ABSTRACT
BACKGROUND
Undertreatment of osteoporosis has been recognized as a common problem in selected patient subgroups. However, primary prevention has been hampered by limited risk assessment tools that can be applied to large populations.
OBJECTIVES
Using clinical risk factors with a new tool from the World Health Organization (FRAX) and recommendations from the National Osteoporosis Foundation (NOF), we evaluated fracture risk and osteoporosis treatment in a US cohort.
PARTICIPANTS
African Americans and Caucasians recruited from 2003–7 across the US as part of a longitudinal study.
DESIGN
Cross-sectional.
MEASURES
The number of persons receiving prescription osteoporosis medications was assessed by race, sex, and fracture risk. Multivariable logistic regression evaluated the association between receipt of osteoporosis medications and fracture risk after controlling for potential confounders.
RESULTS
Among 24,783 participants, estimated fracture risk was highest for Caucasian women. After multivariable adjustment for fracture-related risk factors, the likelihood of receipt of osteoporosis medications among African Americans was lower than among Caucasians [odds ratio (OR) = 0.44, 95% confidence interval (CI) 0.37, 0.53] and for men compared to women (OR = 0.08, 95% CI 0.06–0.10). Even for the highest risk group, Caucasian women with 10-year hip fracture risk ≥3% (n = 3,025, 39.7%), only 26% were receiving treatment.
CONCLUSIONS
A substantial gap exists between 2008 NOF treatment guidelines based on fracture risk and the receipt of prescription osteoporosis medications. This gap was particularly notable for African Americans and men. FRAX is likely to be useful to assess risk at a population level and identify high-risk persons in need of additional evaluation.
KEY WORDS: osteoporosis, fracture, African American, Caucasian, epidemiology, FRAX, bisphosphonate
INTRODUCTION
Osteoporosis is an important public health problem and results in approximately 1.6 million fractures each year in the United States1. The direct costs of osteoporosis-related fractures in 2005 were estimated at more than 19 billion dollars2. Although historically considered a disease of women, approximately 20% of fractures occur in men1. Fracture rates among Caucasians are higher than for other racial and ethnic groups3,4; however, the prevalence of osteoporosis among non-Caucasians is expected to increase at a faster rate than for Caucasians as the population ages2. Outcomes following fracture are worse among men and non-Caucasians compared to Caucasian women5.
Previous research has suggested that disparities in the evaluation and management of osteoporosis exist for high-risk patients. For example, one study showed that African Americans with prior fracture were 83% less likely to be treated with prescription medications compared to Caucasian women6. The challenge with studies focused on sex and racial disparities is that osteoporosis risk assessment in the absence of bone mineral density (BMD) testing is problematic since men and African-American women on average have higher bone mineral density than Caucasian women7,8. For that reason, previous investigations of osteoporosis disparities generally have been limited to high-risk groups: persons with prior fragility fractures or using certain medications (e.g., glucocorticoids). In the absence of these two strong risk factors, fracture risk assessment has historically required use of central dual-energy X-ray absorptiometry (DXA)9. However, recent population-based US data have shown that a minority of patients age ≥65 have undergone DXA testing10. Only 31% of Caucasian women, 17% of African-American women, and fewer than 5% of men over age 65 have undergone such testing. Therefore, DXA is infeasible as a requirement to screen large populations11,12.
Strategies to quantify fracture risk based only on clinical risk factors have been proposed13,14. The newest of these fracture risk assessment tools from the World Health Organization (WHO) is called FRAX and, even without BMD, can validly estimate the 10-year absolute risk for hip and major fracture (i.e., hip, clinical vertebral, forearm, humerus)15. Many large health-care organizations (e.g., Kaiser Permanente, the Veterans Administration) have access to the clinical data required by FRAX. Physicians with access to state-of-the-art health information technology systems also could apply this approach in a semi-automated fashion to risk-stratify their patients. Higher risk patients would be prioritized for further osteoporosis evaluation (including BMD testing) and more aggressive quality improvement efforts.
Using data from a large US cohort of African-American and Caucasian men and women, we (1) assessed the impact of using commonly available clinical data to evaluate fracture risk estimated by FRAX (without BMD) and (2) evaluated possible gender- and race-associated osteoporosis treatment disparities in accordance with revised National Osteoporosis Foundation (NOF) recommendations based on FRAX16.
