Abstract
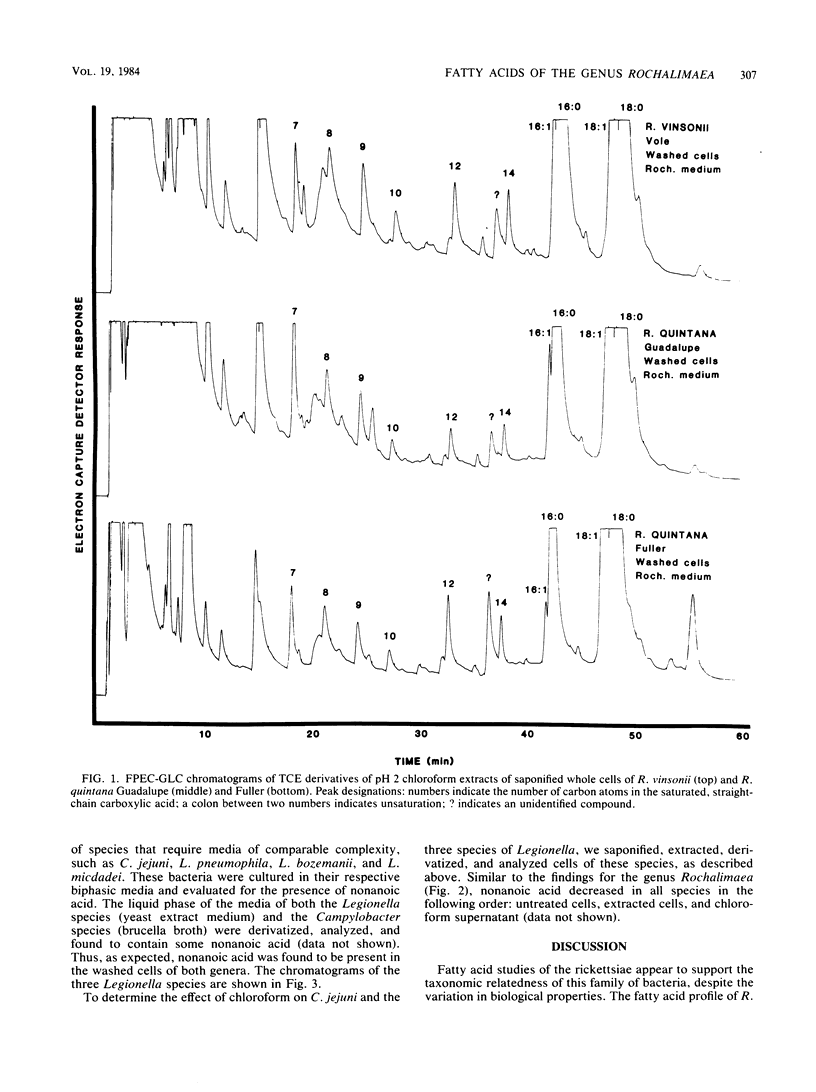
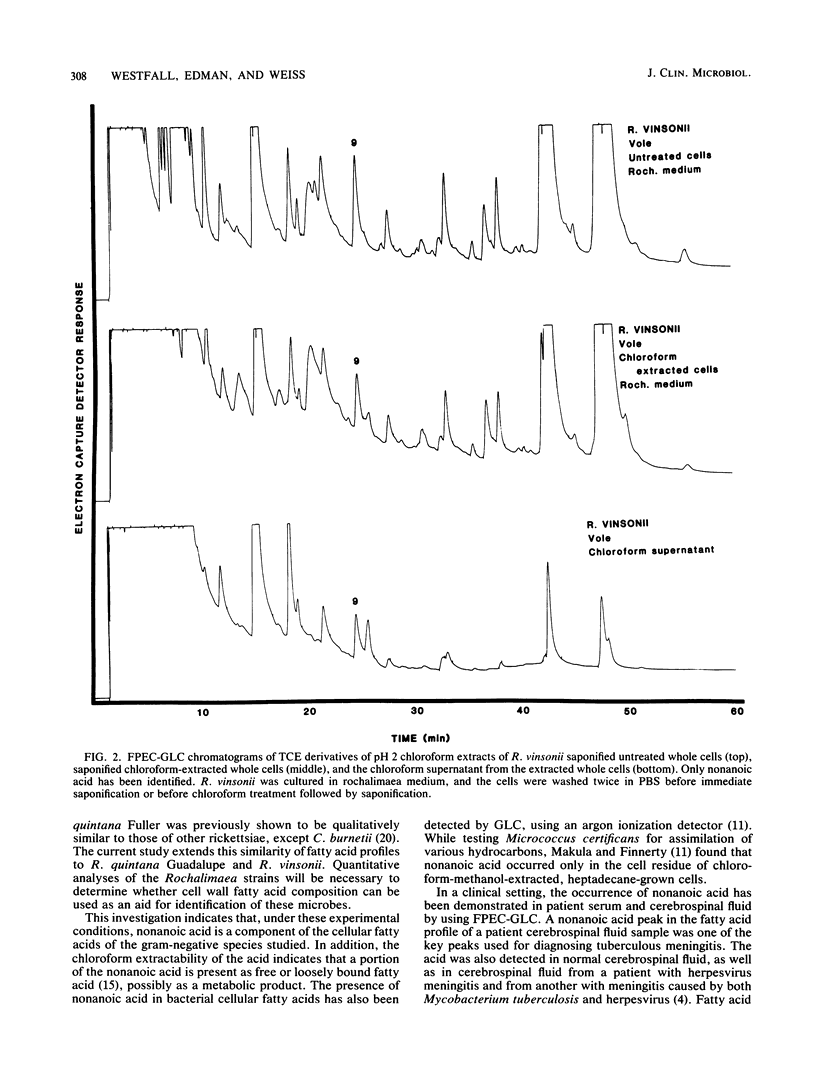
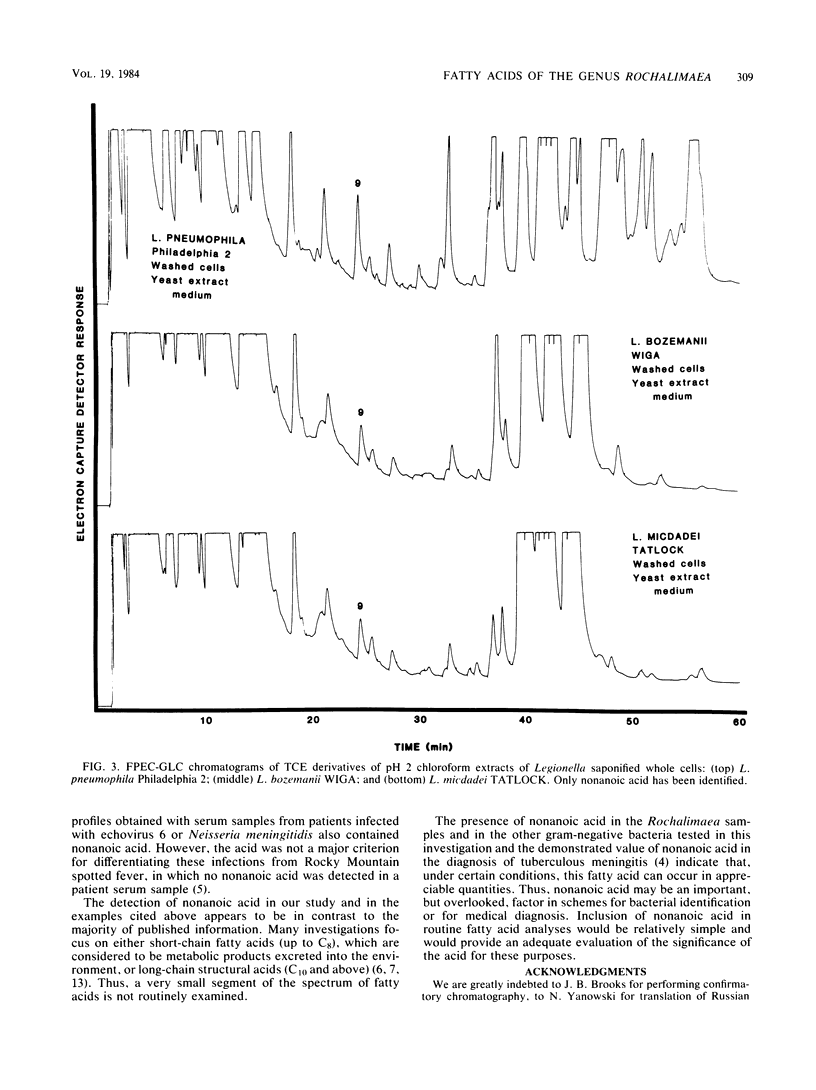
The fatty acid compositions of Rochalimaea quintana, strains Fuller and Guadalupe, and R. vinsonii, the Canadian vole agent, were determined in an effort to further characterize these bacteria. The cells were saponified with 5% NaOH in 50% methanol and acidified to pH 2. The methanolysates were extracted with chloroform, derivatized with 2,2,2-trichloroethanol, and analyzed using a Hewlett-Packard gas chromatograph equipped with a frequency pulse-modulated electron capture detector and a 3% OV-101 packed-glass column. The fatty acid profiles of the three Rochalimaea strains were similar, with octadecenoic acid (C18:1) the most abundant, followed by octadecanoic (C18:0) and hexadecanoic (C16:0) acids. Moderate to trace amounts of other acids were also present. Unexpectedly, well-defined peaks of nonanoic acid (C9) were found consistently. A portion of this acid, but not all, was extractable with chloroform. Since C9 is not reported as a usual component of bacteria and most analyses do not include a search for this fatty acid, this study was extended to three strains of Legionella and one of Campylobacter. Comparable results were obtained. Since these bacteria were grown in complex media which contain some C9, it is possible that the medium is the source of bacterial C9. Whether this compound can be synthesized by the bacteria remains to be investigated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alley C. C., Brooks J. B., Kellogg D. S., Jr Electron capture gas-liquid chromatographic-mass spectral identification of acids produced by Neisseria meningitidis in a defined medium. J Clin Microbiol. 1979 Jan;9(1):97–102. doi: 10.1128/jcm.9.1.97-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Gerloff R. K., Thomas L. A., Mann R. E., Bickel W. D. Immunological properties of Rickettsia rickettsii purified by zonal centrifugation. Infect Immun. 1975 Jun;11(6):1203–1209. doi: 10.1128/iai.11.6.1203-1209.