The limited clinical success of stem-cell injections for the treatment of myocardial infarction [1], [2] has been mainly attributed to the low retention and survival of injected cells. Alternative methods for improving the efficiency of cell delivery include the following: 1) injection of a mixture of bioactive hydrogels and cells followed by cell-hydrogel polymerization in situ [3] and 2) the epicardial implantation of a tissue-engineered cardiac patch [4]. Although surgically more complex than cell or cell-hydrogel injection, the patch implantation is also expected to yield improved survival of delivered cells, and potentially, a more efficient structural and functional tissue reconstruction at the infarct site.
The tissue engineering of a functional biomimetic cardiac patch in vitro is a highly challenging problem because of the following: 1) the limited proliferative potential and high metabolic demand of differentiated cardiac cells, 2) the requisite presence of functional intercellular connections, and 3) the complex anisotropic architecture and electromechanical function of heart tissue. Since the late 1990s, research in the cardiac tissue engineering field has primarily involved the use of neonatal or embryonic cardiomyocytes to create three-dimensional (3-D) heart tissue equivalents for use in in vitro experimental studies [5]–[9] and, more recently, for the treatment of myocardial infarction in animal models [10]–[12]. These studies have shown that the structure and function of a cardiac tissue patch depend on the animal species from which the cells are derived [13]–[15], the composition of the seeded cells [6], [16], [17], the initial seeding density [6], [14], [18], [19], the scaffold characteristics [20]–[26], the composition of the culture medium [22], [27], the bioreactor type [18], [22], and the nature of the applied physical forces [13], [28], [29]. Although these studies have established a number of useful design rules for the engineering of a functional cardiac tissue patch, it is well recognized that the use of differentiated cardiomyocytes dissociated from heart tissue will remain limited to in vitro model systems and proof-of-concept in vivo studies. On the other hand, tissue patches made of stem cells offer a potential for translation to clinical practice and as such have been recently utilized in several studies for the functional repair of heart injury. Therefore, this short review is aimed at describing recent advances in the emerging field of stem-cell-based cardiac tissue engineering, with an emphasis on the potential use of cardiogenic stem cells for the construction of electrically conducting and contractile cardiac tissue patches. For the first time, the ability of genetically selected embryonic stem-cell-derived cardiomyocytes (ESC-CMs) to support continuous action potential propagation over a few-square centimeter area will be demonstrated using optical mapping of membrane potentials.
Noncardiogenic Stem-Cell Tissue Patches for the Repair of Myocardial Infarction
Autologous stem cells currently used in clinical trials lack significant potential to differentiate into functional cardiac myocytes [30]. Nevertheless, these cells represent a natural first choice in the engineering of a tissue patch for treatment of myocardial infarction. Recent studies have thus utilized tissue patches made of skeletal myoblasts [31]–[35], bone marrow-derived stem cells [36]–[42], or endothelial progenitor cells [43] for the repair of heart damage. Compared with the injection of a cell suspension, the implantation of tissue sheets composed of skeletal myoblasts has been proven more advantageous for the treatment of myocardial infarction in rats [32] and dilated cardiomyopathy in hamsters [31]. In particular, implantation of the engineered myoblast sheets over an infarction site yielded improved neovascularization, attenuated left ventricular dilatation, decreased fibrosis, improved fractional shortening, and prolonged animal survival compared to the delivery of the same number of myoblasts by cell injection. These benefits were mainly attributed to the improved survival of cells when implanted as a tissue sheet, and consequently, to the increased secretion of different paracrine factors including stromal-derived factor 1, hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF) [32]. In a follow-up study, the functional benefits of implanted myoblast sheets were also demonstrated in a pacing-induced canine heart failure model [33]. In an independent study, Siepe et al. compared the implantation of skeletal myoblasts seeded on polyurethane scaffolds with direct cell injection in the treatment of rat myocardial infarction [34]. Although cell survival was significantly higher in tissue patches compared with direct injection (80% vs. 10%–20%), both treatment groups exerted similar improvements of contractile heart function relative to untreated controls. In their most recent study, the same group demonstrated significantly improved fractional shortening in infarcted rat hearts after implantation of a tissue patch made of differentiated skeletal myoblasts and a mixture of collagen gel and matrigel [35]. In all described studies, the functional improvements were attributed to paracrine action of the implanted myoblasts.
