Abstract
Adipose tissue consists of mature adipocytes, preadipocytes and mesenchymal stem cells (MSCs), but a culture system for analyzing their cell types within the tissue has not been established. We have recently developed “adipose tissue-organotypic culture system” that maintains unilocular structure, proliferative ability and functions of mature adipocytes for a long term, using three-dimensional collagen gel culture of the tissue fragments. In this system, both preadipocytes and MSCs regenerate actively at the peripheral zone of the fragments. Our method will open up a new way for studying both multiple cell types within adipose tissue and the cell-based mechanisms of obesity and metabolic syndrome. Thus, it seems to be a promising model for investigating adipose tissue biology and regeneration. In this article, we introduce adipose tissue-organotypic culture, and propose two theories regarding the mechanism of tissue regeneration that occurs specifically at peripheral zone of tissue fragments in vitro.
Key words: adipose tissue-organotypic culture, three-dimensional, tissue fragments, peripheral zone, central zone, mature adipocytes, preadipocytes (immature adipocytes), mesenchymal stem cells, adipokines, tissue regeneration
Introduction
Adipose tissue is subdivided into white and brown adipose tissues. White adipose tissue (called adipose tissue in this article) consists of mature adipocytes, preadipocytes (immature adipocytes) and endothelial cells. Recently, adipose tissue is also shown to have mesenchymal stem cells (MSCs) that produce various cell types, e.g., adipocytes, osteoblasts, myocytes, chondrocytes, neurons and hepatocytes.1 In general, adipose tissue is a specific organ that stores excess energy in the form of lipid droplet. The tissue plays a central role in lipid metabolism including triglyceride storage and fatty acid release.2 Also, adipose tissue has been well known to be an endocrine organ that affects the biological behavior of various cell types through its production of adipokines, including leptin, adiponectin, tumor necrosis factor-α (TNF-α), heparin-binding epidermal growth factor, insulin-like growth factor II, adipsin and other cytokines.3–5 Thus, adipose tissue with multiple cell types above seems critical for the maintenance of body homeostasis.
Culturing mature adipocytes in vitro has been technically difficult, because mature adipocytes have large lipid droplets and thus do not attach to the surface of culture dish due to their buoyancy in culture medium. To resolve this problem, we have been demonstrated the following primary culture systems of isolated mature adipocytes: (1) ceiling culture6,7 and (2) three-dimensional collagen gel culture.8 These methods are useful for studying the proliferation and differentiation of isolated mature adipocytes, but they do not allow us to investigate the biological behavior of multiple cell types within adipose tissues.
To challenge this interesting issue and to overcome the difficulty in culturing adipose tissue, we have recently established a new culture system of adipose tissue fragments embedded in a three-dimensional collagen gel, which is able to easily entrap buoyant adipose tissue.9 Our method named “adipose tissue-organotypic culture” maintains the unilocular structure and active function of mature adipocytes for more than 4 weeks. Furthermore, both preadipocytes and MSCs develop actively around adipose tissue fragments. At the peripheral zone of the tissue fragments, but not at the central zone, the tissue remodeling actively take place. Thus, our method also seems to be a promising model for investigating tissue regeneration and organogenesis and their regulation mechanisms. In this article, we introduce a new organotypic culture system of adipose tissue, and discuss its application to the study of adipose tissue biology and regeneration.
