Abstract
Recent studies have significantly improved our ability to investigate cell transplantation and study the physiology of transplanted cells in cardiac tissue. Several previous studies have shown that fully-immersed heart slices can be used for electrophysiological investigations. Additionally, ischemic heart slices induced by glucose and oxygen deprivation offer a useful tool to investigate mechanical integration and to measure forces of contraction of engrafted cells, at least for short term analysis. A recent and novel model of heart slices, prepared from rat and human tissues, can be maintained in culture for up to two months. This new heart slice model can be used for long term in vitro cell transplantation studies and for pharmacological evaluation. This review will focus on describing these models and demonstrating the use of organotypic heart slices as a novel tool for drugs for studying electrophysiology and developing cellular therapeutic approaches to alleviate cardiac tissue damage.
Key words: heart, organotypic, culture, stem cells, transplantation, electrophysiology, pharmacology
Introduction
Most chronic heart diseases develop after cardiac stroke, cardiomyopathies and muscle myopathies.1 The clinical picture of these diseases is non-specific and includes the presence of degenerative myocytes and increased interstitial fibrosis.2,3 Pharmacology-based treatment of heart failure is still limited and some approved drugs show signs of toxicity, prompting new therapeutic strategies including regenerative cell therapies.
Several experimental and clinical observations suggest that cell transplantation could be beneficial in restoring cardiac function. Cell lines from different origins, including mesenchymal stem cells from bone marrow,4–6 smooth and skeletal muscle7–12 and embryonic stem cells,13 have been used in preclinical animal models, human embryonic stem (hES) cells, which can divide indefinitely and give rise to many cellular types including cardiomyocytes, represent a very promising tool for a cell therapeutic approach to treat heart failures.14 In the last few years, several studies have demonstrated that transplantation of ES cells into injured heart generates functional cardiomyocytes and improves cardiac functions.15–17 Key factors for cellular therapy to be effective are efficient cell engraftment, differentiation into the cell type appropriate for the damaged tissue, and an ability to interact with existing cardiac tissue cells in order to restore long-term cardiac function. However, it has been difficult to study these factors due to the lack of appropriate models that allow long-term assessment of transplanted ES cells. Animal models represent the most relevant system to study new pharmacological compounds, to perform toxicity tests and to study transplanted therapeutic cells. However, pharmacological and cellular transplant experiments in animal models are limited by the need for complex experimental design, surgical procedures and the difficulty to monitor transplanted cells over time. In addition, they are expensive and unpopular on ethical grounds.18
For these reasons it was clear that better experimental platforms were required in order to provide the rational groundwork before future therapeutics trials for cardiovascular diseases to be carry out in animal models.19 Ex-vivo approaches, such as organotypic slice cultures, provide very important tools for the drug discovery process and for cell therapy approach. For example, brain organotypic cultures have been used for a wide spectrum of application, including physiology, morphology, pharmacology and toxicology20 this review will focus on the different techniques of heart organotypic slices and their possible applications for novel therapeutics.
Organotypic Heart Slices Cultures and Cell Transplantation
Pillekamp and colleagues established the first cardiac in vitro model in order to assess stem cell-derived cardiomyocytes transplantation.22 Their heart slice cultures were generated from neonatal mice (3 to 4 days old) and most slices contracted spontaneously. The ventricles were sliced in a plane orthogonal to the long axis, resulting in rings of ventricular myocardium.
Different studies showed that the damaged myocardium produced cellular signals important for the cardiac differentiation of grafted cells.23,24 To mimic myocardial infarction, the heart slices were damaged irreversibly by oxygen and glucose deprivation using hypoxia chamber. The ischemic heart slices showed complete loss of spontaneous beating activity. This model was used as an in vitro model to study mechanical integration of, and to measure forces of contraction in, hESC-derived cardiomyocytes. Within the first 7 days of the co-culture the beating cells clustered to fill fissures and cavities on the surface of the murine ventricular slices. The contraction of the beating area resulted in movements of the whole preparation demonstrating mechanical interaction of the hESC-derived cardiomyocytes with the ventricular slices. However, microscopic analysis did not show any desmosomes or fasciae adhaerens between transplanted cells and host cardiac tissue, arguing against a direct connection between transplanted cells and the host. More importantly, the cells transplanted were already differentiated into cardiomyocytes, explaining why these authors could use slices surviving only 7 days.