Similar to the skeletal myoblast studies, several groups have attempted to treat myocardial injury by implanting a tissue patch made of either whole population or just the mesenchymal fraction of bone marrow cells (BMCs). In particular, in studies by Duplàa et al., abdominal muscle patches were formed into a cup shape to hold collagen gel mixed with either unfractionated BMCs [36], BMCs and mesenchymal stem cells (MSCs), or BMCs and endothelial progenitor cells [43], and implanted over one-month-old murine cryoin-farcts. Two weeks after patch implantation, angiogenesis, cell survival, scar thickness, and pressure waveforms were improved in all treatment groups, but only MSCs were found to invade the scar. Fukuhara et al. have implanted polyglycolic acid scaffolds impregnated with BMCs and collagen I gel, with or without basic fibroblast growth factor (bFGF) in one-month-old rat infarcts [37]. Four weeks after implantation, the capillary growth as well as the amplitudes and slopes of pressure waveforms were most improved in the bFGF/BMC group (relative to cell-free scaffolds), whereas no BMCs were found to invade the scar area. Using the cell sheet engineering approach, Miyahara et al. have implanted tissue sheets made of adipose-derived MSCs or dermal fibroblasts over four-week-old rat infarcts [38]. Eight weeks after implantation, MSC, but not fibroblast, tissue sheets reversed wall thinning, promoted angiogenesis, and significantly improved cardiac function and survival compared to untreated controls. Because implanted MSCs were found to express endothelial and smooth muscle rather than cardiac markers, the observed functional benefits were attributed to MSC secretion of VEGF and HGF. In a series of studies by Sung et al., decellularized bovine pericardia crosslinked with genipin were seeded with a concentrated suspension of 5-azacytidine-activated MSCs [39] or sandwiched with multilayered MSC sheets [40], [44], and implanted over a surgically created right ventricular defect and a four-week-old infarct in rats. Twelve weeks after implantation, MSCs within the patch predominantly differentiated into either smooth muscle cells or myofibroblasts, to a lesser extent into endothelial cells, and only occasionally into early but not mature cardiomyocytes. The expression of angiogenic (bFGF and platelet-derived growth factor beta polypeptide) and cardioprotective (insulin-like growth factor 1 and HGF) factors by MSCs was found to be the main contributor to significant patch vascularization and improved heart function [44]. In a recent study by Simpson et al., a tissue patch made of human MSCs embedded in collagen I gel was implanted 10 min after coronary ligation in rats [41]. Despite the improved ventricular remodeling and fractional shortening relative to treatment with a nonviable patch, no MSCs were found at the implantation site four weeks after infarction. These results suggest that the initial paracrine actions of implanted cells may be sufficient to exert at least a short-term positive effect on cardiac function. Finally, in the most recent study by Potapova et al., urinary bladder extracellular matrix was seeded with a suspension of human MSCs or with 3-D MSC spheroids and implanted over a surgically created right ventricular defect in canine hearts [42]. Interestingly, MSCs in spheroids, but not in regular monolayer cultures, exhibited increased expression of cardiac markers with 16% of the cells containing L-type Ca currents resembling those of adult ventricular myocytes. Eight weeks after implantation, MSC spheroid-seeded patches improved regional systolic contraction and stroke work compared to MSC suspension-seeded and cell-free patches.
The expression of angiogenic and cardioprotective factors by mesenchymal stem cells was found to be the main contributor to significant patch vascularization and improved heart function.
Tissue Patches Made of Cardiogenic Stem Cells
Although a noncardiogenic patch is expected to exert functional benefits mainly through concentrated paracrine actions, cardiogenic stem cells capable of generating large numbers of contractile cardiomyocytes can additionally augment heart function through direct electromechanical coupling with host cells. The resulting therapeutic benefits in this case would be mainly derived from significant remuscularization of the infarct or peri-infarct area rather than revascularization alone. Ideally, a functional cardiac tissue patch made for safe, efficient, and sustained repair of myocardial damage should comprise the following: 1) support the anisotropic architecture and electromechanical function characteristic of native cardiac muscle and 2) rapidly vascularize and integrate upon implantation. Among the cardiogenic sources being considered for therapy [45]–[49], recently identified autologous resident cardiac progenitor cells with a restricted potential to differentiate into cardiomyocytes, endothelial, and smooth muscle cells [45], [47], [49] appear to be an ideal candidate for the engineering of a functional cardiac patch. However, the ability of these cells to generate sufficient numbers of functional cardiomyocytes in vitro and without the contact with differentiated cardiomyocytes (e.g., from neonatal rat) is still questionable. ESC-CMs, on the other hand, can be generated in relatively large numbers in vitro [50], [51], and upon implantation, electromechanically integrate and successfully form new cardiac muscle [46], [52]. In light of the recent discovery of induced pluripotent stem cells [53]–[55], the ethical and immunogenic concerns related to the use of ESCs could be eliminated, and the future of cardiac tissue engineering therapy may involve the reprogramming of a patient's own cells into stem cells suitable for cardiogenic differentiation [56], [57] followed by the construction and implantation of functional cardiac patch.