Summary of Adipose Cell Types and Their Conventional Culture Systems
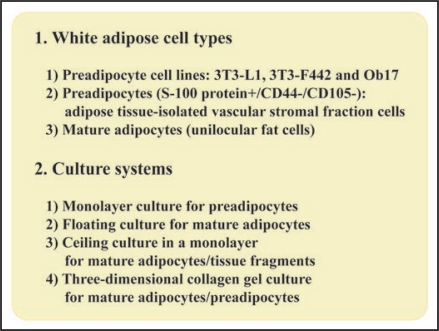
Firstly, we would like to summarize adipose cell types and their conventional culture systems in order to well understand a new organotypic culture of adipose tissue fragments. Figure 1 summarizes adipose cell types and their suitable culture systems. 3T3-L1 and 3T3-F442A cells, which were all cloned from mouse embryo-originated Swiss 3T3 cells, are the most frequently used preadipocyte cell lines.10–12 Ob17 preadipocyte cell line, which was originated from epididymal adipose tissue of ob/ob obese mice, is less commonly utilized.13 Recently, the new preadipocyte cell line DFAT-GFP cells, which were derived from mature adipocytes of GFP transgenic mice, have been established by H. Nobusue and K. Kano.14 DFAT-GFP cells would be useful for studying adipocyte biology, because they form adipose tissue after their implantation into subcutaneous tissue of mice more easily and stably than 3T3-L1 cells.14 Adipose tissue-isolated vascular stromal fraction cells with fine lipid droplets have been used as primary preadipocytes,15 although CD44+/CD105+ MSCs have been shown to exist in adipose tissue.16 These preadipocyte types are easily cultured in a conventional monolayer system, because they are not buoyant in culture medium. Adipose tissue-isolated mature adipocytes have been cultured in a floating system due to their buoyancy only for a short term (24 to 72 hours),17 because they rapidly undergo necrosis and apoptosis in this system. To resolve this disadvantage of the floating culture, H. Sugihara developed for the first time, ceiling culture system of mature adipocytes (Fig. 2), applying their buoyancy to culturing the cells.6,7 Also, he established three-dimensional culture of isolated mature adipocytes8 and ceiling culture of adipose tissue fragments in a monolayer.18 These methods established by H. Sugihara allow us to investigate proliferation and differentiation of mature adipocytes and preadipocytes in vitro for a long term. Recently, H.H. Zhang and M.C. Eggo have shown that ceiling culture of human mature adipocytes is useful for investigating adipocyte functions.17 By a combination of adipose tissue fragments with three-dimensional collagen gel, we have recently developed a new organotypic culture of adipose tissue fragments,9 as described below.
Figure 1.
Adipose cell types and their suitable culture systems. As preadipocyte cell lines, 3T3-L1 and 3T3-F442 cells are used more frequently than Ob17 cells. Primary preadipocytes (S-100 protein+/CD44−/CD105−) and mature adipocytes that are all isolated from adipose tissue are also utilized. Preadipocyte types are easily monolayer-cultured. Floating culture of mature adipocytes is used, but it maintains viable mature adipocytes only for a short term. In contrast, ceiling culture maintains viable mature adipocytes for a long term and is useful for studying their growth and differentiation. Ceiling culture is able to be applied to culture of tissue fragments in monolayer. Three-dimensional collagen gel culture is useful for both mature adipocytes and preadipocytes.
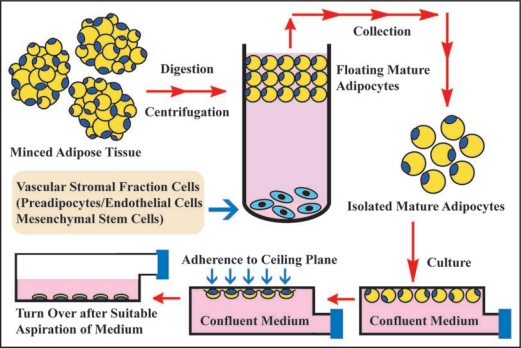
Figure 2.
Ceiling culture method. Minced adipose tissue is digested by collagenase and then centrifuged. After centrifugation, mature adipocyte layer floats at top of test tube, while vascular stromal fraction cell types that consist of preadipocytes, endothelial cells and MSCs precipitate at bottom. After isolated mature adipocytes are collected from mature adipocyte layer, they are pored into culture bottle filled with medium. The bottle is placed in upside-down fashion in culture incubator. After mature adipocytes adhere to ceiling plane, medium is aspirated and the bottle is placed in upside-down fashion. In this way, ceiling culture of mature adipocytes is set up.
Method of Adipose Tissue-Organotypic Culture
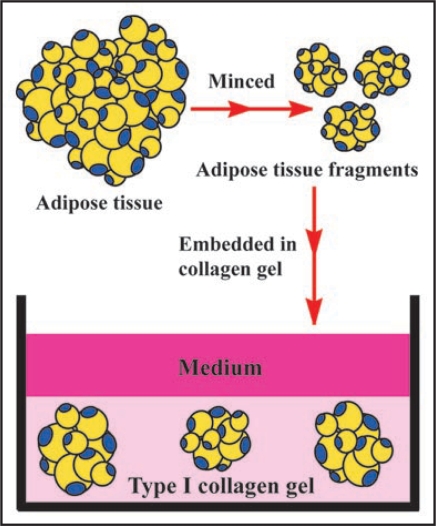
This method9 allows us to culture subcutaneous and visceral adipose tissues that have mature adipocytes, preadipocytes and MSCs for a long term. These adipose tissues are processed as follows. Figure 3 illustrates this culture method.