METHODS
Study Population
The REasons for Geographic And Racial Differences in Stroke (REGARDS) study is a National Institutes of Health-funded, population-based cohort study. REGARDS is the parent for several ancillary studies, including one specifically focusing on fracture risk that collected osteoporosis-related information. Potential REGARDS participants were recruited from across the US from January 2003 through October 2007 without respect to stroke risk17. Participants were randomly selected from commercially available lists obtained from credit-reporting services and were contacted using a combination of mail and telephone. The only inclusion criteria were age ≥45, race (African American/Caucasian), and geography. Approximately 20% were recruited from the stroke belt buckle (coastal plain region of North Carolina, South Carolina, and Georgia), 30% from stroke belt states (the remainder of these states, plus Alabama, Mississippi, Tennessee, Arkansas, and Louisiana), and the remainder from the other 40 contiguous states. Potential participants were ineligible if they were undergoing treatment for active cancer, resided in a nursing home, had severe cognitive impairment, or had medical conditions that precluded long-term participation.
The proportion of households contacted with an eligible person who agreed to participate was 45.3%. Baseline data were collected via computer-assisted telephone interview. An in-home visit subsequently collected physical measurements, blood pressure, and blood and urine samples. Participants retrieved bottles of all prescription and over-the-counter medications taken within 2 weeks of the in-home visit. These medications were recorded, confirmed, and later classified by registered pharmacists. Follow-up phone contact was made at 6-month intervals in order to collect additional data and to identify suspected events (e.g., fracture). Participants gave informed consent for the study, which was approved by the Institutional Review Boards at participating institutions.
Assessment of Osteoporosis Risk Factors and Associated Medication Use
The main demographic factor of interest was self-reported race (African American or Caucasian). The risk factors needed by FRAX were collected and assessed as: alcohol (≥3 units per day), BMI measured at the in-home visit, and smoking (current, past, or never). Previous fracture was assessed using the validated question “Please tell me which bones you have broken, fractured, or crushed since the age of 45?”18. Parental history of fracture was assessed as, “Did your mother or father break or fracture their hip after age 35?” Falls were assessed via a question asking how many times they had fallen in the previous 6 months. Medications of interest included oral glucocorticoids and osteoporosis medications (i.e., alendronate, risedronate, ibandronate, teriparatide, raloxifene, and calcitonin).
A-priori, we selected other factors potentially related to fracture or the likelihood of receiving osteoporosis treatment. These factors included age, sex, region (stroke belt, stroke buckle, other), annual income (<$25 thousand (k), 25–35 k, 35–75 k, or >$75 k), education (<high school, high school, some college, college degree), and medical insurance. We also considered several general health variables: the Study Short Form-12 (SF)-12 mental component summary scale (MCS), the SF-12 physical component summary scale (PCS), SF-12 overall general health (poor, fair, good, very good, excellent), perceived stress scale (PSS), and the Center for Epidemiologic Studies Depression (CESD-4) scale (normal <4 versus depressed ≥4)19. Diabetes, heart disease, prior stroke, and dyslipidemia were examined as potential confounders (definitions per Appendix). At the time of this report, 24,783 REGARDS participants had provided relevant information and completed the in-home examination, and they were included in these analyses.
Assessment of Fracture Risk using FRAX and Clinical Risk Factors
In 2008, the WHO published a novel approach to fracture risk assessment. They evaluated more than 60,000 persons participating in large cohort studies and created a risk prediction model that provides patient-specific estimates of the 10-year risk for hip fracture and major fracture (hip, clinical vertebral, wrist/forearm, or humerus fracture). A race-specific calculator that provides these risk estimates is available in a simple Internet tool called FRAX20. FRAX integrates multiple clinical risk factors plus BMD testing, or if not available, body mass index (BMI)15. The NOF recommended thresholds for intervention with prescription osteoporosis therapies based upon FRAX16. These data were generated based upon cost-effectiveness analyses21, which are likely to be country-specific. The NOF recommends that persons who have a ≥3% 10-year risk for hip fracture, or >20% 10-year risk for major fracture, receive a prescription osteoporosis medication16. In our study, US-based, race-specific estimated fracture risk was calculated by FRAX using a computer program that provided each of the FRAX risk factors described above to the FRAX web site, and we obtained the results for the 10-year predicted risk for hip and major fracture.
Statistical Analysis
Differences in demographics and fracture risk factors within each race/sex group were examined across geographic region. There were no clinically significant differences in the distribution of risk factors by region, so data were aggregated. We evaluated the proportion of persons recommended for treatment by the NOF using various estimated 10-year hip fracture risk cutpoints to determine the ability of FRAX to risk-stratify patients within various race/sex/age groups. Multivariable logistic regression was used to describe the relationship between the use of an OP medication (modeled dichotomously as the outcome of interest) and FRAX-estimated fracture risk (modeled as a continuous variable) as the main independent variable22,23. Although FRAX includes age, sex, and race, these factors were included in the model to determine whether participants were more or less likely to receive treatment for OP based upon these factors, independent of predicted fracture risk. We further fit incremental logistic regression models to determine the influence that additional groups of potentially confounding covariates might have on the relationship between use of OP medications and race, including variables described above to maximally control for confounding. We initially ran a bivariate logistic regression model, then added demographics, followed by socioeconomic factors, then general health variables, then comorbidities, and finally fracture risk factors.