To date, only a handful of studies have described attempts to engineer embryonic stem-cell-derived tissue patches for cardiac repair. Ke et al. have implanted polyglycolic acid scaffolds seeded with undifferentiated mouse ESCs 15 min after coronary ligation in mice [58]. Eight weeks after implantation, scar size and ventricular dilatation were reduced, whereas hemodynamic functional indices and survival rate were improved relative to the use of cell-free patches. Interestingly, no tumor formation was reported despite the use of undifferentiated cells. In studies by Guo et al., differentiating mouse ESCs were enriched for cardiomyocytes using Percoll gradient, embedded in matrigel-supplemented collagen rings, and statically cultured for five days followed by seven days of 2-Hz cyclic stretch [59]. The resulting tissue constructs contained aligned and cross-striated cardiomyocytes, as well as neural, endothelial, and fibroblastic cells, and exhibited twitch amplitudes and pharmacological responses similar to those previously measured in constructs made of neonatal rat cardiomyocytes. No signs of tumorigenesis were found after four weeks of subcutaneous implantation. In studies by Caspi et al., microdissected beating areas from differentiating human ESCs were enzymatically dissociated into single cells, mixed with human umbilical vein endothelial cells and mouse embryonic fibroblasts, embedded in matrigel, and seeded in porous poly-L-lactic/polyglycolic acid scaffolds [60]. After two weeks of culture, a synchronously contracting cardiac tissue patch with endothelial vessel networks was formed. The three cocultured cell types acted in synergy to promote cell survival, proliferation, and stabilization of blood vessels. In a recent study by Gwak et al., mouse ESC-derived beating cells were enzymatically dissociated from embryoid bodies, seeded on porous, elastic poly(lactide-co-caprolactone) scaffolds, exposed to cyclic stretch for two weeks, and implanted over three-week-old cryoin-farcts in rats [61]. Six weeks after implantation, the cyclically strained patches exhibited reduced fibrosis and apoptosis, higher VEGF expression and capillary formation, and upregulation of cardiac markers compared to unstretched ESC control patches made using nonelastic poly(lactide-co-glycolide) scaffolds. No assessment of tumorigenicity or cardiac function was performed. Finally, in the most recent study by Shimko et al., mouse ESC-CMs were purified based on -myosin heavy chain promoter driven resistance to neomycin, embedded in fibronectin-supplemented collagen gel rings, and after seven days of static culture, cyclically stretched at different frequencies for three days [62]. Cyclic stretch at 3 Hz, but not 1 Hz, upregulated the expression of sarcomeric cardiac genes and yielded improved cell density and alignment as well as the distribution of the gap junctional protein connexin-43. Despite the enhanced cardiac gene expression, no spontaneous beating was observed in these cultures.
Future Challenges of Stem-Cell-Based Cardiac Tissue Engineering
It may not be necessary or desirable to culture a noncardiogenic tissue patch in vitro for extended time periods before implantation, whereas the use of cardiogenic cells to create tissue patches with structure and function resembling those of native cardiac muscle will require specialized and well-controlled culture conditions over a period of several weeks. Fortunately, the experience gained with the engineering of tissue patches made of differentiated (neonatal or embryonic) cardiomyocytes can be utilized to promote in vitro cardiogenic stem-cell survival, differentiation, alignment, and electromechanical coupling to yield the formation of a cardiac tissue patch that supports fast action potential propagation and large contractile forces. Achieving this goal will be contingent upon establishing a well-defined and reproducible culture practice that will be based on our thorough understanding of the key cellular, biochemical, and physical determinants of cardiac growth and differentiation.
For example, although ethical and immunogenic issues related to use of ESCs may be resolved by the discovery of induced pluripotent stem cells, tumorigenic risks from implantation of undifferentiated cells still remain [63]. To date, the most efficient methods to obtain pure populations of differentiated or already committed ESCs involve selection based on genetically acquired resistance to antibiotics driven by the activation of cardiogenic promoters [64]–[67]. However, which cardiogenic promoter will yield a tissue patch with the optimal electrical and mechanical function remains unknown. Although pure ESC-CMs selected using different cardiac promoters (e.g., -MHC, NCX1, MLC-2v, ANF) may prove adequate as a source for future cell injection therapies, it is not clear if on their own they are capable of sufficiently differentiating and remodeling the surrounding matrix to form a functional tissue patch in vitro. On the other hand, ESC-derived cardiovascular progenitor cells (ESC-CPCs) selected for the combination of mesodermal (Brachyury or T) and cardiovascular transcription factors (Isl1, Nkx2-5) and cell surface receptors (Flk1, c-Kit, CXCR4) can differentiate into a mixture of cardiomyocytes, smooth muscle, and endothelial cells [68]–[70]. In theory, these cells could reconstitute both a cardiac muscle patch and its vasculature by providing a more natural cardioinductive environment. Eschenhagen et al. demonstrated that an increased proportion of nonmyocytes (mostly fibroblasts, but also endothelial and smooth muscle cells) relative to neonatal rat cardiomyocytes improved the mechanical function of their engineered heart tissues [13]. However, we showed that an excess of nonmyocytes deteriorated the electrical properties of a neonatal rat cardiac patch leading to an increased occurrence of arrhythmic activity [6]. Therefore, although the ESC-CPC derived vascular cells in the cardiac tissue patch may be beneficial for the differentiation and contractile function of individual cardiomyocytes [71], [72], they may also hamper the establishment of electrical coupling and successful formation of a functional syncytium. Taken together, the optimal balance of electrical and mechanical function within a cardiac tissue patch may require a specific proportion of cardiomyocytes and noncardiomyocytes. Ideally, these different cell types would be obtained from a common cell source rather than multiple tissues.
In addition, the development of safe and efficient cardiac tissue engineering therapies will be dependent on our ability to promote the functional maturation of stem-cell-derived cardiomyocytes in vitro. In essence, the functional competence of the cardiac patch is expected to directly correlate with the ability of derived CMs to conduct action potentials with a high velocity and exert a strong contraction through a Ca2+-dependent process of excitation–contraction (E-C) coupling. Relatively mature E-C coupling has been demonstrated in 20-day-old mouse ESC-CMs [73], [74], whereas one- to two-month-old human ESC-CMs exhibited immature sarcoplasmic reticulum (SR) function including negative force–frequency relationship, dependence of Ca2+ transients on sarcolemmal calcium inflow rather than SR release, lack of postrest potentiation, and no phospholamban or calsequestrin expression [75], [76]. Other studies, however, demonstrated more mature Ca2+-handling properties of the same age human ESC-CMs [77]. Although a longer culture time, 3-D cardiac patch environment, or postimplantation environment in vivo may promote maturation of the SR function in human ESC-CMs, the genetic manipulation strategies may be necessary to significantly improve the ability of a cardiac tissue patch to effectively contribute contractile forces in an infarcted heart.
