directing cues in the blocked task (left panel) and in the mixed task (right panel), overlaid onto a brain rendered in 3D. Areas activated by only location cues are shown in red, only color cues in blue, and those areas activated by both cues are shown in green. Cue-related activations are rendered on a partially inflated reconstruction of a spatially normalized anatomical volume using Caret (Van Essen et al., 2001; http://brainmap.wustl.edu/caret) and displayed using a height threshold of P <0.005 (uncorrected) and an extent threshold of 8 contiguous voxels.
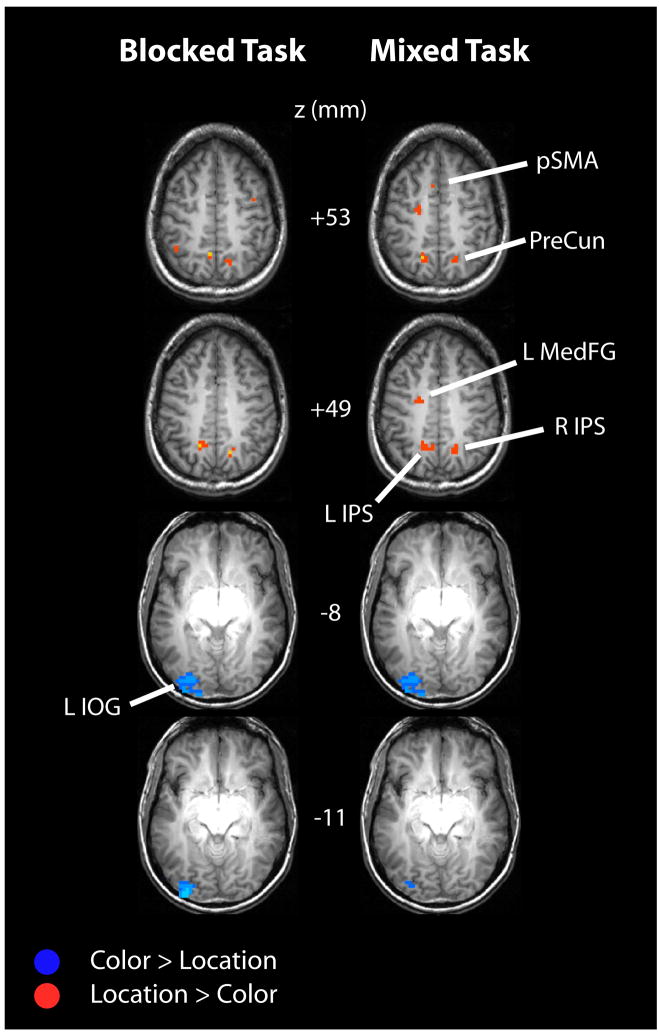
The direct comparison of color and location cue-related activity, shown in Figure 3, revealed that some brain areas were more specific to one type of attention compared to the other (see Table 3 for their coordinates and F-values). In the mixed task, regions that were selectively activated in response to location cues included: parietal cortex along the intraparietal sulcus, extending medially into the precuneus in both hemispheres; and left frontal cortex, at the intersection of the superior frontal gyrus and the precentral sulcus; and dorso-medial frontal cortex. These location-specific brain areas are very similar to the ones identified by Giesbrecht et al. (2003) using the same within-subject statistical comparison of color versus location cue-elicited activity. In the blocked task, both the left and right intraparietal sulcus and the precuneus also showed stronger activation in response to location cues than to color cues. The atlas coordinates of the location-cue-specific activations found in both the blocked and mixed task were generally very comparable to each other (see Table 3). In addition to these location-specific regions of frontal and parietal cortex, an area in left inferior occipital cortex exhibited greater activation to color than location cues in both the mixed and blocked tasks.
Figure 3.
Dimension-specificity in attentional control. Brain areas showing dimension-specific activity in the blocked (left column) and mixed (right column) task. Brain areas that were more strongly activated when location compared to color was cued are shown in red. Brain areas that were more strongly activated when color compared to location was cued are shown in blue. Results (p<.05) from repeated measures ANOVAs with factors Dimension (location, color) and MR Frame (1.5–13 s), performed for each task separately (and only for those voxels that were activated by location OR color cues in the first place; p<.005). Abbreviations: IPS: intraparietal sulcus, IOG: inferior occipital gyrus, pSMA: pre-supplementary motor area, PreCun: precuneus, MedFG: medial frontal gyrus, L: left, and R: right.
Table 3.
MNI coordinates and F-values are listed for brain areas showing significantly greater activity to location compared to color cues (Loc > Col; top part table) or to color compared to location cues (Col > Loc; bottom part table), separately for the mixed and blocked tasks (all P’s < .05). Abbreviations: IPS=intraparietal sulcus, pSMA: pre-supplementary motor area.
| Loc > Col | x | y | z | F-value | |
|---|---|---|---|---|---|
| Mixed task | Left IPS | −19 | −68 | 56 | 3.2 |
| Right IPS | 19 | −64 | 49 | 2.9 | |
| Precuneus | 0 | −56 | 56 | 2.5 | |
| Left pSMA | −8 | 11 | 60 | 4.1 | |
| Left Medial Frontal | −23 | −11 | 49 | 2.9 | |
|
| |||||
| Blocked task | Left IPS | −19 | −60 | 49 | 3.0 |
| Right IPS | 15 | −68 | 49 | 3.1 | |
| Precuneus | −4 | −64 | 49 | 3.5 | |
|
| |||||
| Col > Loc | x | y | z | F-value | |
|
| |||||
| Mixed task | Left Occipital | −30 | −98 | −11 | |
|
| |||||
| Blocked task | Left Occipital | −30 | −94 | −4 | |
The next analysis examined whether the blocked presentation of spatial and nonspatial attention-directing conditions may cause participants to rely more on dimension-specific task preparation strategies. If true, then the blocked and mixed tasks should differ in terms of dimension-specific cue-related activity. This was statistically tested using a repeated-measures ANOVA with the within-subjects factors Task (blocked, mixed), Dimension (color, location), and MR Frame (eight TR frames, from 1.5–13.5 seconds). This direct statistical comparison of dimension-specific activity in the blocked and mixed task did not reveal any brain areas showing an interaction between Task, Dimension and MR Frame or an interaction between Task and Dimension (all p’s>.05). These results argue against the possibility that participants relied differentially on dimension-specific task preparation strategies when spatial and nonspatial attention conditions were presented intermixed within the same run compared to when they were presented in different runs.