Figure 3.
Adipose tissue-organotypic culture and its organization procedure. After rinsing adipose tissue, it was minced in about 0.5 mm in diameter. The minced tissue fragments are embedded in type I collagen gel. After the gel is fully firm, the gel is covered with medium and cultured.
After rinsing with MEM (Minimum essential medium, Nissui Co. Ltd., Tokyo, Japan), adipose tissue is minced in about 0.5 mm diameter. A total of 0.1 ml of the minced tissue fragments was embedded in 1.0 ml type I collagen gel solution (Nitta Gelatin Co. Ltd., Osaka, Japan), as described below.
Prepare the collagen gel solution as follows. Mix 8 volumes of acid-soluble type I collagen with one volume of ×10 concentrated Ham's F-12 medium and 1 volume of reconstructive buffer (2.2 g NaHCO3 and 4.77 g HEPES in 100 ml 0.05 M NaOH) and keep this mixture on ice.
Gently and fully mix the cold collagen solution with adipose tissue fragments above.
Pour 1 ml of this mixture in a 12- or 6-well culture dish, and immediately warm the dish to 37 C for at least 20 minutes in a 5% CO2 incubator to allow a gel to form.
After at least 20 minutes, when the gel is fully firm, cover the gel with 3 ml/dish Ham's F-12 medium supplemented with 10 % fetal calf serum and 50 µg/ml gentamicin.
Incubate the dish at 37 C in a 5% CO2/95% air incubator.
In culture assembly, cellular behaviors are able to be easily analyzed by histochemistry, immunohistochemistry, electron microscopy, ELISA and gene-investigated methods (RT-PCR, real-time RT-PCR, and Northern and Southern blots). For western blot analysis, serum-free medium is recommended to be used in culture, because much serum-containing albumin interferes with visible detection of targeted proteins. Finally, this method can be applied to cultures of various organ-related (e.g., heart and kidney) and bone marrow adipose tissues. To characterize both various organ-specific and bone marrow adipose tissues, several studies are proceeding in our laboratory.
Adipose Tissue-Organotypic Culture
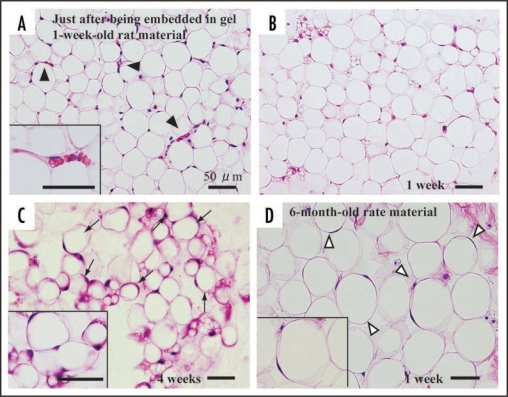
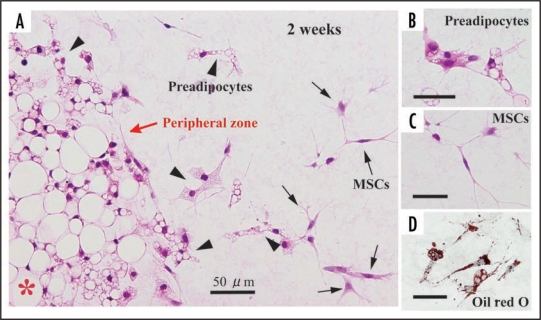
In this article, we mainly describe cellular behaviors of 1-week-old rat-derived subcutaneous adipose tissue in our culture system. Just after being embedded in collagen gel, adipose tissue fragments apparently have mature adipocytes and capillaries (Fig. 4A). Mature adipocytes containing a single large lipid droplet in their cytoplasm show a unilocular structure, and they are well maintained for more than 4 weeks in culture (Fig. 4, B and C). Systemic capillary network disappears by 7 days (Fig. 4B), although a few capillaries are sporadically retained. Central parts of adipose tissue fragments have no drastic change throughout culture term, but the peripheral zones of the fragments undergo prominent changes as follows. After 2 days in culture, two spindle-shaped cell types gradually begin to develop at the peripheral zones of adipose tissue fragments, and thereafter they actively grow (Fig. 5). The one cell type preadipocytes have fine lipid droplets and express S-100 protein (Fig. 5, A, B and D). The other cell type MSCs without any lipid droplets express both CD44 and CD105 (Fig. 5, A and C; supplementary data-1) that are well known to be markers of MSCs . The ratio of preadipocytes (85.0 ± 3.4%) to MSC-like cells (15.0 ± 3.4%) is 5.7:1 at 1 week in culture. In this system, MSCs do not spontaneously differentiate