In addition to the challenges of developing mature contractile properties of the ESC-CM patches, the establishment of efficient electrical conduction will also be crucial. The main determinants of conduction velocity in cardiac tissue are the availability of sodium current for propagation (which depends both on sodium conductance and cell resting potential), strength of gap junctional coupling, and cell size. Interestingly, although studies by several groups have shown that single human and mouse ESC-CMs exhibit sodium current densities comparable to those of neonatal or fetal ventricular myocytes [78], [79], conduction velocities of ES-CMs within the beating embryoid body outgrowths were measured to be only 1–5 cm/s in both human and mouse cells [52], [79]–[82]. For comparison, isotropic cultures of neonatal rat ventricular myocytes exhibit conduction velocities of 20 cm/s, comparable to those of neonatal rat ventricles [22], [83]. In human ESC-CMs, low-conduction velocities can be partially explained by limited expression of inward rectifier K+ currents and partial sodium current inactivation due to depolarized resting potential [79], [84], whereas in mouse ESC-CMs, the resting potential after a few weeks of differentiation is comparable to that of neonatal rat cardiomyocytes [65], [78], [85]. Alternatively, low-conduction velocities in outgrowth ESC-CMs may be caused by their small size or potentially strong coupling with surrounding nonmyocytes.
Our recent studies, however, suggest that a very important determinant of conduction velocity in mouse ES-CMs is the distribution and functionality of gap junctions. Specifically, pure ES-CMs selected based on their puromycin resistance driven by an α-myosin heavy chain promoter were dissociated into single cells after ten days of embryoid body differentiation and seeded on fibronectin-coated coverslips. After seven days of culture, the obtained confluent monolayers consisted of pure, interconnected, and cross-striated cardiomyocytes [Figures 1(a) and (b)]. Unlike the previous conduction measurements in ESC-CMs that utilized extracellular microelectrode arrays with only an approximately 2-mm2 field of view, propagation of action potentials in the resulting ESC-CM monolayers was optically mapped over an approximately 3-cm2 area [Figures 1(c) and (d)] using a 504-channel photodiode array [86]. As seen in Figure 1, we demonstrate, for the first time, that large numbers of pure ESC-CMs can establish functional gap junctions to support relatively uniform and continuous action potential propagation over a few-square centimeter area. This result suggests that the formation of a relatively large and functional ESC-CM patch is feasible. Furthermore, it is important to note that two very similar cell differentiation and isolation procedures from the same ESC clone have yielded significantly different conduction velocities after the same number of days in culture. The observed conduction velocity of 17.6 cm/s [Figure 1(d)] is comparable to those reported in isotropic monolayers of neonatal rat cardiomyocytes [83] and significantly higher than previously reported velocities in ESC-CM outgrowths [52], [79]–[82]. Although other differences between the two ESC-CM populations in Figures 1(a) and (b) may exist, one obvious difference is the spatial distribution and abundance of connexin-43 gap junctions. Sporadic and mainly punctate distribution within and between cardiomyocytes in slower cultures [Figure 1(a)] is contrasted with the presence of long intercellular gap junctional plaques (also characteristic of neonatal cardiomyocytes [87]) in faster cultures [Figure 1(b)]. These results also indicate that specific details of cell differentiation, selection, isolation, and culture procedure may play a crucial role in our ability to reproducibly engineer mature electrophysiological properties in ESC-derived cardiac patches.
Fig. 1.
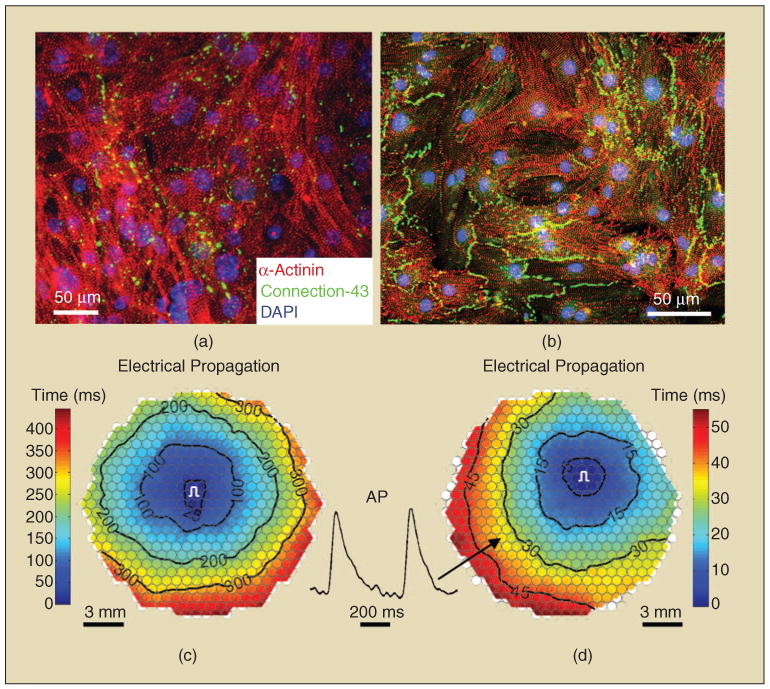
Mouse embryonic stem-cell-derived cardiomyocyte monolayers. (a) and (b) Two different cardiomyocyte preparations with distinctly different patterns of connexin-43 expression. (c) and (d) Corresponding isochrone maps of action potential propagation. Note a significantly lower conduction velocity (longer propagation time) in (c) (2.5 cm/s) compared with (d) (17.6 cm/s). Black circles in propagation maps denote 504 recording sites. Hexagonal field of view has a diameter of approximately 20 mm. Pulse signs denote the sites of electrical stimulation. AP inset shows optical action potential traces from one of the recording sites during the 3-Hz stimulation.