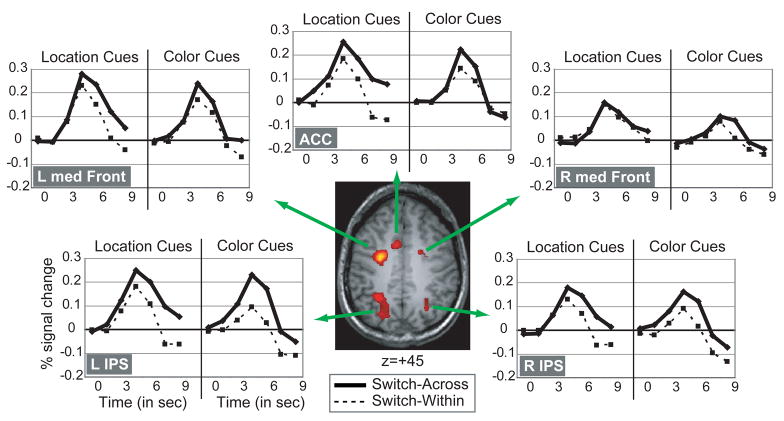
The final analysis examined the generality of the attention shift mechanism. To examine this issue, we identified brain areas that were more activated on switch-across versus switch-within cue-only trials in the mixed task. Any brain areas isolated by comparing activity on switch-across versus switch-within trials cannot be associated with processes related to the encoding of a new cue because both trial types involved encoding a cue stimulus that was different from the one presented in the preceding trial. Instead, these areas more likely mediate additional processes related to shifting attention to a new dimension different from the dimension cued in the previous trial (cf. Slagter et al., 2006). We used the brain areas that showed greater activity on switch-across versus switch-within trials to define functional regions of interest (ROIs) within which the presence of dimension-specific attention-shift-related activity was tested. Seven regions, all in dorsal frontal and parietal cortex, were more strongly activated when attention shifted across dimension versus when attention was repeated to the same dimension (see Table 4 for their coordinates and F-values). Of central importance, none of these ROIs showed a significant interaction between Dimension (color, location) and Switch Type (switch-across, switch-within) (all F’s < 1), suggesting a dimension-general mechanism of attentional control. In Figure 4, the color and location cue-triggered hemodynamic time courses are plotted for switch-across and switch-within trials separately for several of the frontal and parietal ROIs. As can be seen in this figure, shifting attention to color and shifting attention to location were associated with similar levels of percent signal change in these brain areas. In line with these ROI results, a voxel-wise comparison of location and color cue-only responses on switch-across and switch-within trials also did not reveal any brain area showing a significant interaction between Switch Type (switch-across, switch-within) and Dimension (color, location) (all p’s >.05). Interestingly, although some brain areas showed both a main effect of Switch Type and a main effect of Dimension, no brain areas showed dimension-specific shift-related preparatory activity (i.e., an interaction between Switch Type and Dimension). As can be seen Figure 5, the brain areas showing only a main effect of Switch Type and/or of Dimension included bilateral IPS and a more medial dorsal frontal area in the left hemisphere.
Table 4.
MNI coordinates and F-values of brain areas showing greater cue-triggered activity on switch-across vs. switch-within trials (collapsed across color and location cue trials) in the mixed task. Abbreviations: IPS: intraparietal sulcus, Med: medial, ACC: anterior cingulate cortex
| Brain Area | x | y | z | F-value | |
|---|---|---|---|---|---|
| Parietal | Left IPS | −23 | −60 | 53 | 5.2 |
| −26 | −79 | 41 | 3.5 | ||
| Right IPS | 34 | −60 | 60 | 4.2 | |
| 34 | −71 | 41 | 3.4 | ||
| Frontal | Left Med Frontal | −26 | −8 | 45 | 7.4 |
| Right Med Frontal | 34 | −8 | 41 | 2.9 | |
| ACC | −8 | 8 | 41 | 4.2 |
Figure 4.
Brain areas involved in cued attention shifting. Group cue-related time courses for several frontal and parietal brain areas in the mixed-task condition, showing greater cue-related activity on switch-across vs. switch-within trials (P<.005), for location and color switch-across and switch-within trials separately. When statistically comparing cue-related peak percent (%) signal change values, no area showed a significant interaction between Switch (switch-across, switch-within) and Dimension (location, color), thereby supporting the conclusion that much of this activity reflects a generic attention switching mechanism. Abbreviations: L=left, R=right, IPS=intraparietal sulcus, ACC=anterior cingulate, med Front = medial frontal.
Figure 5.