into any cell types other than preadipocytes. In fact, any of bone, cartilage and muscle tissues are not detected in culture assembly throughout culture term. The lipogenesis factor insulin increases the number of immature adipocytes (supplementary data-2), suggesting that insulin causes MSCs to differentiate into immature adipocytes. In general, the differentiation of isolated MSCs into various mesenchymal cell types, i.e. adipocytes, osteoblasts, myocytes and chondrocytes, is well known to require various differentiating factors.1 To elucidate whether MSCs detected in our culture system are able to differentiate into various mesenchymal cell types and others, further studies are in order in our laboratories. Finally, we also cultured 6-month-old rat-derived adipose tissue fragments in order to test the validity of our culture method. Mature adipocytes within these tissue fragments were well maintained (Fig. 4D), although the development rate of spindle-shaped cell types decreased to about 30% of that of 1-week-old rat-derived materials.
Figure 4.
Histology of adipose tissue in adipose tissue-organotypic cultures of 1-week-old (A, B and C) and 6-month-old rat materials (D). (A) Adipose tissue fragment just after being embedded in collagen gel has viable mature adipocytes with a large single lipid droplet and a peripherally located nucleus, hence have been called unilocular fat cells. Capillary network (arrowheads and inset) is seen among mature adipocytes. Note that erythrocytes are seen in capillaries. (B) At 1 week in culture, viable mature adipocytes are maintained at the center of the tissue fragment and they have no drastic morphological change. However, capillary network disappears within the tissue fragments. Spindle-shaped cells do not develop at the central part of the fragment. (C) Even at 4 weeks in culture, mature adipocytes are well retained within the fragment, and spindle-shaped cells are also not observed at the center. (D) Mature adipocytes within the fragments derived from 6-month-old rats are also well retained at 1 week in culture. Notice that the size of 6-month-old rat-derived mature adipocytes is about 1.5 to 2 times that of 1-week-old rat-derived mature adipocytes (B). A, B, C and D, H–E staining.
Figure 5.
Development of preadipocytes and MSCs at the peripheral zone of adipose tissue fragments derived from 1-week-old rats. (A) At 2 weeks in culture, preadipocytes and MSCs appear actively at the peripheral part of the fragment. Preadipocytes (arrowheds) have fine lipid droplets in the cytoplasm. MSCs without lipid droplets (C) are also seen. (D) Lipid droplets (red in color) are confirmed by oil red O staining. (A, B and C), H-E staining. *central zone of tissue fragment.
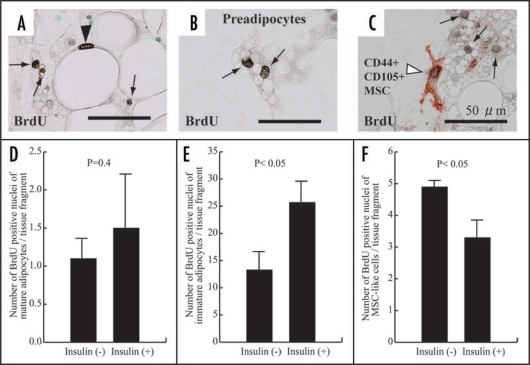
In this system, the growth marker bromodeoxyuridine (BrdU) uptakes of both mature and preadipocytes are clearly detected (Fig. 6, A and B), although the uptake of preadipocytes is prominently higher than that of mature adipocytes (Fig. 6, D and E). This suggests that not only preadipocytes but also mature adipocytes have proliferative ability. Insulin tends to increase BrdU intake of mature adipocytes (Fig. 6D), but it significantly increases the uptake of preadipocytes (Fig. 6E). Insulin inhibits BrdU uptake of MSCs (Fig. 6, C and F). Namely, insulin is a growth-promoting factor for preadipocytes, while it is a growth-suppressing agent for MSCs.