Along with the choice of cardiogenic cell source and factors that can promote the functional maturation and 3-D assembly of derived cardiomyocytes in vitro, other important issues for the future of cardiac tissue engineering therapy relate to the transition from in vitro to in vivo environment after implantation. In particular, the extent to which the architecture or biochemical environment (including incorporation of bioactive molecules) of a tissue patch can be manipulated to promote patch vascularization, survival, and electromechanical integration has yet to be fully addressed. Additional questions that need to be answered include the following: 1) What time after infarction will the implantation of an ESC-derived cardiac tissue patch exert the most benefit? 2) Which surgical procedure will allow optimal survival and integration of the patch? 3) Is the alignment of cells within the patch and their specific orientation relative to the host tissue during implantation relevant and ultimately beneficial to successful cardiac repair? Although obtaining definite answers to these and other important questions will require a systematic and long-term effort from multiple research groups, only through the establishment of well-defined and reproducible design rules and practices for the engineering and implantation of stem-cell-derived cardiac tissues will we be able to realize the full therapeutic potential of cardiac tissue engineering.
Acknowledgments
This work is supported by National Institute of Health Grant HL080469.
References
- 1.Welt FG, Losordo DW. Cell therapy for acute myocardial infarction: Curb your enthusiasm? Circulation. 2006 Mar;113:1272–1274. doi: 10.1161/CIRCULATIONAHA.105.613034. [DOI] [PubMed] [Google Scholar]
- 2.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation. 2008 Mar;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 3.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006 May;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann WH, Didie M, Doker S, Melnychenko I, Naito H, Rogge C, Tiburcy M, Eschenhagen T. Heart muscle engineering: An update on cardiac muscle replacement therapy. Cardiovasc Res. 2006 Apr; doi: 10.1016/j.cardiores.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Rau T, Nose M, Remmers U, Weil J, Weissmuller A, Davia K, Harding S, Peppel K, Koch WJ, Eschenhagen T. Overexpression of wild-type Galpha(i)-2 suppresses beta-adrenergic signaling in cardiac myocytes. FASEB J. 2003 Mar;17:523–525. doi: 10.1096/fj.02-0660fje. [DOI] [PubMed] [Google Scholar]
- 6.Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, Vunjak-Novakovic G, Freed LE. Cardiac muscle tissue engineering: Toward an in vitro model for electrophysiological studies. Amer J Physiol. 1999 Aug;277:H433–H444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- 7.Zong X, Bien H, Chung CY, Yin L, Fang D, Hsiao BS, Chu B, Entcheva E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials. 2005 Sept;26:5330–5338. doi: 10.1016/j.biomaterials.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 8.Baar K, Birla R, Boluyt MO, Borschel GH, Arruda EM, Dennis RG. Self-organization of rat cardiac cells into contractile 3-D cardiac tissue. FASEB J. 2005 Feb;19:275–277. doi: 10.1096/fj.04-2034fje. [DOI] [PubMed] [Google Scholar]
- 9.Yost MJ, Baicu CF, Stonerock CE, Goodwin RL, Price RL, Davis JM, Evans H, Watson PD, Gore CM, Sweet J, Creech L, Zile MR, Terracio L. A novel tubular scaffold for cardiovascular tissue engineering. Tissue Eng. 2004 Jan–Feb;10:273–284. doi: 10.1089/107632704322791916. [DOI] [PubMed] [Google Scholar]
- 10.Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM. Survival and function of bioengineered cardiac grafts. Circulation. 1999 Nov;100:II63–II69. doi: 10.1161/01.cir.100.suppl_2.ii-63. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006 Apr;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 12.Furuta A, Miyoshi S, Itabashi Y, Shimizu T, Kira S, Hayakawa K, Nishiyama N, Tanimoto K, Hagiwara Y, Satoh T, Fukuda K, Okano T, Ogawa S. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res. 2006 Mar;98:705–712. doi: 10.1161/01.RES.0000209515.59115.70. [DOI] [PubMed] [Google Scholar]
- 13.Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000;14:669–679. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann W, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac cells. Biotechnol Bioeng. 2000;68:106–114. [PubMed] [Google Scholar]
- 15.Carrier RL, Papadaki M, Rupnick M, Schoen FJ, Bursac N, Langer R, Freed LE, Vunjak-Novakovic G. Cardiac tissue engineering: Cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol Bioeng. 1999 Sept;64:580–589. doi: 10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann WH, Eschenhagen T. Cardiac tissue engineering for replacement therapy. Heart Fail Rev. 2003 July;8:259–269. doi: 10.1023/a:1024725818835. [DOI] [PubMed] [Google Scholar]
- 17.Iyer RK, Chiu LL, Radisic M. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J Biomed Mater Res A. 2008 Apr; doi: 10.1002/jbm.a.32014. [DOI] [PubMed] [Google Scholar]