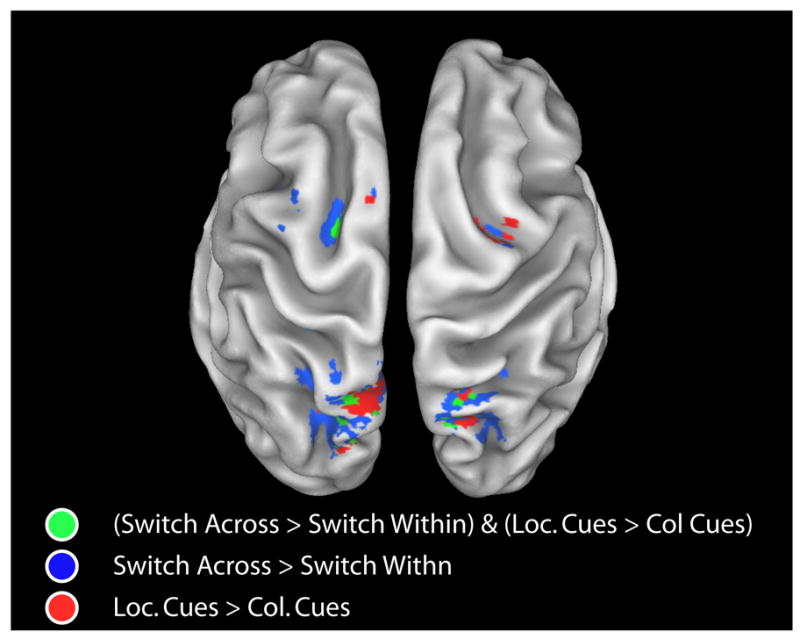
Generalization and specialization within the fronto-parietal control network. Frontal and parietal brain areas that were more strongly activated on switch-across vs. switch-within trials (p<.05) AND that were more strongly activated on location vs. color trials (p<.05) in the mixed task are shown in green. Brain areas that were more strongly activated on switch-across vs. switch-within trials are shown in blue (p<.05). Brain areas that were more strongly activated on location vs. color trials in the mixed task are shown in red (p<.05). Cue-related activations are rendered on a partially inflated reconstruction of a spatially normalized anatomical volume using Caret (Van Essen et al., 2001; http://brainmap.wustl.edu/caret).
2.3. Eye Movements
Analyses of the cue-related electro-oculogram (EOG) signal recorded from electrodes located lateral to each eye showed that participants did not significantly move their eyes in any of the task blocks during the training session. That is, the EOG traces did not significantly deviate from zero in the postcue interval in any of the tested conditions, including the left-cue and right-cue conditions (all p’s >.05).
3. Discussion
A central question in the study of selective attention is to what degree the mechanisms that control voluntary orienting are generalized with respect to the type of information that is to be attended. The aim of the present study was to investigate two issues that pertain to the generality of spatial and nonspatial control of visual attention. First, we wanted to determine whether dimension-specific orienting activity was more prevalent when spatial and nonspatial attention-directing conditions are presented in separate runs as opposed to intermixed in the same run. Second, we wished to determine the generality of mechanism by which the focus of attention is shifted. Overall, our findings indicate that attentional control in spatial and non-spatial paradigms relies upon similar neural mechanisms.
3.1 Intermixed presentation of spatial and nonspatial cues does not reduce dimension-specific preparation
Previous studies have observed overlap of brain regions activated for spatial and nonspatial cueing of attention under mixed design conditions (Giesbrecht et al., 2003). The present finding that the mixed and blocked tasks exhibited similar patterns of cue-related dimension-specific anticipatory brain activity argues against the possibility that participants rely on dimension-specific task preparation strategies to a lesser degree when attention-directing conditions are mixed within the same trial block. Instead, the present results support the notion that spatial and nonspatial control rely on common operations, which include processes that are sufficiently abstract to represent different types of inputs (e.g., Shulman et al., 2002a). Consistent with this interpretation, we recently found that the magnitude of observed attention shift-specific preparatory activity is dependent on the number of possible stimulus features that can be presented in a given trial block (Slagter et al., 2006). Thus, when the present findings are considered jointly with our previous work, they suggest that whereas the global task context can influence the recruitment of shift-related preparatory processes, it may not affect the recruitment of dimension-specific task preparation strategies.
In line with results from a previous event-related fMRI study (Giesbrecht et al., 2003), directing attention to a location in space compared to a color was associated with enhanced activation in more medial dorsal parietal and frontal regions. Recent research has suggested that parietal and frontal areas exhibit topographic representations of the visual field and that the voluntary deployment of attention can induce spatially selective attentional modulations within these regions (Serences & Yantis, 2007; Silver et al., 2005). The location-specific brain areas identified in the current study are located close to these spatially selective areas, which include the intraparietal sulcus and frontal eye fields. It is thus possible that they represent salience maps that provide the spatial coordinates of the behaviorally relevant location in the visual field. In addition, part of the location-specific activity observed in the current study may reflect aspects of the top-down attentional deployment itself as opposed to location coding, as some overlap was observed between the brain areas that were specifically activated by location cues and the brain areas that exhibited shift-specific preparatory activity. Thus, it may be that directing attention to location may have called more strongly on attention shift processes in general. Further replicating Giesbrecht et al.’s (2003) findings, left ventral occipital cortex exhibited more activity when attention was directed to a color versus a location. As visual cortex is generally considered likely to be a target of top-down signals, rather than a source of such signals (Corbetta & Shulman, 2002), it is conceivable that the observed pattern of selectivity in response to the color cues in visual cortex reflects the enhancement of color-specific areas in preparation for the target display (e.g., Chawla et al., 1999; Giesbrecht et al., 2006). These data thus indicate that the selection of the target rectangle was based on the feature information (i.e., specific color) provided by the cue.