RESULTS
The characteristics of the participants are shown in Table 1. Approximately half were ≥65 years old; 30–50% of individuals had a BMI ≥30 kg/m2. Five percent of African-American men and nine percent of African-American women reported prior fracture since age 50. These proportions were two-fold greater among Caucasians. The prevalence of current smoking was 11–19%, and 2–3% of participants were using systemic glucocorticoids. Between 9 and 19% of participants reported at least one fall in the previous 6 months. Approximately 7–8% of participants had at least mild cognitive impairment (not shown).
Table 1.
Characteristics of Fracture Risk Factors Among REGARDS Participants, by Sex and Race (n = 24,783)
| Caucasian (n = 14,958) | African American (n = 9,825) | |||
|---|---|---|---|---|
| Men (n = 7,339) | Women (n = 7,619) | Men (n = 3,545) | Women (n = 6,280) | |
| Age, years* | ||||
| 45–54 | 636 (9%) | 1,016 (13%) | 446 (13%) | 917 (15%) |
| 55–64 | 2,695 (37%) | 2,860 (38%) | 1,431 (40%) | 2,527 (40%) |
| 65–74 | 2,577 (35%) | 2,416 (32%) | 1,158 (33%) | 1,975 (31%) |
| 75–84 | 1,254 (17%) | 1,179 (15%) | 469 (13%) | 766 (12%) |
| ≥85 | 177(2%) | 148 (2%) | 41 (1%) | 95 (2%) |
| Body mass index (BMI), kg/m2 | ||||
| <20 | 114 (2%) | 381 (5%) | 94 (3%) | 127 (2%) |
| 20-25 | 1,643 (22%) | 2,256 (30%) | 707 (20%) | 816 (13%) |
| 25−30 | 3,348 (46%) | 2,507 (33%) | 1,415 (40%) | 1,844 (29%) |
| 30–35 | 1,540 (21%) | 1,447 (19%) | 842 (24%) | 1,651 (26%) |
| >35 | 692 (9%) | 1,022 (13%) | 486 (14%) | 1,834 (29%) |
| Previous fracture since age 50 | 773 (11%) | 1,386 (18%) | 176 (5%) | 525 (9%) |
| Parent fractured hip | 1,005 (14%) | 1,213 (16%) | 228 (7%) | 437 (7%) |
| Current smoker | 801 (11%) | 976 (13%) | 680 (19%) | 936 (15%) |
| Alcohol use ≥3 units per day† | 372 (5%) | 106 (1%) | 117 (3%) | 46 (1%) |
| Systemic glucocorticoids | 203 (3%) | 236 (3%) | 78 (2%) | 176 (3%) |
| Any anti-osteoporosis drug** | 79 (1%) | 1,032 (14%) | 12 (<0.5%) | 272 (4%) |
| Alendronate | 59 (0.8%) | 536 (7%) | 11 (0.3%) | 132 (2%) |
| Ibandronate | 1 (0.01%) | 15 (0.2%) | 0 | 2 (0.03%) |
| Risedronate | 14 (<0.5%) | 214 (3%) | 1 (<0.5%) | 69 (1%) |
| Calcitonin | 5 (0.07%) | 49 (0.6%) | 0 | 10 (0.2%) |
| Teriparatide | 0 | 7 (0.09%) | 0 | 0 |
| Raloxifene | 0 | 211 (3%) | 0 | 59 (1%) |
| Systemic estrogens | n/a | 1,044 (14%) | n/a | 449 (7%) |
| Number of unique medications (excluding osteoporosis medications) | ||||
| 0–1 | 1,158 (16%) | 837 (11%) | 695 (20%) | 866 (14%) |
| 2–4 | 2,166 (30%) | 1,916 (25%) | 1,154 (33%) | 1,863 (30%) |
| 5–8 | 2,462 (34%) | 2,633 (35%) | 1,106 (31%) | 2,277 (35%) |
| >8 | 1,553 (21%) | 2,233 (29%) | 590 (17%) | 1,324 (21%) |
| Falls | ||||
| Any in last 6 months | 892 (13%) | 1,362 (19%) | 307 (9%) | 782 (13%) |
| Number in last 6 months (median, range) | 1 (1,50) | 1 (1,35) | 1 (1,20) | 1 (1,15) |
n/a = Not applicable
*Participants aged >90 years were considered 90 for the purpose of the fracture risk calculation
†A unit of alcohol is defined as a standard glass of beer or a medium-sized glass of wine
**Numbers do not sum to total since 23 persons were taking more than one of these medications
Table 2 describes FRAX-estimated 10-year hip fracture risk, stratified by race, sex, and age. FRAX effectively risk-stratified Caucasian women ages 65–75 across a wide distribution of fracture risk; in contrast, almost all Caucasian women age 75–84 were identified as having hip fracture risk >3% (considered high risk by NOF recommendations). For African Americans, FRAX classified almost all of them as being at lower risk (hip fracture risk <3%) until they reached age 75 or older.