- 18.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003 May;82:403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 19.Dar A, Shachar M, Leor J, Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002 Nov;80:305–312. doi: 10.1002/bit.10372. [DOI] [PubMed] [Google Scholar]
- 20.Ozawa T, Mickle DA, Weisel RD, Koyama N, Ozawa S, Li RK. Optimal biomaterial for creation of autologous cardiac grafts. Circulation. 2002 Sept;106:I176–I182. [PubMed] [Google Scholar]
- 21.Pego AP, Poot AA, Grijpma DW, Feijen J. Biodegradable elastomeric scaffolds for soft tissue engineering. J Control Release. 2003 Feb;87:69–79. doi: 10.1016/s0168-3659(02)00351-6. [DOI] [PubMed] [Google Scholar]
- 22.Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: Molecular, structural, and electrophysiological studies. Amer J Physiol Heart Circ Physiol. 2001 Jan;280:H168–H178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 23.Shachar M, Cohen S. Cardiac tissue engineering, ex-vivo: Design principles in biomaterials and bioreactors. Heart Fail Rev. 2003 July;8:271–276. doi: 10.1023/a:1024729919743. [DOI] [PubMed] [Google Scholar]
- 24.Yin L, Bien H, Entcheva E. Scaffold topography alters intracellular calcium dynamics in cultured cardiomyocyte networks. Amer J Physiol Heart Circ Physiol. 2004 Apr; doi: 10.1152/ajpheart.01120.2003. [DOI] [PubMed] [Google Scholar]
- 25.Bursac N, Loo Y, Leong K, Tung L. Novel anisotropic engineered cardiac tissues: Studies of electrical propagation. Biochem Biophys Res Commun. 2007 Oct;361:847–853. doi: 10.1016/j.bbrc.2007.07.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YC, Khait L, Birla RK. Contractile three-dimensional bioengineered heart muscle for myocardial regeneration. J Biomed Mater Res A. 2007 Mar;80:719–731. doi: 10.1002/jbm.a.31090. [DOI] [PubMed] [Google Scholar]
- 27.Huang YC, Khait L, Birla RK. Modulating the functional performance of bioengineered heart muscle using growth factor stimulation. Ann Biomed Eng. 2008 Aug;36:1372–1382. doi: 10.1007/s10439-008-9517-9. [DOI] [PubMed] [Google Scholar]
- 28.Akhyari P, Fedak PW, Weisel RD, Lee TY, Verma S, Mickle DA, Li RK. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation. 2002 Sept;106:I137–I142. [PubMed] [Google Scholar]
- 29.Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, Vunjak-Novakovic G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Amer J Physiol Heart Circ Physiol. 2004 Feb;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 30.Murry CE, Field LJ, Menasche P. Cell-based cardiac repair: Reflections at the 10-year point. Circulation. 2005 Nov;112:3174–3183. doi: 10.1161/CIRCULATIONAHA.105.546218. [DOI] [PubMed] [Google Scholar]
- 31.Kondoh H, Sawa Y, Miyagawa S, Sakakida-Kitagawa S, Memon IA, Kawaguchi N, Matsuura N, Shimizu T, Okano T, Matsuda H. Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc Res. 2006 Feb;69:466–475. doi: 10.1016/j.cardiores.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, Sakakida SK, Kondoh H, Aleshin AN, Shimizu T, Okano T, Matsuda H. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005 Nov;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Hata H, Matsumiya G, Miyagawa S, Kondoh H, Kawaguchi N, Matsuura N, Shimizu T, Okano T, Matsuda H, Sawa Y. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J Thorac Cardiovasc Surg. 2006 Oct;132:918–924. doi: 10.1016/j.jtcvs.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Siepe M, Giraud MN, Pavlovic M, Receputo C, Beyersdorf F, Menasche P, Carrel T, Tevaearai HT. Myoblast-seeded biodegradable scaffolds to prevent post-myocardial infarction evolution toward heart failure. J Thorac Cardiovasc Surg. 2006 July;132:124–131. doi: 10.1016/j.jtcvs.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 35.Giraud MN, Ayuni E, Cook S, Siepe M, Carrel TP, Tevaearai HT. Hydrogel-based engineered skeletal muscle grafts normalize heart function early after myocardial infarction. Artif Organs. 2008 Sept;32:692–700. doi: 10.1111/j.1525-1594.2008.00595.x. [DOI] [PubMed] [Google Scholar]
- 36.Barandon L, Couffinhal T, Dufourcq P, Alzieu P, Daret D, Deville C, Duplaa C. Repair of myocardial infarction by epicardial deposition of bone-marrow-cell-coated muscle patch in a murine model. Ann Thorac Surg. 2004 Oct;78:1409–1417. doi: 10.1016/j.athoracsur.2003.12.078. [DOI] [PubMed] [Google Scholar]
- 37.Fukuhara S, Tomita S, Nakatani T, Fujisato T, Ohtsu Y, Ishida M, Yutani C, Kitamura S. Bone marrow cell-seeded biodegradable polymeric scaffold enhances angiogenesis and improves function of the infarcted heart. Circ J. 2005 July;69:850–857. doi: 10.1253/circj.69.850. [DOI] [PubMed] [Google Scholar]
- 38.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006 Apr;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 39.Wei HJ, Chen SC, Chang Y, Hwang SM, Lin WW, Lai PH, Chiang HK, Hsu LF, Yang HH, Sung HW. Porous acellular bovine pericardia seeded with mesenchymal stem cells as a patch to repair a myocardial defect in a syngeneic rat model. Biomaterials. 2006 Nov;27:5409–5419. doi: 10.1016/j.biomaterials.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Chen CH, Wei HJ, Lin WW, Chiu I, Hwang SM, Wang CC, Lee WY, Chang Y, Sung HW. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovasc Res. 2008 Oct;80:88–95. doi: 10.1093/cvr/cvn149. [DOI] [PubMed] [Google Scholar]