One potential explanation for the location specific activations is that subjects were making eye movements during the task. Although this possibility cannot fully be excluded because eye movement were not recorded during the scanning session, there are two reasons that make it unlikely that the observed location-specific activations resulted from overt eye movements to the cued location during scanning. First, eye movements were monitored during the training session to ensure that subjects were able to maintain fixation, and no significant differences in eye movements were observed between the spatial and nonspatial orienting conditions. Second, dorsal frontal and parietal areas have been shown to be involved in covert eye movements in humans (Corbetta et al., 1998; Kelley et al., in press; Nobre et al., 2000; Gitelman et al, 1999), an observation that is in line with findings from recent single-unit studies in monkeys showing that the visuo-oculomotor system of primates has evolved the capacity to amplify target-visual signals in the absence of the overt deployment of eye movements (Awh et al., 2006; Moore et al., 2003; Thompson et al., 2005). It therefore seems unlikely that the increased activation in parietal and frontal regions to location cues observed in the present study was due to overt eye movements. Rather, together with results from Giesbrecht et al. (2003) and other studies implicating these areas for covert attentional control, the current findings suggest a specialization of a subset of dorsal frontal and parietal brain areas in the orienting of attention to a location in space, the recruitment of which appears to be independent of the intermixed versus blocked presentation of spatial and nonspatial orienting conditions.
3.2 The generality of the attention shift mechanism
In the present work, preparatory shifts of spatial and nonspatial visual attention were associated with similar levels of activation in various subregions of the fronto-parietal control network. This finding provides support for a dimension-general role of these regions in the initiation of an attentive state, and extends previous fMRI studies of attention shifting which could not directly compare spatial and nonspatial attention shift-specific preparatory activity within the same experiment and subject group (Le et al., 1998; Liu et al., 2003; Serences et al., 2004b; Shulman et al., 2002a; Vandeberghe et al., 2001; Yantis et al., 2002). More specifically, as on switch-across trials, the task-relevant information had to be updated at both the feature and dimension levels, while on switch-within trials, this only had to be done at the feature level, it is likely that the observed dimension-general shift-specific preparatory activity reflects processes related to updating or switching attentional set (cf. Slagter et al., 2006).
The conclusion that preparatory shifts of spatial and nonspatial visual attention rely on the same neural system is at odds with results from a previous across-studies comparison, which had raised the possibility that functional compartments may exist within the parietal lobule that are specialized for the control of shifts of attention within distinct perceptual domains (Serences et al., 2005). One could argue that the current study may not have isolated the full pattern of dimension-specific activation, as it is unclear to what extent participants fully directed their attention in advance of the presentation of the target display in every trial. Although this possibility cannot be fully excluded, our finding of differential activation in the cue-target interval between the color and location attention-directing conditions indicates that participants did use the information provided by the cue to select the relevant feature before the target display was presented. Another, perhaps more likely explanation for this discrepancy may involve the fact that the fMRI studies (Liu et al., 2004; Serences et al., 2004; Yantis et al., 2002) included in the across-studies comparison, all used different stimuli. It is thus possible that perceptual differences in the stimuli used in those studies contributed to variable patterns of activation depending on the stimulus selectivity of different parietal regions.
Interestingly, in the current study, cue-related effects of shifting attention were confined to relatively medial dorsal frontal and parietal brain regions. More lateral dorsal frontal and parietal regions have recently been implicated in the analysis and interpretation of the functional significance of the cue-symbol, while more medial dorsal frontal and parietal regions were more specifically associated with the orienting of spatial attention (Woldorff et al., 2004). Our study extends these previous findings by showing that more medial dorsal brain regions in the fronto-parietal network subserve dimension-general processes involved in shifting visual attention. This observation is in line with prior work showing that the attentional control functions of dorsal posterior parietal and dorsal frontal cortices are not limited to the visual domain, but also show activity related to the control of auditory attention (Wu et al., 2007) and to audio-visual cross-modal shifts of attention (Shomstein et al., 2004). More work is necessary to determine to what extent our findings generalize to other types of nonspatial visual attention and other attentional domains.
The conclusion that the operation of shifting attention is independent of the dimension (spatial, nonspatial) of the cued information is in line with results from recent event-related potential (ERP) studies, showing that the temporal sequence of activation within brain regions involved in directing the attentional focus is very similar for spatial and nonspatial visual attention (Slagter et al., 2005a, 2005b). In these ERP studies, differences between spatial and nonspatial attention-directing conditions in scalp-recorded brain potentials and their neural generators as estimated using dipole source modeling were generally not observed until relatively late in the cue-target interval (i.e., after 580 ms post-cue (Slagter et al., 2005a) and after 640 ms (Slagter et al., 2005b)). These late dimension-specific differences in brain activity appeared to be more related to differences between the two types of attention in the specific brain areas involved in maintaining the biased attentive state, rather than to the fronto-parietal control signals per se. These ERP results combined with the present results support the conclusion that the specific brain areas involved in shifting visual attention, as well as the temporal pattern of activation within these brain areas may be unaffected by the sensory attributes of the cued information, whereas later processes related to the maintenance of the selective attentional state and their neural substrates may be dependent on the type of to-be-attended information.
4. Conclusion
The current findings reveal two key points. First, we observed overlap in the spatial and nonspatial attention conditions whether the conditions were intermixed within a block or presented in separate blocks. Thus, it is unlikely that the overlapping activity in top-down control regions observed here and in previous studies (e.g., Giesbrecht et al., 2003) is due to an artifact of intermixing spatial and nonspatial attention-directing conditions within the same task block. Secondly, activity in subregions of the fronto-parietal control network appear to reflect the shifting of attention more generally, independent of the dimension (spatial, nonspatial) of the task-relevant information and any role these regions may play in the maintenance of the cued location. In addition to these generalized regions, some regions in the fronto-parietal network were more strongly activated when attention was directed to a location in space than to a color. In contrast, left ventral occipital cortex exhibited more activity when attention was directed to a color than to a location. Together with data from previous studies, the present findings support the idea that some regions in the fronto-parietal network are specialized for the content (e.g., location in space) of the task-relevant information, while other regions are specialized for the type of process performed – e.g., attentional switching more generally -- independent of the content of the to-be-attended information.