Figure 6.
Proliferation of mature adipocytes (A and D), preadipocytes (B and E) and MSCs (C and F) at 1 week in cultures of 1-week-old rat materials with or without insulin. In cultures with insulin, its stimulation was carried out at culture day 2, 4 and 6. Cell growth is assessed by immunohistochemistry with BrdU. A mature adipocyte shows intranuclear BrdU uptake in black (arrowhead in panel A). Preadipocytes show intranuclear BrdU uptake (allows in panels A, B and C). CD44+/CD105+ MSCs in brownish red (C) has intranuclear BrdU intake (arrowhead in C). (D) There is no significant difference of number of BrdU-positive mature adipocytes between cultures with and without insulin, although insulin tends to enhance BrdU uptake of mature adipocytes. (E and F) Insulin enhances BrdU uptake of preadipocytes (E), while it inhibits BrdU uptake of MSCs (F), indicating their significant difference between that of immature adipocytes or MSC-like cells with and without insulin.
Functional Properties of Adipose Tissue in its Organotypic Culture
In adipose tissue-organotypic culture, triglyceride is produced in culture supernatants. The lipogenesis-promoting factor insulin enhances its production, increasing size of mature adipocytes and number of preadipocytes. Also, adipokines of adiponectin and leptin are produced in culture supernatants, and insulin prominently enhances their production (supplementary data-3, A and B). The lipolysis-related factor BRL and the inflammation-related agent TNF-α inhibit the production of both adiponectin and leptin (supplementary data-3, A and B), whereas the inflammation-related factor LPS does not affect their production (supplementary data-3, A and B). This suggests that insulin may be a powerful differentiation-promoting player for adipose tissue, whereas TNF-α, LPS and BRL may be a differentiation-suppressing factor for the tissue at least in their comparison with insulin. In general, adipose tissue produces many adipokines other than adiponectin and leptin, and adipokines are suggested to be closely associated with various pathologic conditions.4,5 Analyzing adipokine profile in response to various agents in this system would be thus promising for investigating mechanisms of obesity, metabolic syndrome, aging19–21 and adipose tissue-related cancer.22–24
Also, mRNAs of adiponectin, leptin and PPARγ are expressed in this system (supplementary data-3, C, D and E). Insulin increases the expression of these genes, whereas TNF-β and LPS inhibit it (supplementary data-3, C, D and E). BRL prohibits mRNA expression of leptin, while BRL does not affect that of adiponectin and PPARγ (supplementary data-3, C, D and E). BRL- and LPS-induced protein expression of adiponectin is inconsistent with BRL- and LPS-induced mRNA expression of adiponectin, while LPS-induced protein expression of leptin is discrepant with LPS-induced mRNA expression of leptin (supplementary data-3). This indicates the discrepancy between protein and mRNA expressions of adiponectin and leptin by BRL or LPS. Although the precise reason for the discrepancy is unclear, the following possibilities may be involved in the cause of the discrepancy: (1) more rapid change of mRNA than that of protein secretion; (2) regulatory issues of protein secretion and transcription, translation and posttranslation of mRNA. Overall, our results suggest that adipose tissue maintained in our culture system is respondent to several lipogenesis-, lipolysis- and inflammation-related molecules. Thus, our adipose tissue-organotypic culture method seems to be a useful tool for investigating adipose tissue-based mechanisms of obesity and metabolic syndrome.