- 41.Simpson D, Liu H, Fan TH, Nerem R, Dudley SC., Jr A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007 Sept;25:2350–2357. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potapova IA, Doronin SV, Kelly DJ, Rosen AB, Schuldt AJ, Lu Z, Kochupura PV, Robinson RB, Rosen MR, Brink PR, Gaudette GR, Cohen IS. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. Amer J Physiol Heart Circ Physiol. 2008 Oct; doi: 10.1152/ajpheart.00219.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derval N, Barandon L, Dufourcq P, Leroux L, Lamaziere JM, Daret D, Couffinhal T, Duplaa C. Epicardial deposition of endothelial progenitor and mesenchymal stem cells in a coated muscle patch after myocardial infarction in a murine model. Eur J Cardiothorac Surg. 2008 Aug;34:248–254. doi: 10.1016/j.ejcts.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 44.Wei HJ, Chen CH, Lee WY, Chiu I, Hwang SM, Lin WW, Huang CC, Yeh YC, Chang Y, Sung HW. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008 Sept;29:3547–3556. doi: 10.1016/j.biomaterials.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007 Aug;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007 Sept;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 47.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007 Feb;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 48.Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, Penicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004 Feb;94:223–239. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 49.Limana F, Zacheo A, Mocini D, Mangoni A, Borsellino G, Diamantini A, De Mori R, Battistini L, Vigna E, Santini M, Loiaconi V, Pompilio G, Germani A, Capogrossi MC. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007 Dec;101:1255–1265. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- 50.Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E, Zandstra PW. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2008 July; doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- 51.Niebruegge S, Nehring A, Bar H, Schroeder M, Zweigerdt R, Lehmann J. Cardiomyocyte production in mass suspension culture: Embryonic stem cells as a source for great amounts of functional cardiomyocytes. Tissue Eng Part A. 2008 Oct;14:1591–1601. doi: 10.1089/ten.tea.2007.0247. [DOI] [PubMed] [Google Scholar]
- 52.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004 Oct;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 53.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007 Dec;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 55.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008 Jan;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 56.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008 July;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 57.Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, Yamanaka S, Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008 July;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 58.Ke Q, Yang Y, Rana JS, Chen Y, Morgan JP, Xiao YF. Embryonic stem cells cultured in biodegradable scaffold repair infarcted myocardium in mice. Sheng Li Xue Bao. 2005 Dec;57:673–681. [PubMed] [Google Scholar]
- 59.Guo XM, Zhao YS, Chang HX, Wang CY, E LL, Zhang XA, Duan CM, Dong LZ, Jiang H, Li J, Song Y, Yang XJ. Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation. 2006 May;113:2229–2237. doi: 10.1161/CIRCULATIONAHA.105.583039. [DOI] [PubMed] [Google Scholar]
- 60.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007 Feb;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 61.Gwak SJ, Bhang SH, Kim IK, Kim SS, Cho SW, Jeon O, Yoo KJ, Putnam AJ, Kim BS. The effect of cyclic strain on embryonic stem cell-derived cardiomyocytes. Biomaterials. 2008 Mar;29:844–856. doi: 10.1016/j.biomaterials.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 62.Shimko VF, Claycomb WC. Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue Eng Part A. 2008 Jan;14:49–58. doi: 10.1089/ten.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB J. 2007 doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 64.Klug MG, Soonpaa MH, Koh GY, Field LJ. Proc Keystone Symp—Tissue Eng. Taos; New Mexico: 1996. Purification of cardiac myocytes differentiated from embryonic stem cells for use in intracardiac grafting. [Google Scholar]
- 65.Muller M, Fleischmann BK, Selbert S, Ji GJ, Endl E, Middeler G, Muller OJ, Schlenke P, Frese S, Wobus AM, Hescheler J, Katus HA, Franz WM. Selection of ventricular-like cardiomyocytes from ES cells in vitro. FASEB J. 2000 Dec;14:2540–2548. doi: 10.1096/fj.00-0002com. [DOI] [PubMed] [Google Scholar]