5. Experimental procedure
5.1. Participants
Fourteen healthy participants (mean age 23.4 years; seven male) were recruited from the Duke University community and gave informed consent in accordance with the guidelines set by the Duke University Medical Center Institutional Review Board. All participants were right-handed, had normal or corrected-to-normal vision, and had no history of neurological trauma or disorders.
5.2. Design and procedure
5.2.1. Apparatus
A computer was used for stimulus presentation and for the recording of response data. Stimuli were viewed through an MR-compatible, fiber-optic goggle system. Responses were recorded with an MR compatible response box. The timing of the stimuli and the recording of the responses were controlled by commercially available software (Presentation, Neurobehavioral Systems, Inc.).
5.2.2. Attention task
On each trial participants were presented with a cue (i.e., a single digit: 3, 4, 5, or 6; visual angle, 0.28° × 0.38°; duration, 100 ms) that instructed them to attend to a particular spatial location (i.e., left or right) or color (i.e., yellow or blue) in an upcoming target display (see Fig. 1). To prevent physical differences among the cue stimuli from confounding the results, the mapping of the cue identity and attended feature was counterbalanced across participants (i.e., for some subjects a “3” cue meant attend left, while for others is meant attend right, and so on). On cue-plus-target trials, a target display (duration = 100 ms) was presented 1500 ms after the onset of the cue. The target display consisted of four rectangles: two green rectangles presented 4 degrees lateral to and 3.5 degrees above fixation in the upper left and upper right visual fields; and two rectangles presented overlapped at fixation, one yellow and one blue. One of the peripheral rectangles was always oriented vertically, the other horizontally. One of the central rectangles was always oriented vertically, the other horizontally. The target display was followed by a 1400 ms interval, during which the participant was to respond. The participant’s task was to indicate the orientation (i.e., horizontal or vertical) of the rectangle with the cued feature by pressing one of two buttons with their right index or middle finger. To equate the difficulty of the color and location tasks, the aspect ratio of the vertical and horizontal axes of the rectangles was adjusted after performance was assessed for each run, separately for the peripheral (location task) and central (color task) rectangles (Giesbrecht et al., 2003, 2006). The size of the rectangles presented at fixation ranged from 0.88° × 0.63° to 1.56° × 0.63°. The size range of the peripheral rectangles was increased to 1.75° × 1.25° to 3.13° × 1.25° to compensate for the lower acuity in the peripheral visual field) (Sereno et al., 1995). All stimuli were presented on a dark grey background.
Two additional types of trials were interleaved with the cue-plus-target trials described above. First, there were cue-only trials that were the same as the cue-plus-target trials except that no target display was presented. These trials were included to provide a measure of top-down attentional control activity not contaminated by target-related processing. Second, there were trials in which a fifth digit cue (‘0’) was presented. On these trials, referred to as ‘catch’ cue-only trials, participants were instructed to press a (third) button with their right ring finger as fast as possible upon presentation of the cue. Catch cue-only trials were randomized into the trial sequence in order to be able to examine whether participants were identifying the cues in the different tasks (see below) similarly. The cue-only and catch-cue only trials had the exact same trial length as the cue-plus-target trials.
Interleaved with the stimulus trials were trials that were of the same duration as the stimulus trials, but in which nothing was presented to the participant. These no-stimulus, or “no-stim”, trials were included to facilitate the decomposition of the overlapping hemodynamic responses to each of the other trial types in our fast-rate event-related design (Burock et al., 1998; Woldorff et al., 2004).
In all trials, the fixation point (a hollow, white square: size, 0.09° × 0.09° of visual angle) was filled in as soon as the cue stimulus was presented. The fixation point remained filled during cue-plus-target trials until the offset of the target display (duration of fixation-fill = 1600 ms) or until the equivalent time in cue-only trials. The reversion to a hollow fixation point at the same time in cue-plus-target and cue-only trials served to signal the end of the trial and was performed to equate the duration of cue-triggered attentional orienting processes in cue-plus-target and cue-only trials (e.g., Corbetta et al., 2000). In no-stim trials, the hollow fixation point remained at fixation for the entire trial. The subjects were not aware of the no-stim trials as to the subjects these were only periods of slightly longer delay between subsequent cued trials, yielding the impression that the inter-trial interval was variable.
Each 72-trial run consisted of 24 cue-plus-target trials, 24 cue-only trials, six catch cue-only trials, and 18 no-stim trials. The trial types were presented in a pseudorandom order such that, on average, each trial type was preceded by the same event distribution one trial back (Woldorff, 1993; Buckner et al., 1998; Burock et al., 1998; Woldorff et al., 2004).
5.2.3. Design
The voluntary orienting task was presented in two types of trial blocks: a block in which color and location cues were presented intermixed (mixed task) and a block in which the two types of cues were presented in separate blocks (blocked task). In the mixed task, the location and color cues were randomly interleaved so that on any given trial subjects could potentially be directed to attend to a rectangle with any one of the four possible stimulus features across both stimulus dimensions (i.e., blue, yellow, left, or right). Accordingly, in 25% of trials the cued feature repeated (e.g., attend blue trial followed by attend blue trial), in 25% of trials participants needed to switch between two features of the same dimension (e.g., attend blue trial followed by attend yellow trial), and in 50% of trials participants needed to switch between features and dimensions (e.g., attend blue trial followed by attend left trial). In the blocked task, the location and color conditions were presented in separate blocks, within which the cue randomly (i.e., from trial to trial) instructed participants to attend to the rectangle with either of two predefined features within the same stimulus dimension (i.e., blue or yellow in color blocks; left or right in location blocks). Since the cue-types occurred in random order, the cued feature repeated (e.g., attend blue trial followed by attend blue trial) in approximately 50% of trials, while participants needed to switch between two features of the same dimension (e.g., attend blue trial followed by attend yellow trial) in the other 50% of trials.