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. B., Edman D. C., Alley C. C., Craven R. B., Girgis N. I. Frequency-pulsed electron capture gas-liquid chromatography and the tryptophan color test for rapid diagnosis of tuberculous and other forms of lymphocytic meningitis. J Clin Microbiol. 1980 Aug;12(2):208–215. doi: 10.1128/jcm.12.2.208-215.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. B., McDade J. E., Alley C. C. Rapid differentiation of rocky mountain spotted fever from chickenpox, measles, and enterovirus infections and bacterial meningitis by frequency-pulsed electron capture gas-liquid chromatographic analysis of sera. J Clin Microbiol. 1981 Aug;14(2):165–172. doi: 10.1128/jcm.14.2.165-172.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. B., Weaver R. E., Tatum H. W., Billingsley S. A. Differentiation between Pseudomonas testosteroni and P. acidovorans by gas chromatography. Can J Microbiol. 1972 Sep;18(9):1477–1482. doi: 10.1139/m72-226. [DOI] [PubMed] [Google Scholar]
- Dees S. B., Moss C. W. Cellular fatty acids of Alcaligenes and Pseudomonas species isolated from clinical specimens. J Clin Microbiol. 1975 May;1(5):414–419. doi: 10.1128/jcm.1.5.414-419.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman D. C., Craven R. B., Brooks J. B. Gas chromatography in the identification of microorganisms and diagnosis of infectious diseases. Crit Rev Clin Lab Sci. 1981;14(2):133–161. doi: 10.3109/10408368109106453. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Myhre E. Differential staining of bacteria in clinical specimens using acridine orange buffered at low pH. Acta Pathol Microbiol Scand B. 1977 Aug;85(4):249–254. doi: 10.1111/j.1699-0463.1977.tb01970.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makula R. A., Finnerty W. R. Microbial assimilation of hydrocarbons: cellular distribution of fatty acids. J Bacteriol. 1972 Oct;112(1):398–407. doi: 10.1128/jb.112.1.398-407.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell B. R., Weiss E., Dasch G. A. Morphological and cell association characteristics of Rochalimaea quintana: comparison of the Vole and Fuller strains. J Bacteriol. 