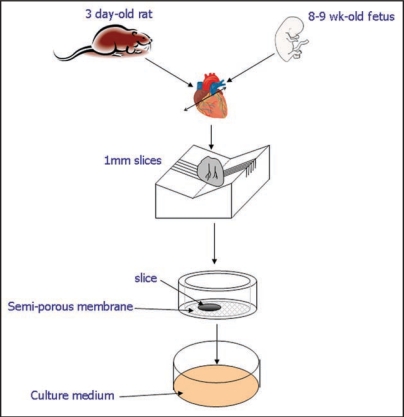
A modification of this model was used to evaluate the pacemaker activity of mESC-derived cardiomyocytes. For this aim, ventricular slices were generated from murine embryonic heart (day 16.5 post-coitum). The authors showed that the transplanted of mESC-derived cardiomyocytes were able to behave as a biological pacemaker, increasing the beating rate with low efficiency.25 These models looked promising and suitable for studying transplantation of already differentiated cells, as the survival time of the tissue was less than two weeks, but they were not suitable with the differentiation time of hES cells which, even when committed to cardiac fate using BMP2 (Bone Morphogenetic Protein 2), require a longer time to differentiate into cardiomyocytes.13,28 We surmise, in accordance with previous studies concerning the organotypic brain slice cultures,20 that the poor preservation of cardiac tissue heart slices over time was connected with immersion in the culture media. Brain organotypic slice cultures have been cultured for long terms by replacing immersion techniques with slow rotation to generate change in oxygenation of slice culture26 or by culturing the brain slices at an air-medium interface on a semiporous membrane.27 We developed the latter technique because it was applicable for intra-slice cell transplantation.28 In fact, the principle of membrane interface culture methods is to maintain the slices on a porous membrane filter at the interface between culture medium and a humidified atmosphere. The culture medium provides adequate nutrition to tissue slice through the membrane via capillary action.29 The cultures were prepared from ventricular section of 3-day old rat or from human fetuses (8–9.5 week-old). The heart slices were transferred to a semiporous membrane and cultured for more than two months (Fig. 1). The ventricular slices exhibited a rhythmic contraction and responded to activation of β-adrenergic receptors in a dose-dependent manner (Fig. 2).28
Figure 1.
A schematic representation of the preparation of organotypic heart slice cultures. Ventricles of neonatal rat or fetal human are placed on heart matrix and cut in sagittal slices at a thickness of 1 mm. The slices are placed on Millipore membrane and cultures in 6 well-plates at 37°C in 5% CO2 humidified atmosphere for up two month.
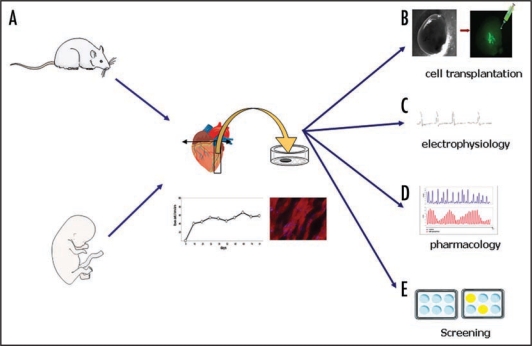
Figure 2.
Applications of organotypic heart slices cultures. (A) The heart slice cultures showed normal histology and exhibited spontaneous and regular rhythmic contractions during up 2 months of culture. (B) Intra-slice injection of GFP positive cells. Epifluorescence observation, using stereomicroscope, showed the presence of GFP positive cell in the cardiac parenchyma close to the injection site. (C–E) Application of heart slice cultures in physiological and pharmacological studies.
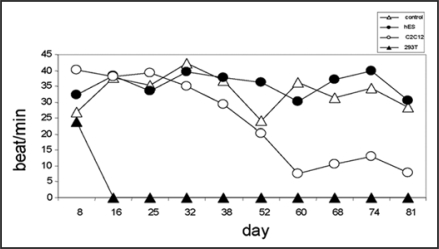
The distinctiveness of an air-medium interface on semi-porous membrane cultures had another attractive point. It was possible to inject single cells into heart slices without losing them into the media as it would be in the case of immersed tissue slice cultures. In this new model it was possible to monitor GFP positive cells using an epifluorescence stereomicroscope without fixation of cardiac tissue (Fig. 2). In our study, the injection of several cell line types [hESC, mouse skeletal myoblastic cell line (C2C12)] did not effect spontaneous contraction over the 81 days of observation, excepted when very tumor-producing, proliferating cells (HEK) were injected (Fig. 3).
Figure 3.
Spontaneous beating frequencies recorded over time. Following implantation of either human embryonic stem cells (hESC), mouse skeletal myoblastic cells (C2C12) or human embryonic kidney cells (HEK-293T).