Each color and location cue-plus-target and cue-only trial was classified according to whether it was presented in the blocked or the mixed task. These trials were further categorized as instructing participants to attend to the rectangle with (1) the same feature that was cued in the previous trial (repeat trial), (2) a different feature in the same dimension (switch-within trial), or (3) a different feature in a different dimension (switch-across trial). Each no-stim trial was categorized according to the nature (spatial, nonspatial) of the feature cued in the preceding trial.
5.2.4. Procedure
Subjects participated in three sessions: a training session and two fMRI sessions. During the training session, participants performed four runs of each type of task to become familiar with the specific task requirements and to ensure that they were able to maintain fixation. Fixation was verified during training by recording each participant’s electrooculogram (EOG) from bipolar electrodes placed on the left and right of the outer canthi to monitor horizontal movements and above and below the left eye to monitor blinks and vertical movements. Electrode impedances were maintained below 10 kΩ. The two EOG channels were continuously recorded with a band pass filter of 0.01 to 100 Hz, a gain of 1000 (SynAmps amplifiers from Neuroscan, Inc.), and digitized with a sampling rate of 250 Hz. Recordings took place in an electrically shielded, sound attenuated, dimly lit room.
After training, participants participated in two separate fMRI sessions. The interval between these sessions was generally two to four weeks. During one of the fMRI sessions, participants performed the blocked bask and a second task, in which subjects were cued to the same feature on each trial. The results from this other task are reported elsewhere (Slagter et al., 2006). There were eight runs of the blocked task, four for each dimension (location and color). The order of the attended dimension within blocked task runs was counterbalanced across participants. During the other fMRI session, participants performed eight runs of the mixed task. The order of the two fMRI sessions was counterbalanced across participants.
5.3. Imaging methods
Functional images were acquired on a General Electric 4-T scanner using an inverse spiral imaging sequence (TR=1.5 s, TE=31 ms, flip angle=60°). During every task block, 164 brain volumes were collected, each of which contained 32 contiguous, 3.75-mm thick slices (in-plane resolution: 3.75 mm × 3.75 mm). Structural images were collected using a T1-weighted spin echo sequence (TR=12.2 ms, TE=5.3 ms, inversion time=300 ms, flip angle=20°). The first six functional images of each run contained no trials and were discarded prior to analysis of the functional data.
The software analysis package SPM’99 (Friston et al., 1995) was used to correct functional images for asynchronous slice acquisition and head motion, to normalize functional images to MNI (Montreal Neurological Institute) standard space, and to spatially smooth the functional data with a Gaussian filter (full width half maximum (FWHM) = 8 mm in the x, y, and z dimensions).
5.4. Statistical analyses
5.4.1. Behavior
Effects of the intermixed vs. blocked presentation of color and location cues were evaluated by determining whether behavioral performance on color and location cue-plus-target trials varied as a function of task (blocked, mixed). To this end, reaction times, error rates, and omitted response rates were first collapsed across the different within-task trial types (repeat, switch-within, switch-across) and then entered into separate repeated measures ANOVAs using the within-subjects factors of Task (blocked, mixed) and Dimension (color, location).
Effects of dimension (color, location) on attentional orienting behavior were measured using reaction times, error rates, and omitted response rates from the blocked and mixed tasks. For the mixed task, reaction times, error rates, and omitted response rates were each analyzed with a repeated measures analysis of variance (ANOVA). Each ANOVA contained the within-subjects factors of Dimension (color, location) and Switch (repeat, switch-within, switch-across). The behavioral data from the blocked task were entered into similar repeated-measures ANOVAs, but the Switch factor only had two levels: repeat and switch-within. Prior to all analyses, error rates and omitted response rates were arc-sin transformed to adjust to a normal distribution of data assumed by ANOVA (Snedecor & Cochran, 1989).
5.4.2. fMRI analyses
Selective averaging was used to estimate the average hemodynamic response produced by each trial type (cf. Buckner et al., 1998) starting 1.5 seconds before and ending 13.5 seconds after trial onset. These average responses were calculated separately at every voxel and for each participant and were converted to units of percent change from baseline that included the average signal intensity at trial onset and the immediately preceding time point. To remove overlap from adjacent trials caused by the fast-rate presentation of the trials, we subtracted from each cue-only response the average response for the no-stim trial type that was preceded by the same event-distribution (Buckner et al., 1998; Burock et al., 1998; Woldorff et al., 2004).
The first aim of this study was to examine whether the blocked presentation of spatial and nonspatial attention-directing conditions may lead individuals to rely more on dimension-specific orienting mechanisms. To this end, we first investigated the brain regions activated by color and/or location cues in the blocked and/or mixed task by entering the overlap-corrected color and location cue-only time-courses into separate voxel-level, one-way repeated-measures ANOVAs. This was done for each type of cue (color, location) and task (blocked, mixed) separately. Activated brain regions were determined by a main effect of MR Frame for these time courses (eight TR frames (1.5–13.5 seconds); F(7,91)=3.15, p<0.005; extent threshold of eight voxels).