- 66.Kolossov E, Lu Z, Drobinskaya I, Gassanov N, Duan Y, Sauer H, Manzke O, Bloch W, Bohlen H, Hescheler J, Fleischmann BK. Identification and characterization of embryonic stem cell-derived pacemaker and atrial cardiomyocytes. FASEB J. 2005 Apr;19:577–579. doi: 10.1096/fj.03-1451fje. [DOI] [PubMed] [Google Scholar]
- 67.Fijnvandraat AC, van Ginneken AC, Schumacher CA, Boheler KR, Lekanne Deprez RH, Christoffels VM, Moorman AF. Cardiomyocytes purified from differentiated embryonic stem cells exhibit characteristics of early chamber myocardium. J Mol Cell Cardiol. 2003 Dec;35:1461–1472. doi: 10.1016/j.yjmcc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008 Feb;132:537–543. doi: 10.1016/j.cell.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008 May;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 70.Nelson TJ, Faustino RS, Chiriac A, Crespo-Diaz R, Behfar A, Terzic A. CXCR4+/FLK-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem Cells. 2008 June;26:1464–1473. doi: 10.1634/stemcells.2007-0808. [DOI] [PubMed] [Google Scholar]
- 71.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: Implications for cardiac regeneration. Circulation. 2004 Aug;110:962–968. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Wamel AJ, Ruwhof C, van der Valk-Kokshoorn LJ, Schrier PI, van der Laarse A. Rapid effects of stretched myocardial and vascular cells on gene expression of neonatal rat cardiomyocytes with emphasis on autocrine and paracrine mechanisms. Arch Biochem Biophys. 2000 Sept;381:67–73. doi: 10.1006/abbi.2000.1947. [DOI] [PubMed] [Google Scholar]
- 73.Sauer H, Theben T, Hescheler J, Lindner M, Brandt MC, Wartenberg M. Characteristics of calcium sparks in cardiomyocytes derived from embryonic stem cells. Amer J Physiol Heart Circ Physiol. 2001 July;281:H411–H421. doi: 10.1152/ajpheart.2001.281.1.H411. [DOI] [PubMed] [Google Scholar]
- 74.Fu JD, Li J, Tweedie D, Yu HM, Chen L, Wang R, Riordon DR, Brugh SA, Wang SQ, Boheler KR, Yang HT. Crucial role of the sarcoplasmic reticulum in the developmental regulation of Ca2+ transients and contraction in cardiomyocytes derived from embryonic stem cells. FASEB J. 2006 Jan;20:181–183. doi: 10.1096/fj.05-4501fje. [DOI] [PubMed] [Google Scholar]
- 75.Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, Itskovitz-Eldor J, Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes: Intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006 Feb;24:236–245. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Fu JD, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: Insights for driven maturation. Stem Cells. 2007 Dec;25:3038–3044. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 77.Satin J, Itzhaki I, Rapoport S, Schroder EA, Izu L, Arbel G, Beyar R, Balke CW, Schiller J, Gepstein L. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008 Aug;26:1961–1972. doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- 78.Maltsev V, Wobus A, Rohwedel J, Bader M, Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res. 1994;75:233–244. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- 79.Satin J, Kehat I, Caspi O, Huber I, Arbel G, Itzhaki I, Magyar J, Schroder EA, Perlman I, Gepstein L. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol. 2004 Sept;559:479–496. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caspi O, Itzhaki I, Arbel G, Kehat I, Gepstien A, Huber I, Satin J, Gepstein L. In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cells Dev. 2008 May; doi: 10.1089/scd.2007.0280. [DOI] [PubMed] [Google Scholar]
- 81.Malan D, Reppel M, Dobrowolski R, Roell W, Smyth N, Hescheler J, Paulsson M, Bloch W, Fleischmann BK. Lack of laminin {gamma}1 in ES cell-derived cardiomyocytes causes inhomogeneous electrical spreading despite intact differentiation and function. Stem Cells. 2008 Oct; doi: 10.1634/stemcells.2008-0335. [DOI] [PubMed] [Google Scholar]
- 82.Hescheler J, Halbach M, Egert U, Lu ZJ, Bohlen H, Fleischmann BK, Reppel M. Determination of electrical properties of ES cell-derived cardiomyocytes using MEAs. J Electrocardiol. 2004;37:110–116. doi: 10.1016/j.jelectrocard.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 83.Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: A model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002 Dec;91:e45–e54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- 84.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: Action potential characterization. Circ Res. 2003 July;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 85.Bursac N, Papadaki M, White JA, Eisenberg SR, Vunjak-Novakovic G, Freed LE. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 2003 Dec;9:1243–1253. doi: 10.1089/10763270360728152. [DOI] [PubMed] [Google Scholar]
- 86.Klinger R, Bursac N. Cardiac cell therapy in vitro: Reproducible assays for comparing the efficacy of different donor cells. IEEE Eng Med Biol Mag. 2008 Jan–Feb;27:72–80. doi: 10.1109/MEMB.2007.913849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pimentel RC, Yamada KA, Kleber AG, Saffitz JE. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res. 2002 Apr;90:671–677. doi: 10.1161/01.res.0000014823.75393.4d. [DOI] [PubMed] [Google Scholar]