Table 2.
Prevalence of FRAX-Estimated 10-Year Hip Fracture Risk by Sex, Race, and Age, %*
| Age group, years | Estimated 10- year hip fracture risk | Caucasian (n = 14,958) | African American (n = 9,825) | ||
|---|---|---|---|---|---|
| Men (n = 7,339) | Women (n = 7,619) | Men (n = 3,545) | Women (n = 6,280) | ||
| 45–54 | <1.4% | 97 | 91 | >99 | 99% |
| 1.4%–3.0% | 3 | 7 | <0.5** | 1** | |
| 3.0%–5.0% | <0.5** | 1% | 0%** | <0.5** | |
| 5.0%–10% | 0** | <0.5%** | 0** | 0** | |
| >10% | <0.5** | 0** | 0** | 0** | |
| 55–64 | <1.4% | 88 | 60 | 99 | 97 |
| 1.4%–3.0% | 11 | 28 | 1 | 3 | |
| 3.0%–5.0% | 1 | 9 | <0.5** | <0.5** | |
| 5.0%–10% | <0.5** | 3 | <0.5** | <0.5** | |
| >10% | 0** | <0.5 | 0** | <0.5** | |
| 65–74 | <1.4% | 32 | 11 | 89 | 67 |
| 1.4%–3.0% | 40 | 33 | 11 | 26 | |
| 3.0%–5.0% | 17 | 23 | 1 | 5 | |
| 5.0%–10% | 8 | 22 | <0.5** | 2 | |
| >10% | 2 | 11 | 0** | <0.5** | |
| 75–84 | <1.4% | 0** | <0.5* | 31 | 4 |
| 1.4%–3.0% | 10 | 1 | 57 | 43 | |
| 3.0%–5.0% | 38 | 8 | 11 | 34 | |
| 5.0%–10% | 40 | 43 | 3 | 15 | |
| >10% | 12 | 48 | 1** | 4 | |
| ≥85 | <1.4% | 0** | 0** | 0** | 2** |
| 1.4%–3.0% | 2** | 0** | 80 | 34 | |
| 3.0%–5.0% | 43 | 3** | 15** | 43 | |
| 5.0%–10% | 37 | 50 | 2** | 18 | |
| >10% | 18 | 46 | 2** | 3** | |
Hip fracture risk estimates were computed using the WHO FRAX tool with body mass index (http://shef.ac.uk/FRAX)
*Proportions are computed within each race/sex/age strata; the sum of the proportions within each race/sex/age strata may total more than 100% due to rounding
**Fewer than ten people were represented in this cell; proportions may not be stable
Table 3 describes the proportion of individuals taking osteoporosis medications, stratified by race, sex, and fracture risk. Although some cell sizes were small, for all demographic groups except African-American men, FRAX-estimated fracture risk was significantly associated with the use of osteoporosis therapies. However, even for the highest risk group (Caucasian women with ≥10% hip fracture risk), only 26% were receiving prescription osteoporosis medications.
Table 3.
Number and Proportion of REGARDS Participants Receiving Osteoporosis Therapies* by FRAX 10-Year Hip Fracture Risk**, Sex, and Race
| Estimated 10-year hip fracture risk | Caucasian n = 14,958 | African American n = 9,825 | ||
|---|---|---|---|---|
| Men† n = 7,339 | Women† n = 7,619 | Men‡ n = 3,545 | Women† n = 6,280 | |
| <1.4% | 15/3,281 (0.4%) | 174/2,897 (6%) | 10/3,004 (0.3%) | 145/4,708 (3%) |
| 1.4–<3.0% | 16/1,478 (1%) | 213/1,697 (13%) | 2/444 (0.5%) | 69/942 (7%) |
| 3.0–<5.0% | 20/1,024 (2%) | 154/926 (17%) | 0/72 (0%) | 37/417 (9%) |
| 5.0–<10.0% | 20/776 (3%) | 222/1,196 (19%) | 0/20 (0%) | 15/167 (9%) |
| ≥10.0% | 8/240 (3%) | 235/903 (26%) | 0/5 (0%) | 1/46 (2%) |
Data shown as number treated/number eligible (%)
*Including alendronate, risedronate, ibandronate, teriparatide, raloxifene, or calcitonin
**Hip fracture risk estimates were computed using the WHO FRAX tool with body mass index (http://shef.ac.uk/FRAX)
†p < 0.001 for column trend
‡p = 0.96
Table 4 describes the relationship between receipt of osteoporosis medications and age, sex, and race, controlling for FRAX-estimated hip fracture risk. As shown, FRAX hip fracture risk was significantly associated with receipt of osteoporosis therapies. However, African Americans were significantly less likely than Caucasians to receive osteoporosis medication, even after adjusting for hip fracture risk. Men were significantly less likely to be treated compared to women.