Organotypic Culture as a Model for Tissue Regeneration and Organogenesis
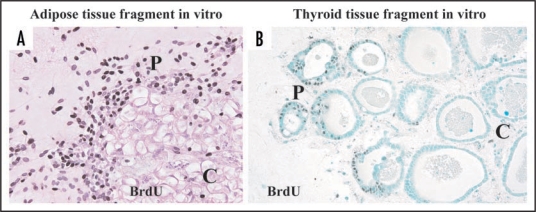
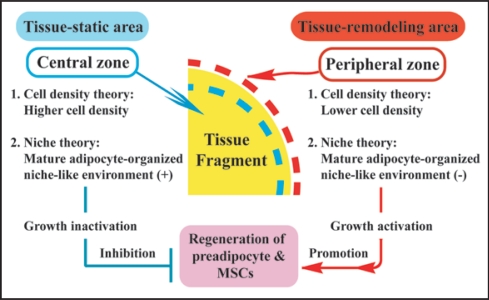
In organotypic culture of adipose tissue fragments (Suppl. Fig. 4), both preadipocytes and MSCs actively regenerate at the peripheral zone of the fragments, whereas they are not produced at the central zone.9 In fact, cellular growth at the peripheral zone is extensively higher than that at the central zone (Fig. 7A). Although the precise mechanism of these phenomena is unclear, we would like to propose the following possible theories for their explanation: (1) cell density theory and (2) niche theory (Fig. 8). Firstly, we explain a “cell density theory.” Namely, the cell density of the central zone of the tissue fragments is prominently higher than that of the peripheral zone. In general, increased cell density in a microenvironment inhibits the regeneration and growth of cells that are subjected to contact inhibition of cell growth.25,26 Thus, it is conceivable that decreased cell density of the peripheral zone may contribute to active development of preadipocytes and MSCs, supporting our previous study that thyroid folliculogenesis with active proliferation of thyrocytes (Fig. 7B) takes place at the peripheral zone, while the phenomena does not occur at the central zone, using three dimensional collagen gel culture of thyroid tissue fragments.27,28 Secondly, we explain a “niche theory.” That is, mature adipocytes, which are concentrated in the central zone of adipose tissue fragments, may organize a niche-like microenvironment for preadipocytes and MSCs, while the microenvironment may be lost at the peripheral zone of the tissue fragments due to the loss of mature adipocytes. In general, a niche microenvironment for stem cell types maintains their resting state.29 Thus, it seems likely that the niche-like microenvironment organized by mature adipocytes may inhibit regeneration of preadipocytes and MSCs at the center, while its loss at the peripheral zone may contribute to their reproduction. In addition, the combination of “cell density theory” and “niche theory” may be involved in active regeneration of preadipocytes and MSCs at peripheral zone of adipose tissue fragments. Since these theories seem promising for explaining the mechanisms of tissue regeneration and remodeling, further studies are in order in our laboratory.
Figure 7.
Immunohistochemistry with BrdU in adipose (A) and thyroid (B) tissue-organotypic culture after 48 hour incubation with 30 µg/ml BrdU. Intranuclear BrdU uptakes of adipose cell types and thyrocytes at peripheral zones of adipose (A) and thyroid tissue fragments (B) are extensively greater than those at the central zones. P, peripheral zone. C, central zone.
Figure 8.
Two theories regarding the mechanism of preadipocyte and MSC regeneration that occurs specifically at peripheral zone of the tissue fragments in vitro. In regard to “cell density theory,” central zone of adipose tissue fragments is characterized by higher cell density, whereas the peripheral zone is characterized by lower cell density. In general, increased cell density in a microenvironment inhibits the regeneration and growth of cells that are subjected to contact inhibition of cell growth. Namely, central zone is tissue-static area with cell growth inactivation, while peripheral zone is tissue-remodeling area with cell growth activation. Thus, lower cell density of the peripheral zone may contribute to active development of preadipocytes and MSCs. In regard to “niche theory,” central zone concentrated by mature adipocytes may be subjected to mature adipocyte-organized niche-like environment, whereas peripheral zone with sparse population of mature adipocytes may lose the environment. In general, a niche environment for stem cell types maintains their resting state. Thus, the niche-like environment formed by mature adipocytes may inhibit regeneration of preadipocytes and MSCs at the center, while its loss at the peripheral zone may contribute to their regeneration.