We next examined whether some of these cue-related brain areas were specifically activated by one type of cue (e.g., spatial) versus the other (i.e., nonspatial), by directly comparing color and location cue-only responses, separately for the blocked and mixed tasks. As our question was whether the blocked versus intermixed presentation of color and location attention-directing conditions led subject to rely more on dimension-specific task preparation strategies, in the mixed task only those cue-only trials which were preceded by a trial in which the same dimension was cued (i.e., repeat and switch-within trials), were included in the location- versus color-cue comparison. This was done because in the blocked task only these cue-only trial types were possible, and in this way the comparison of color-cue and location-cue-related responses was identical for the blocked and mixed tasks, with only the global task context differing.
The direct comparison of color and location cue-related responses was performed for each task separately using a two-way repeated-measures ANOVA, restricted to the voxels or regions of interest (ROIs) that were generated by the union of the location and color cue-related statistical maps (thresholded as above; excluding trials in which attention was shifted across dimensions in the mixed task). This approach created functional ROIs that reduced the search volume while maximizing sensitivity to areas activated to one cue or the other. Activated regions were determined by an interaction effect between Dimension (color, location) and MR Frame (eight TR frames from 1.5–13.5 seconds). Because this procedure was implemented to test a priori predictions, the statistical threshold for the contrasts within the ROIs was set to p < .05 (F(7,91)=2.11) with an extent threshold of eight contiguous voxels.
To examine the effects of intermixed versus blocked presentation on dimension-specific preparatory brain activity, a within-subjects direct statistical comparison was conducted. To reveal such effects, voxels that were activated in the conjunction of the location cue and color cue-related statistical maps (thresholded as above) were identified. The percent signal change values for each voxel in this conjunction map were then entered into a three-way ANOVA with Task (blocked, mixed), Dimension (color, location) and MR Frame (eight TR frames (1.5–13.5 seconds) as factors. The resulting interaction map was thresholded at p < .05; F(7,91)=2.11.
The second question of this study pertained to the generality of the attention shifting mechanism. To examine differences in the neural systems involved in shifts of spatial and nonspatial visual attention, we first identified brain areas that were more strongly activated in the mixed task on switch-across compared to switch-within cue-only trials, independently of the cued attribute (i.e., collapsed across color and location conditions; p<.005). These attention-shift related brain areas were used to functionally define regions of interest. Each ROI was centered on a local maximum in the activation map for the shift-specific cue-related response, and consisted of a 3 × 3 × 3 cube of voxels. In every ROI, the overlap-corrected response for location and color cues on switch-across and switch-within trials was derived (see above) and averaged across all voxels within the ROI for each participant separately. Peak activity for the different trial types was computed by averaging the percent change values at the fourth and fifth time point of the BOLD response (4.5 to 7.5 seconds after cue onset and these values were entered into a repeated-measures ANOVA). A two-way interaction between the within-subject factors Switch Type (switch-across, switch-within) and Dimension (color, location) was taken as indicative of differences between spatial and nonspatial attention in preparatory aspects of shifting attention (p<.05/number of ROIs).
5.4.3. Eye Movement Analysis
The continuous electrooculogram (EOG) data were segmented into epochs starting 100 ms before and ending 1450 ms after the cue (regardless of whether the cue was followed by a target). Trials in which artefacts occurred (e.g., blinks) were identified using the vertical EOG channel (+/− 40 μV) and excluded from the analysis. The remaining trials were averaged according to cue type (left, right, or color [collapsed across blue and yellow]) and task (blocked, mixed), yielding a total of six trial types. We collapsed across cues coding for blue and yellow, as both types of color cues encouraged participants to maintain fixation. To examine the presence of horizontal eye moments across the cue-target interval, the 1450 ms cue-target interval was divided into thirty-six time bins of 40 ms each (10 sample points) for each trial type. For each time bin, the average voltage amplitude at the horizontal EOG channel was then computed for every trial type separately. To investigate whether participants moved their eyes, one-sample t-tests were performed on these mean voltage values, separately for attend left cue trials, attend right cue trials, and attend color cue trials in blocked and mixed tasks. Because of multiple interrelated comparisons, and hence the likelihood of false-spurious significant effects, eye movement effects were only considered present if they persisted for at least three successive time bins (40 ms each, (corrected) p-value < .05).
Acknowledgments
We would like to thank Allen Song for advice and assistance. This research was supported by a Dutch NWO grant (42520206) to A.K. and J.L.K., the Psychology Research Institute of the University of Amsterdam (A.K.), a postdoctoral National Research Service Award to D.H.W. (1 F32 NS41867-01), and research grants from NIMH (R01-MH055714) to G.R.M., from NINDS (PO1-NS41328, Proj 2) to M.G.W. and G.R.M, and from NIMH (RO1-MH60415) to M.G.W.