1978 Aug;135(2):633–640. doi: 10.1128/jb.135.2.633-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Dees S. B. Cellular fatty acids and metabolic products of Pseudomonas species obtained from clinical specimens. J Clin Microbiol. 1976 Dec;4(6):492–502. doi: 10.1128/jcm.4.6.492-502.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers W. F., Wisseman C. L., Jr, Fiset P., Oaks E. V., Smith J. F. Taxonomic relationship of vole agent to Rochalimaea quintana. Infect Immun. 1979 Dec;26(3):976–983. doi: 10.1128/iai.26.3.976-983.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'leary W. M. THE FATTY ACIDS OF BACTERIA. Bacteriol Rev. 1962 Dec;26(4):421–447. doi: 10.1128/br.26.4.421-447.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins D. M., Coolbaugh J. C., Walker R. I., Weiss E. Biphasic culture system for rapid Campylobacter cultivation. Appl Environ Microbiol. 1983 Jan;45(1):284–289. doi: 10.1128/aem.45.1.284-289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N. Lipid composition as a guide to the classification of bacteria. Adv Appl Microbiol. 1974;17(0):63–108. doi: 10.1016/s0065-2164(08)70555-0. [DOI] [PubMed] [Google Scholar]
- Somov G. P., Dzadzieva M. F., Boikova E. A. Izuchenie khimicheskogo sostava D. sibiricus. Soobshchenie II. Lipidy rikketsii D. sibiricus. Zh Mikrobiol Epidemiol Immunobiol. 1973 Jul;50(7):126–131. [PubMed] [Google Scholar]
- Tzianabos T., Moss C. W., McDade J. E. Fatty acid composition of rickettsiae. J Clin Microbiol. 1981 Mar;13(3):603–605. doi: 10.1128/jcm.13.3.603-605.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Dasch G. A., Woodman D. R., Williams J. C. Vole agent identified as a strain of the trench fever rickettsia, Rochalimaea quintana. Infect Immun. 1978 Mar;19(3):1013–1020. doi: 10.1128/iai.19.3.1013-1020.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Mamay H. K., Dasch G. A. Ornithine metabolism in the genus Rochalimaea. J Bacteriol. 1982 Apr;150(1):245–250. doi: 10.1128/jb.150.1.245-250.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Miller E. T. Phospholipid composition of Rickettsia prowazeki grown in chicken embryo yolk sacs. J Bacteriol. 1978 Oct;136(1):175–178. doi: 10.1128/jb.136.1.175-178.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