In this long-term heart slice culture, undifferentiated embryonic stem cells differentiated and expressed human-specific cardiac markers up to 2 months after transplantation. The novelty of this system is that the complex natural structural base of the cardiac tissue is preserved and provides a scaffold of live cell cues and the appropriate microstructure to allow hES cells to engraft and differentiate into the appropriate cell type.19 Further studies will be needed to determine the effectiveness of the engrafted hES cells in their ability to help repair tissue damage.
Electrophysiological Analyses and Heart Slices
In 1996, Stoppini and colleagues reported the possibility of using the microelectrode arrays (MEA) system to monitor the electrical activity of hippocampal organotypic cultures.30 The possibility to place cardiac ventricular slices on planar microelectrode arrays offers a powerful technique to study cardiac impulse propagation.31
The characterization of the physiological activity of ventricular slice cultures demonstrated the preservation of cardiac tissue in organotypic cultures obtained from embryonic mouse hearts (16.5–18.5 post coitum) and cultured for 1 week. Action potentials in slices generated from embryonic stage heart were compared to current clamp recordings of isolated ventricular cardiomyocytes from embryos of the same stage of differentiation. Moreover, the induction of a delayed repolarization after administration of Linopiridine, a specific KCNQ channels blocker, demonstrate the utility of this model for pharmacological studies.32 The characteristic structures of adult myocardium (e.g., cell size and gap junction distribution) as well as its typical electrophysiological properties cannot be stimulated sufficiently by means of immature cardiac tissue.33 However, the heart slices of murine adult ventricles were electrophysiologicaly intact and reacted normally to cardio-active drugs. The application of lidocain, a Na+ channel blocker, evoked a decline of relative conduction speed and amplitude. Moreover, the 4-aminopyridine, a blocker of voltage-dependent K+ channels, prolonged APD50 (Action potential duration at 50% repolarization) of adult heart slices.34 The researchers concluded that this adult myocardium model could be important in the study of stem cell functional integration after transplantation into an infarcted heart in vivo.
The aim of all cell transplantation studies for cardiac disease is the conservation of electrical interaction between grafted cells and the host cardiomyocytes. Typical in vitro co-cultures are not representative for the in vivo situation. The physiology of the mammalian heart depends on the functional alignment of cardiomyocytes, and controlling cell alignment is an important parameter in biomaterial designs for cardiac tissue engineering and research.35,36 The cardiomyocytes in ventricular myocardium are linked by perimisyal collagen in a complex lamina structures. The voltage fields induced by intramural current injection are influenced by microfiber direction and by transmural arrangement of muscle layer.37 For this reason, the three-dimensional architectures of heart slices could be more precise than in an in vitro coculture to study the electrical coupling between grafted cells and host parenchyma. The powerful technique of ventricular slices cultures was confirmed by investigation of electrophysiological maturation and integration of immature cardiomyocytes after transplantation. The transplantation of murine fetal cardiomyocytes into healthy or cryo-injured ventricular mouse slices was related to the expression of connexin 43, indicating the electrical coupling between grafted cell and host parenchyma. The electrical integration of transplanted fetal cardiomyocytes required embedding in viable tissue; in fact, when transplanted cells were surrounded by cryo-injured tissue, an electrical integration was never observed. This indicated an exclusive regulation of maturation by soluble and diffusible cytokine by ventricular heart slices.38 The combination of long-term culture and easily electrophysiological recording of heart slices represent a powerful tool in the investigation of electrical integration of transplanted cells with host tissue and in the cardiac physiological studies.