Table 4.
Factors Associated with Receipt of Osteoporosis Medications (Multivariable Logistic Regression)
| Odds ratio (95% CI) | |
|---|---|
| African American (Caucasian referent) | 0.36 (0.31–0.42) |
| Age, years | |
| <55 | 0.29 (0.21–0.40) |
| 55–<65 | 1.0 (referent) |
| 65–<75 | 1.46 (1.27–1.67) |
| 75–<85 | 1.38 (1.14–1.67) |
| ≥85 | 1.38 (0.95–2.00) |
| Men (women referent) | 0.08 (0.06–0.10) |
| 10-year hip fracture risk (per 5% increase)* | 1.05 (1.04–1.06) |
Interaction term between race and sex (p = 0.99) was not included
*Using FRAX with body mass index (incorporates fracture risk factors as described in the text)
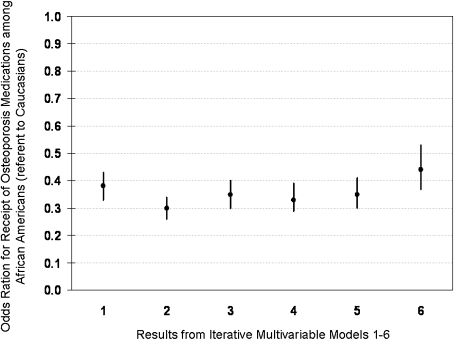
Figure 1 shows the results from the unadjusted logistic model and the incremental models that incorporated additional factors. None of these factors had a major effect on the relationship between receipt of osteoporosis medications and race. The result from the fully adjusted model showed that African Americans were significantly less likely (OR = 0.44, 95% CI 0.37–0.53) to receive osteoporosis medications compared to Caucasians.
Figure 1.
Multivariable model showing the relationship between receipt of osteoporosis medications and race. Model 1 is the univariate model, with race only. Model 2 includes race, age, sex, and geographic region. Model 3 includes model 2 variables and socioeconomic factors (income, education, insurance). Model 4 includes model 3 variables and general health (MCS and PCS scores, perceived stress, depression, and overall health; see text for details). Model 5 includes model 4 variables, comorbidities (diabetes, heart disease, prior stroke and dyslipidemia, cognitive status), and number of unique medications. Model 6 includes model 5 variables and fracture risk factors (alcohol use, smoking, BMI, previous fracture, parental history of a hip fracture, use of glucocorticoids, and fall in the previous 6 months).
DISCUSSION
The principal findings from this large, population-based study demonstrate that African Americans were substantially less likely to receive prescription osteoporosis medications than Caucasians, even after adjusting for socioeconomic factors and clinical risk factors for fracture. Men were also significantly less likely to be treated compared with women. Although this finding has been observed previously in selected high-risk groups (e.g., patients with prior fracture), disparities research has previously not been able to assess primary and secondary osteoporosis prevention in large community settings very well given that osteoporosis treatment recommendations related to fracture risk estimated using only clinical risk factors have heretofore not been available. Our approach also demonstrates the feasibility and impact of using FRAX with BMI to assess fracture risk of a large population as a mechanism for risk-stratifying patients. These results might be used as the first step in a two-step approach to identify persons at higher fracture risk on the basis of clinical risk factors; these individuals would therefore be more efficiently targeted for additional osteoporosis evaluation, including DXA testing. Indeed, we have recently demonstrated in a small cohort of 324 high-risk patients that FRAX without BMD provides a sensitive screening test, and the second step (incorporating DXA data) enhances specificity24.
Our results are consistent with prior literature in more geographically restricted settings demonstrating that African Americans are less likely to be screened or treated for osteoporosis. One study evaluated 275 women participating in a family medicine research network in North Carolina and found that Caucasians were two- to five-fold more likely than African Americans to receive osteoporosis-related counseling, bone-density testing, calcium, or bisphosphonates, even after adjusting for age, weight, fracture, and family history25. These findings were similar to another study conducted among 8,909 older women in Alabama that found that African Americans were 0.4-fold as likely as Caucasians to receive BMD testing or osteoporosis medications, even after controlling for weight, income, insurance, glucocorticoid use, and prior fracture26. Consistent observations have been found in more selected populations, such as persons with prior fracture or long-term glucocorticoid users27–31. Although the reasons for undertreatment are likely varied, lack of uniformity in osteoporosis screening guidelines32 and interpretation of BMD results for non-Caucasians33–35 may contribute to the problem. Physicians also may be aware of the well-established data showing that on average, African Americans are at lower risk for osteoporosis than Caucasians. It is possible that they may generalize this data even to high-risk African Americans and fail to appropriately assess and manage osteoporosis for these individuals. Risk stratification using FRAX with clinical risk factors may help combat this misperception.