To maintain body homeostasis, stem cells are considered to produce tissue-specific differentiating cell types (hematopoietic, intestinal, epidermal cells, etc.) in response to daily cellular loss.29 Partial defect of tissue is well known to initiate tissue regeneration such as liver regeneration after its partial defect by injury.30 Thus, the defect of cell population and tissue is considered to be essential for initiation of these phenomena. As described above, a tissue fragment is largely subdivided into the following two parts: (1) peripheral zone with lower density of cell population and (2) central zone with higher density of cell population (Fig. 8). On the basis of this fact, organotypic culture of tissue fragments seems to be a promising model for investigating tissue regeneration and remodeling in vitro. However, only several organotypic cultures of tissue fragments, including thyroid,27,28 brain31,32 and adipose tissues,9 are successfully established. Thus, various tissues other than these tissues above should be applied to organotypic culture system. In addition, injection of various stem cell types, including embryonic stem cells33 and iPS cells,34,35 into tissue fragments may allow us to study in vitro organogenesis with their proliferation and differentiation in a tissue microenvironment-dependent way. Since these issues are critical for regenerative medicine, further extensive studies are inevitably needed.
In Conclusion
In this article, we have introduced adipose tissue-organotypic culture system that maintains the unilocular structure, proliferative ability and function of mature adipocytes, generating both preadipocytes (immature adipocytes) and CD44+/CD105+ MSCs from the tissue fragments. Our culture method will open up a new way for investigating multiple cell types within adipose tissue and for studying the cell-based mechanisms of obesity and metabolic syndrome. Since our method retains viable adipose tissue in vitro for a long term, it is a useful tool for studying interactions between adipose tissue and other tissues or various cell types. In addition, organotypic culture system of tissue fragments, including adipose, brain and thyroid tissue fragments seems to be a promising model for studying tissue regeneration and organogenesis in vitro.
Acknowledgments
This work was supported in part by Grants-in-Aid from Japanese Ministry of Education, Culture, Sports, Science and Technology for Scientific Research Nos. 18591871 and 20592023 and personal Grants from Koike Hospital, Sasebochuo Hospital and Yamada Clinic (to Prof. Shuji Toda). We thank Messrs. H. Ideguchi, S. Nakahara, F. Mutoh and Mrs. M. Nishida for their helpful technical assistance.
Abbreviations
- BRL
b3-agonist BRL-37344
- LPS
lipopolysaccharide
- MEM
minimum essential medium
- MSCs
mesenchymal stem cells
- TNF-α
tumor necrosis factor-α
Footnotes
Previously published online as an Organogenesis E-publication: www.landesbioscience.com/journals/organogenesis/article/8347
Supplementary Material
References
- 1.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S57–S63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anghel SI, Wahli W. Fat poetry: a kingdom for PPAR gamma. Cell Res. 2007;17:486–511. doi: 10.1038/cr.2007.48. [DOI] [PubMed] [Google Scholar]
- 4.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose Tissue: The new endocrine organ? A Review Article. Dig Dis Sci. 2008 doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 5.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–S73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugihara H, Yonemitsu N, Miyabara S, Yun K. Primary cultures of unilocular fat cells: characteristics of growth in vitro and changes in differentiation properties. Differentiation. 1986;31:42–49. doi: 10.1111/j.1432-0436.1986.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 7.Sugihara H, Yonemitsu N, Miyabara S, Toda S. Proliferation of unilocular fat cells in the primary culture. J Lipid Res. 1987;28:1038–1045. [PubMed] [Google Scholar]
- 8.Sugihara H, Yonemitsu N, Toda S, Miyabara S, Funatsumaru S, Matsumoto T. Unilocular fat cells in three-dimensional collagen gel matrix culture. J Lipid Res. 1988;29:691–697. [PubMed] [Google Scholar]
- 9.Sonoda E, Aoki S, Uchihashi K, Soejima H, Kanaji S, Izuhara K, et al. A new organotypic culture of adipose tissue fragments maintains viable mature adipocytes for a long term, together with development of immature adipocytes and mesenchymal stem cell-like cells. Endocrinology. 2008;149:4794–4798. doi: 10.1210/en.2008-0525. [DOI] [PubMed] [Google Scholar]