Conclusion and Future Perspectives
We and others have developed simple in vitro beating heart models for short-term and for long-term assessment of experimental therapeutics. Previous models have allowed us to study the survival and electrical integration of already differentiated and beating hES-derived cardiomyocytes22,34,38 but lacked the ability to study long term engraftment of cardiac-committed cells. The first application of this novel heart slice culture system has been the rat ventricular tissue for the analysis of stem cell engraftment into the myocardium in vitro and electrophysiological studies. The heart slice was maintained for a long-term period in culture at the air-medium interface on semiporous membrane, so might play an important role in the investigation of key factors concerning cell transplantation, such as migration, integration and functionality of grafted cell in host cardiac parenchyma. We have since expanded this model to use human fetal cardiac tissue which will be a more relevant substrate for human ES cells studies. As this tissue may provide non-cross reactive (rat-human) molecules significant for the development of transplanted cells to develop into the appropriate cell types which can be deduced. This new novel model of in vitro heart slice model provides significant advantages in studying therapeutic cell engraftment. In addition, this system could be adapted for many other applications, for instance pharmacological studies, toxicity assays and drug screening (Fig. 1). In fact, the main advantage of this ex-vivo model was the capacity of long-term monitoring of electrical activity of cardiac tissue. In addition, the use of MEA to record fields potentials could facilitate the investigation of drugs screening for cardiovascular disease. Most of the models have so far used isolated cells (cardiomyocytes or hES-derived cardiomyocytes) and clearly they have presented some limits. Most isolated adult ventricular cardiomyocytes are terminally differentiated and have an extremely limited capacity to divide or grow to confluence in culture. Instead, they rapidly dedifferentiate, losing their characteristic rod-shaped striated morphology and showing marked alterations in contraction and relaxation. Myocytes are commonly cultured for 24 or 48 hr with relative success, but even at these time points some alterations are beginning to be evident. Pharmacological responses are often maintained during short-term (48 hr) culture, but verification of this is necessary for each receptor target.39 Cardiomyocytes derived from hESC could be cultured for a longer time, however their maturation process has not been systematically controlled, resulting in gradual loss of their beating potency and insufficient function.40
In the future, the generation of this novel heart slice model from animals (and more specifically human fetal) with cardiac diseases could offer an exclusive pre-clinical model for drug discovery and screening in the assessment in both normal and disease models.
Acknowledgements
We are grateful to Dr. Michael W. Melkus for his help on this manuscript.
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/article/9091
References
- 1.Megeney LA, Kablar B, Perry RL, Ying C, May L, Rudnicki MA. Severe cardiomyopathy in mice lacking dystrophin and MyoD. Proc Natl Acad Sci USA. 1999;96:220–225. doi: 10.1073/pnas.96.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schonberger J, Seidman CE. Many roads lead to a broken heart: the genetics of dilated cardiomyopathy. Am J Hum Genet. 2001;69:249–260. doi: 10.1086/321978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox GF, Kunkel LM. Dystrophies and heart disease. Curr Opin Cardiol. 1997;12:329–343. [PubMed] [Google Scholar]
- 4.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci. 2001;938:221–229. doi: 10.1111/j.1749-6632.2001.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 5.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108:253–258. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 6.Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, et al. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48:2116–2124. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 7.Yoo KJ, Li RK, Weisel RD, Mickle DA, Li G, Yau TM. Autologous smooth muscle cell transplantation improved heart function in dilated cardiomyopathy. Ann Thorac Surg. 2000;70:859–865. doi: 10.1016/s0003-4975(00)01630-1. [DOI] [PubMed] [Google Scholar]
- 8.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 10.Ghostine S, Carrion C, Souza LC, Richard P, Bruneval P, Vilquin JT, et al. Long-term efficacy of myoblast transplantation on regional structure and function after myocardial infarction. Circulation. 2002;106:131–136. [PubMed] [Google Scholar]
- 11.Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 12.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 13.Menard C, Hagege AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005;366:1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- 14.Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ Res. 2002;91:659–661. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- 15.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663–671. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomescot A, Leschik J, Bellamy V, Dubois G, Messas E, Bruneval P, et al. Differentiation in vivo of cardiac committed human embryonic stem cells in postmyocardial infarcted rats. Stem Cells. 2007;25:2200–2205. doi: 10.1634/stemcells.2007-0133. [DOI] [PubMed] [Google Scholar]