Other strategies for population-based risk assessment included the Fracture Index by Black et. al.13 and more simple screening instruments such as the Osteoporosis Screening Tool (OST)36. The Fracture Index assesses six clinical risk factors for fracture, and the OST requires only age and weight. Neither of these tools requires knowledge of BMD. However, results from these tools in multi-ethnic populations are of uncertain validity since their derivation and validation did not include African Americans, nor have they been rigorously assessed in this racial group since. Moreover, there are no widely agreed upon treatment recommendations promulgated based upon the results of these tools. FRAX overcomes these limitations and provides race-specific fracture risk estimates, and the 2008 NOF recommendations define high-risk individuals by setting treatment thresholds based upon FRAX.
Given that most older persons have not undergone BMD testing, FRAX with BMI could be used as a case finding method to identify persons at higher fracture risk to allocate limited osteoporosis resources (e.g., BMD testing or an osteoporosis quality improvement intervention). However, the usefulness of FRAX with clinical risk factors to risk-stratify patients appears highly dependent on age. Based upon the distributions of FRAX-estimated fracture risk shown in Table 2, this approach would be most useful for Caucasian women at ages 55–75 and Caucasian men ages 65–75. It would not be very useful for African Americans until they reached age ≥75 because at younger ages, most of them would be considered by FRAX to be at low risk.
The strengths of our study include the diverse geographic representation of Americans participating in this large cohort study, with intentional oversampling of African Americans. Additionally, osteoporosis risk assessment has received limited study in a large geographically diverse population of African Americans. Indeed, although the National Health and Nutrition Evaluation Survey evaluated BMD among older persons, these data provide estimates only for 640 African-American women and 598 African-American men. Moreover, BMD data appear somewhat less informative among African Americans. A report from the Study of Osteoporotic Fractures showed that the relationship between hip BMD and fracture risk was largely explained by clinical risk factors, including BMI37,38. Regardless, most older persons in the US have not undergone DXA testing10, so risk assessment at a population level cannot require BMD results.
Limitations of our study include lack of information regarding some of the secondary causes of osteoporosis [e.g., rheumatoid arthritis (RA), malnutrition] represented in FRAX. This would have the effect of underestimating fracture risk for persons with these conditions. Thus, the distribution of risk based upon clinical factors shown in Table 2 is somewhat low. However, these conditions are relatively uncommon in the US; RA, for example, affects only 1% of adults39. Moreover, it is likely that this type of information gap is common to a number of population-based data sources that may be missing one or a few of the less common FRAX risk factors, but this should not meaningfully prohibit use of FRAX as a mechanism to risk-stratify individuals within a population or health-care system. Additionally, we recognize that only selected risk factors for fracture were evaluated, and other conditions (e.g., Parkinson’s disease) were not evaluated. Importantly, though, extra-skeletal risk factors, including those that contribute to falls, may not be amenable to treatment with osteoporosis medications, which is why they were not included in FRAX. We also recognize that the cross-sectional design of the study limits us from knowing whether some individuals started osteoporosis therapies but did not tolerate them, could not afford them, or were not adherent. Likewise, we did not know which persons had previously undergone BMD testing and were not considered in need of osteoporosis treatment. Finally, although REGARDS is a population-based cohort, it is not necessarily representative of the entire US population. This would not affect internal validity, but could affect generalizability.
In conclusion, we found for persons older than age 75, more than three-quarters of Caucasian men and more than one-half of African-American women were suspected to be at high enough osteoporosis risk on the basis of clinical factors to warrant further evaluation and could be considered candidates for prescription osteoporosis medications. However, even for the highest risk Caucasian women, only 26% were receiving osteoporosis therapies. The simple risk factors in FRAX can be used to identify high-risk individuals, even in the absence of a BMD measurement. While this surveillance could occur at a population level and may help ameliorate disparities in osteoporosis management, clinicians also could systematically apply the FRAX tool within their own practice using health information technology systems to identify patients at high risk for fracture.