Footnotes
The current paper presents an analysis of previously unreported aspects of the data from an experiment by Slagter et al. (2006) in order to address new questions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. Literature References
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, et al. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neuroscience. 2000;12(6):988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen BR, Dale AM. Functional–anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent or overlapping neural systems? Proc Natl Acad Sci USA. 1998;95:831–839. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev, Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Tansy AP, Stanley CM, Astafiev SV, Snyder AZ, Shulman GL. A functional MRI study of preparatory signals for spatial location and objects. Neuropsychologia. 2005;43:2041–2056. doi: 10.1016/j.neuropsychologia.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and non-spatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Eason RG, Harter M, White C. Effects of attention and arousal on visually evoked potentials and reaction time in man. Physiol Behav. 1969;4:283–289. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top–down control during spatial and feature attention. NeuroImage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Mangun GR. Identifying the neural systems of top–down attentional control: a meta-analytic approach. In: Itti L, Rees G, Tsotsos J, editors. Neurobiology of Attention. Academic Press/Elsevier; New York: 2005. [Google Scholar]
- Giesbrecht B, Weissman D, Woldorff MG, Mangun GR. Pre-target activity in visual cortex predicts behavioral performance on spatial and feature attention tasks. Brain Research. 2006;1080:63–72. doi: 10.1016/j.brainres.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioral and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Golman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, Mountcastle F, editors. Handbook of Physiology. Vol. 5. The American Physiological Society; Washington DC: 1987. pp. 373–517. [Google Scholar]
- Grent-t-Jong T, Woldorff MG. Timing and sequence of brain activity in top-down control of visual-spatial attention. PLoS Biology. 2007;5:e12. doi: 10.1371/journal.pbio.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter MR, Previc FH. Size-specific information channels and selective attention: visual evoked potential and behavioral measures. Electroencephalogr Clin Neurophysiol. 1978;45:628–640. doi: 10.1016/0013-4694(78)90163-3. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Munte TF. Selective attention to color and location: an analysis with event-related brain potentials. Percept Psychophys. 1984;36:185–198. doi: 10.3758/bf03202679. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top–down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms of shifting and holding visuospatial attention. Cerebral Cortex. doi: 10.1093/cercor/bhm036. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsorge T, Heuer H, Schmidtke V. Assembling a task space: Global determination of local shift costs. Psych Res. 2004;68:31–40. doi: 10.1007/s00426-003-0134-9. [DOI] [PubMed] [Google Scholar]
- LaBerge D. Attentional processing: The brain’s art of mindfulness. Cambridge, MA: Harvard University Press; 1995. [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory function within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Liu T, Slotnick SD, Serences JT, Yantis S. Cortical mechanisms of feature-based attentional control. Cereb Cortex. 2004;13:1334–43. doi: 10.1093/cercor/bhg080. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV. Preparatory deployment of attention to motion activates higher-order motion-processing brain regions. NeuroImage. 2004;22:1515–1522. doi: 10.1016/j.neuroimage.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hansen JC, Hillyard SA. The spatial orienting of attention: sensory facilitation or response bias? In: Johnson R Jr, Rohrbaugh JW, Parasuraman R, editors. Current Trends in Event-Related-Potential Research. Elsevier; New York: 1986. pp. 118–124. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Nobre AC. The attentive homunculus: Now you see it, now you don’t. Neurosci Biobehav Rev. 2001;25:477–496. doi: 10.1016/s0149-7634(01)00028-8. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: Overlapping neural systems. NeuroImage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y, Rafal RD. Neural systems control of spatial orienting. Philos Trans R Soc Long B Biol Sci. 1982;298:187–198. doi: 10.1098/rstb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primage prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal and frontal cortex. Cereb Cortex. 2007;17:284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Serences JT, Liu T, Yantis S. Parietal mechanisms of switching and maintaining attention to locations, objects, and features. In: Itti L, Rees G, Tsotsos J, editors. Neurobiology of Attention. New York: Academic Press; 2005. pp. 35–41. [Google Scholar]
- Serences JT, Schwarzbach J, Courtney SM, Golay X, Yanis S. Control of object-based attention in human cortex. Cereb Cortex. 2004;14:1346–57. doi: 10.1093/cercor/bhh095. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Control of attention shifts between vision and audition in human cortex. J Neurosci. 2004;24:10702–10706. doi: 10.1523/JNEUROSCI.2939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, d’Avossa G, Tansy AP, Corbetta M. Two attentional processes in the parietal lobe. Cereb Cortex. 2002a;12:1124–1131. doi: 10.1093/cercor/12.11.1124. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Tansy AP, Kincade M, Petersen SE, McAvoy MP, Corbetta M. Reactivation of networks involved in preparatory states. Cereb Cortex. 2002b;12:590–600. doi: 10.1093/cercor/12.6.590. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Kok A, Mol N, Kenemans JL. Spatio-temporal dynamics of top-down control: directing attention to location and/or color as revealed by ERPs and source modeling. Cogn Brain Res. 2005a;22:333–348. doi: 10.1016/j.cogbrainres.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Kok A, Mol N, Talsma D, Kenemans JL. Generating spatial and nonspatial attentional control: An ERP study. Psychophys. 2005b;42:428–439. doi: 10.1111/j.1469-8986.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Weissman DH, Giesbrecht B, Kenemans JL, Mangun GR, Kok A, Woldorff MG. Brain regions activated by endogenous preparatory set-shifting as revealed by fMRI. Cognitive, Affective and Behavioral Neuroscience. 2006;6(3):175–189. doi: 10.3758/cabn.6.3.175. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 8. Ames: Iowa State University Press; 1989. [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25(41):9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An integrated software system for surface-based analyses of cerebral cortex. Journal of American Medical Informatics Association. 2001;41:1359–1378. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Woldorff MG, Mangun GR. A role for top–down attentional orienting during interference between global and local aspects of hierarchical stimuli. NeuroImage. 2002;17:1266–1276. doi: 10.1006/nimg.2002.1284. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. The neural mechanisms for minimizing cross–modal distraction. J Neurosci. 2004;24:10941–10949. doi: 10.1523/JNEUROSCI.3669-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KD, Woldorff MG, Mangun GR. Control networks and hemispheric asymmetries in parietal cortex during attentional orienting in different spatial reference frames. NeuroImage. 2005;25:668–683. doi: 10.1016/j.neuroimage.2004.07.075. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Hazlett CJ, Fichtenholtz HM, Weissman DH, Anders AM, Song AW. Functional parcellation of attentional control regions of the brain. J Cogn Neurosci. 2004;16:149–165. doi: 10.1162/089892904322755638. [DOI] [PubMed] [Google Scholar]
- Wu CT, Weissman DH, Roberts KC, Woldorff MG. The neural circuitry underlying the executive control of auditory spatial attention. Brain Res. 2007;1134:187–198. doi: 10.1016/j.brainres.2006.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobiology. 2003;13:187–93. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]