- 10.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 11.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 12.Green H, Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976;7:105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- 13.Negrel R, Grimaldi P, Ailhaud G. Establishment of preadipocyte clonal line from epididymal fat pad of ob/ob mouse that responds to insulin and to lipolytic hormones. Proc Natl Acad Sci USA. 1978;75:6054–6058. doi: 10.1073/pnas.75.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobusue H, Endo T, Kano K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue. Cell Tissue Res. 2008;332:435–446. doi: 10.1007/s00441-008-0593-9. [DOI] [PubMed] [Google Scholar]
- 15.Butterwith SC, Peddie CD, Goddard C. Effects of transforming growth factor-alpha on chicken adipocyte precursor cells in vitro. J Endocrinol. 1992;134:163–168. doi: 10.1677/joe.0.1340163. [DOI] [PubMed] [Google Scholar]
- 16.Morizono K, De Ugarte DA, Zhu M, Zuk P, Elbarbary A, Ashjian P, et al. Multilineage cells from adipose tissue as gene delivery vehicles. Hum Gene Ther. 2003;14:59–66. doi: 10.1089/10430340360464714. [DOI] [PubMed] [Google Scholar]
- 17.Zhang HH, Kumar S, Barnett AH, Eggo MC. Ceiling culture of mature human adipocytes: use in studies of adipocyte functions. J Endocrinol. 2000;164:119–128. doi: 10.1677/joe.0.1640119. [DOI] [PubMed] [Google Scholar]
- 18.Sugihara H, Funatsumaru S, Yonemitsu N, Miyabara S, Toda S, Hikichi Y. A simple culture method of fat cells from mature fat tissue fragments. J Lipid Res. 1989;30:1987–1995. [PubMed] [Google Scholar]
- 19.Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev. 2004;5:13–19. doi: 10.1111/j.1467-789x.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 20.Chapman IM. Endocrinology of anorexia of ageing. Best Pract Res Clin Endocrinol Metab. 2004;18:437–452. doi: 10.1016/j.beem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Duque G. Bone and fat connection in aging bone. Curr Opin Rheumatol. 2008;20:429–434. doi: 10.1097/BOR.0b013e3283025e9c. [DOI] [PubMed] [Google Scholar]
- 22.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi H, Takayama T, Yoneda K, Endo H, Iida H, Sugiyama M, et al. Association of visceral fat accumulation and plasma adiponectin with rectal dysplastic aberrant crypt foci in a clinical population. Cancer Sci. 2009;100:29–32. doi: 10.1111/j.1349-7006.2008.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, et al. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100:578–582. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson PJ, Daniel TO. Emerging targets: molecular mechanisms of cell contact-mediated growth control. Kidney Int. 2002;61:S99–S105. doi: 10.1046/j.1523-1755.2002.0610s1099.x. [DOI] [PubMed] [Google Scholar]
- 26.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 27.Toda S, Watanabe K, Yokoi F, Matsumura S, Suzuki K, Ootani A, et al. A new organotypic culture of thyroid tissue maintains three-dimensional follicles with C cells for a long term. Biochem Biophys Res Commun. 2002;294:906–911. doi: 10.1016/S0006-291X(02)00561-2. [DOI] [PubMed] [Google Scholar]
- 28.Toda S, Aoki S, Suzuki K, Koike E, Ootani A, Watanabe K, et al. Thyrocytes, but not C cells, actively undergo growth and folliculogenesis at the periphery of thyroid tissue fragments in three-dimensional collagen gel culture. Cell Tissue Res. 2003;312:281–289. doi: 10.1007/s00441-003-0718-0. [DOI] [PubMed] [Google Scholar]
- 29.Slack JM. Origin of stem cells in organogenesis. Science. 2008;322:1498–1501. doi: 10.1126/science.1162782. [DOI] [PubMed] [Google Scholar]
- 30.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- 32.Elias L, Kriegstein A. Organotypic slice culture of E18 rat brains. J Vis Exp. 2007:235. doi: 10.3791/235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lui KO, Waldmann H, Fairchild PJ. Embryonic stem cells: overcoming the immunological barriers to cell replacement therapy. Curr Stem Cell Res Ther. 2009;4:70–80. doi: 10.2174/157488809787169093. [DOI] [PubMed] [Google Scholar]
- 34.Park IH, Daley GQ. Human iPS cell derivation/reprogramming. Curr Protoc Stem Cell Biol. 2009 doi: 10.1002/9780470151808.sc04a01s8. Chapter 4:Unit 4A 1. [DOI] [PubMed] [Google Scholar]
- 35.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.