Acknowledgements
Work for this analysis was supported in part by the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (AR053351) and the Arthritis Foundation. REGARDS is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript, but not directly involved in the collection, management, analysis, or interpretation of the data. The authors acknowledge the participating investigators and institutions for their valuable contributions: The University of Alabama at Birmingham, Birmingham, Alabama (Study PI, Statistical and Data Coordinating Center, Survey Research Unit): George Howard DrPH, Leslie McClure PhD, Virginia Howard PhD, Libby Wagner MA, Virginia Wadley PhD, Rodney Go PhD, Monika Safford MD, Ella Temple PhD, Margaret Stewart MSPH, J. David Rhodes BSN; University of Vermont (Central Laboratory): Mary Cushman MD; Wake Forest University (ECG Reading Center): Ron Prineas MD, PhD; Alabama Neurological Institute (Stroke Validation Center, Medical Monitoring): Camilo Gomez MD, Susana Bowling MD; University of Arkansas for Medical Sciences (Survey Methodology): LeaVonne Pulley PhD; University of Cincinnati (Clinical Neuroepidemiology): Brett Kissela MD, Dawn Kleindorfer MD; Examination Management Services, Incorporated (In-Person Visits): Andra Graham; Medical University of South Carolina (Migration Analysis Center): Daniel Lackland DrPH; Indiana University School of Medicine (Neuropsychology Center): Frederick Unverzagt PhD; National Institute of Neurological Disorders and Stroke, National Institutes of Health (funding agency): Claudia Moy Ph.D.
Conflicts of Interest JRC: Consulting: Roche, UCB, Procter & Gamble, CORRONA; speakers bureau: Procter & Gamble, Eli Lilly, Roche, Novartis; research grants: Merck, Procter & Gamble, Eli Lilly, Amgen, Novartis
KGS: Consulting: Merck, Novartis, Procter & Gamble, Amgen, Aventis, Eli Lilly; speakers bureau: Novartis; research grants: Amgen
ED: Research grants: Amgen
Others: None
EO: E.O. has received honoraria from and served as a consultant for Merck. He has received research support from Amgen, Pfizer, Novartis, Zelos Therapeutics, Imaging Therapeutics, and Solvay Pharmaceuticals. He has received research support from and served as a consultant for Ely Lilly & Co. and served as a consultant for Servier.
Appendix
Definitions for Comorbidities of Interest
Diabetes: fasting glucose ≥126 mg/dl, non-fasting glucose ≥200 mg/dl, or self-reported diabetes medication
History of heart disease: self-reported myocardial infarction, coronary artery bypass grafting (CABG), bypass, angioplasty or stenting, OR evidence of myocardial infarction via electrocardiography
Prior stroke: self-report
Dyslipidemia: total cholesterol ≥240, low density lipoprotein ≥160 or high density lipoprotein ≤40, or self-reported lipid medication use
References
- 1.National Osteoporosis Foundation: Clinician’s Guide to Prevention and Treatment of Osteoporosis. 2008 [updated 2008; cited accessed April 10, 2008].
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. [DOI] [PubMed]
- 3.Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80:871–3. [DOI] [PMC free article] [PubMed]
- 4.Kellie S, Brody J. Sex specific and Race specific Hip Fracture Rates. Am J Public Health. 1990;80(3):326–8. [DOI] [PMC free article] [PubMed]
- 5.Jacobsen S, Goldberg J, Miles T, Brody J, Stiers W, Rimm A. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147–50. [DOI] [PMC free article] [PubMed]
- 6.Mikuls TR, Saag KG, George V, Mudano AS, Banerjee S. Racial disparities in the receipt of osteoporosis related healthcare among community-dwelling older women with arthritis and previous fracture. J Rheumatol. 2005;32(5):870–5. [PubMed]
- 7.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–89. [DOI] [PubMed]
- 8.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Proximal femur bone mineral levels of US adults. Osteoporos Int. 1995;5(5):389–409. [DOI] [PubMed]
- 9.Lewiecki EM, Watts NB, McClung MR, Petak SM, Bachrach LK, Shepherd JA, et al. Official positions of the international society for clinical densitometry. J Clin Endocrinol Metab. 2004;89(8):3651–5. [DOI] [PubMed]
- 10.Curtis JR, Carbone L, Cheng H, Hayes B, Laster A, Matthews R, et al. Longitudinal trends in use of bone mass measurement among older americans, 1999–2005. J Bone Miner Res. 2008;23(7):1061–7. [DOI] [PMC free article] [PubMed]
- 11.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288(15):1889–97. [DOI] [PubMed]
- 12.Melton LJ 3rd, Johnell O, Lau E, Mautalen CA, Seeman E. Osteoporosis and the global competition for health care resources. J Bone Miner Res. 2004;19(7):1055–8. [DOI] [PubMed]
- 13.Black DM, Steinbuch M, Palermo L, Dargent-Molina P, Lindsay R, Hoseyni MS, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporos Int. 2001;12(7):519–28. [DOI] [PubMed]
- 14.Schneyer CR, Lopez H, Concannon M, Hochberg MC. Assessing population risk for postmenopausal osteoporosis: a new strategy using data from the Behavioral Risk Factor Surveillance System (BRFSS). J Bone Miner Res. 2008;23(1):151–8. [DOI] [PubMed]
- 15.Kanis JA, for WHO Scientific Group. Assessment of Osteoporosis at the Primary Care Level. Technical Report.: World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK; 2007.