- 17.Singla DK, Hacker TA, Ma L, Douglas PS, Sullivan R, Lyons GE, et al. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Sundstrom L, Morrison B, 3rd, Bradley M, Pringle A. Organotypic cultures as tools for functional screening in the CNS. Drug Discov Today. 2005;10:993–1000. doi: 10.1016/S1359-6446(05)03502-6. [DOI] [PubMed] [Google Scholar]
- 19.Russell B, Collins JM. Hearty slices to plan for future health. Cardiovasc Res. 2009;81:235–236. doi: 10.1093/cvr/cvn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- 21.Scheffler B, Schmandt T, Schroder W, Steinfarz B, Husseini L, Wellmer J, et al. Functional network integration of embryonic stem cell-derived astrocytes in hippocampal slice cultures. Development. 2003;130:5533–5541. doi: 10.1242/dev.00714. [DOI] [PubMed] [Google Scholar]
- 22.Pillekamp F, Reppel M, Rubenchyk O, Pfannkuche K, Matzkies M, Bloch W, et al. Force measurements of human embryonic stem cell-derived cardiomyocytes in an in vitro transplantation model. Stem Cells. 2007;25:174–180. doi: 10.1634/stemcells.2006-0094. [DOI] [PubMed] [Google Scholar]
- 23.Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, et al. Stem cell differentiation requires a paracrine pathway in the heart. Faseb J. 2002;16:1558–1566. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 24.Kofidis T, de Bruin JL, Yamane T, Tanaka M, Lebl DR, Swijnenburg RJ, et al. Stimulation of paracrine pathways with growth factors enhances embryonic stem cell engraftment and host-specific differentiation in the heart after ischemic myocardial injury. Circulation. 2005;111:2486–2493. doi: 10.1161/01.CIR.0000165063.09283.A8. [DOI] [PubMed] [Google Scholar]
- 25.Hannes T, Halbach M, Nazzal R, Frenzel L, Saric T, Khalil M, et al. Biological pacemakers: characterization in an in vitro coculture model. J Electrocardiol. 2008;41:562–566. doi: 10.1016/j.jelectrocard.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- 27.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 28.Habeler W, Pouillot S, Plancheron A, Puceat M, Peschanski M, Monville C. An in vitro beating heart model for long-term assessment of experimental therapeutics. Cardiovasc Res. 2009;81:253–259. doi: 10.1093/cvr/cvn299. [DOI] [PubMed] [Google Scholar]
- 29.Cho S, Wood A, Bowlby MR. Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Curr Neuropharmacol. 2007;5:19–33. doi: 10.2174/157015907780077105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoppini L, Duport S, Correges P. A new extracellular multirecording system for electrophysiological studies: application to hippocampal organotypic cultures. J Neurosci Methods. 1997;72:23–33. doi: 10.1016/s0165-0270(96)00151-3. [DOI] [PubMed] [Google Scholar]
- 31.Pillekamp F, Reppel M, Brockmeier K, Hescheler J. Impulse propagation in late-stage embryonic and neonatal murine ventricular slices. J Electrocardiol. 2006;39:425. doi: 10.1016/j.jelectrocard.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Pillekamp F, Reppel M, Dinkelacker V, Duan Y, Jazmati N, Bloch W, et al. Establishment and characterization of a mouse embryonic heart slice preparation. Cell Physiol Biochem. 2005;16:127–132. doi: 10.1159/000087739. [DOI] [PubMed] [Google Scholar]
- 33.Thomas SP, Bircher-Lehmann L, Thomas SA, Zhuang J, Saffitz JE, Kleber AG. Synthetic strands of neonatal mouse cardiac myocytes: structural and electrophysiological properties. Circ Res. 2000;87:467–473. doi: 10.1161/01.res.87.6.467. [DOI] [PubMed] [Google Scholar]
- 34.Halbach M, Pillekamp F, Brockmeier K, Hescheler J, Muller-Ehmsen J, Reppel M. Ventricular slices of adult mouse hearts—a new multicellular in vitro model for electrophysiological studies. Cell Physiol Biochem. 2006;18:1–8. doi: 10.1159/000095132. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood DN, Akins RE, Jr, Parrag IC, Woodhouse KA, Rabolt JF. Culture on electro-spun polyurethane scaffolds decreases atrial natriuretic peptide expression by cardiomyocytes in vitro. Biomaterials. 2008;29:4783–4791. doi: 10.1016/j.biomaterials.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lunkenheimer PP, Redmann K, Kling N, Jiang X, Rothaus K, Cryer CW, et al. Three-dimensional architecture of the left ventricular myocardium. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:565–578. doi: 10.1002/ar.a.20326. [DOI] [PubMed] [Google Scholar]
- 37.Hooks DA, Trew ML, Caldwell BJ, Sands GB, LeGrice IJ, Smaill BH. Laminar arrangement of ventricular myocytes influences electrical behavior of the heart. Circ Res. 2007;101:103–112. doi: 10.1161/CIRCRESAHA.107.161075. [DOI] [PubMed] [Google Scholar]
- 38.Halbach M, Pfannkuche K, Pillekamp F, Ziomka A, Hannes T, Reppel M, et al. Electrophysiological maturation and integration of murine fetal cardiomyocytes after transplantation. Circ Res. 2007;101:484–492. doi: 10.1161/CIRCRESAHA.107.153643. [DOI] [PubMed] [Google Scholar]
- 39.Harding SE, Ali NN, Brito-Martins M, Gorelik J. The human embryonic stem cell-derived cardiomyocyte as a pharmacological model. Pharmacol Ther. 2007;113:341–353. doi: 10.1016/j.pharmthera.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]