- 16.Dawson-Hughes B, Tosteson AN, Melton LJ 3rd, Baim S, Favus MJ, Khosla S, et al. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19(4):449–58. [DOI] [PubMed]
- 17.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. [DOI] [PubMed]
- 18.Chen Z, Kooperberg C, Pettinger M, Bassford T, Cauley J, LaCroix AZ, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11(3):264–74. [DOI] [PubMed]
- 19.Melchior L, Huba G, Brown V, Reback C. A short depression index for women. Educ Psychol Meas. 1993;53:1117–25. [DOI]
- 20.World Health Organization Fracture Risk Assessment Tool. Available from: http://shef.ac.uk/FRAX. Accessed May 31st, 2008.
- 21.Tosteson AN, Melton LJ 3rd, Dawson-Hughes B, Baim S, Favus MJ, Khosla S, et al. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19(4):437–47. [DOI] [PMC free article] [PubMed]
- 22.Hosmer JDW, Lemeshow S. Applied Logistic Regression: 2nd Edition. John Wiley and Sons; 2000.
- 23.Lemeshow S, Hosmer JDW. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. [DOI] [PubMed]
- 24.Curtis JR, Arora T, Donaldson M, Alarcon G, Callahan L, Moreland L, Bridges L, Mikuls TR. Skeletal health among African Americans with recent onset rheumatoid arthritis. Arthritis Care and Research. In press. [DOI] [PMC free article] [PubMed]
- 25.Gourlay ML, Callahan LF, Preisser JS, Sloane PD. Osteoporosis preventive care in white and black women in community family medicine settings. South Med J. 2007;100(7):677–82. [DOI] [PubMed]
- 26.Mudano A, Casebeer L, Patino F, Allison J, Weissman N, Kiefe C, et al. Racial disparities in osteoporosis prevention in a managed care population. South Med J. 2003;96(5):445–51. [DOI] [PubMed]
- 27.Block AE, Solomon DH, Cadarette SM, Mogun H, Choudhry NK. Patient and physician predictors of post-fracture osteoporosis management. J Gen Intern Med. 2008;23(9):1447–51. [DOI] [PMC free article] [PubMed]
- 28.Solomon DH, Morris C, Cheng H, Cabral D, Katz JN, Finkelstein JS, et al. Medication use patterns for osteoporosis: an assessment of guidelines, treatment rates, and quality improvement interventions. Mayo Clin Proc. 2005;80(2):194–202. [DOI] [PubMed]
- 29.Curtis JR, Westfall AO, Allison JJ, Becker A, Casebeer L, Freeman A, et al. Longitudinal patterns in the prevention of osteoporosis in glucocorticoid-treated patients. Arthritis Rheum. 2005;52(8):2485–94. [DOI] [PubMed]
- 30.Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int. 2004;15(10):767–78. [DOI] [PubMed]
- 31.Hamrick I, Whetstone LM, Cummings DM. Racial disparity in treatment of osteoporosis after diagnosis. Osteoporos Int. 2006;17(11):1653–8. [DOI] [PubMed]
- 32.Morris CA, Cabral D, Cheng H, Katz JN, Finkelstein JS, Avorn J, et al. Patterns of bone mineral density testing: current guidelines, testing rates, and interventions. J Gen Intern Med. 2004;19(7):783–90. [DOI] [PMC free article] [PubMed]
- 33.Binkley N, Kiebzak GM, Lewiecki EM, Krueger D, Gangnon RE, Miller PD, et al. Recalculation of the NHANES database SD improves T-score agreement and reduces osteoporosis prevalence. J Bone Miner Res. 2005;20(2):195–201. [DOI] [PubMed]
- 34.Mikuls TR, Saag KG, Curtis J, Bridges SL Jr., Alarcon GS, Westfall AO, et al. Prevalence of osteoporosis and osteopenia among African Americans with early rheumatoid arthritis: the impact of ethnic-specific normative data. J Natl Med Assoc. 2005;97(8):1155–60. [PMC free article] [PubMed]
- 35.Broussard DL, Magnus JH. Risk assessment and screening for low bone mineral density in a multi-ethnic population of women and men: does one approach fit all? Osteoporos Int. 2004;15(5):349–60. [DOI] [PubMed]
- 36.Koh LK, Sedrine WB, Torralba TP, Kung A, Fujiwara S, Chan SP, et al. A simple tool to identify asian women at increased risk of osteoporosis. Osteoporos Int. 2001;12(8):699–705. [DOI] [PubMed]
- 37.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293(17):2102–8. [DOI] [PubMed]
- 38.Looker AC, Flegal KM, Melton LJ 3rd. Impact of increased overweight on the projected prevalence of osteoporosis in older women. Osteoporos Int. 2007;18(3):307–13. [DOI] [PubMed]
- 39.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182–8. [DOI] [PubMed]