Abstract
Metabolic syndrome (MS) and “low grade” systemic inflammation (LGSI) are very common findings in the older population. Although MS and LGSI have been associated in adults, it is not known what is the real contribution of MS, and its single components, to LGSI in older persons, due to the potential confounding effect of comorbidity and aging.
We investigated the relationship between increased C-reactive protein (CRP) plasma levels, a marker of LGSI, and MS in 1044 older (≥65 years) community dwelling Italian individuals enrolled the InChianti study.
Metabolic syndrome was defined by the NCEP-ATP III-AHA/NHLBI criteria. High sensitivity CRP (hs.CRP) levels were measured by enzyme-linked immunosorbent assay, and defined as high when >3 mg/L.
The overall prevalence of MS was 31%. The prevalence of high hs.CRP was 54.5% in subjects with, and 41.3% in those without MS (p < 0.001). MS was associated with high hs.CRP levels after adjustment for age, gender, and comorbidity (OR: 1.93, 95% CI: 1.46-2.55). Compared to subjects with MS and no LGSI, individuals with MS and LGSI were characterized by higher waist circumference, BMI, and HOMA score.
Multivariate logistic regression analysis confirmed the association between waist circumference and high hs.CRP levels in subjects with MS (waist circumference III vs. I tertile OR: 2.60, 95% CI: 1.79-3.77) independent of age, gender, and important confounding variables including comorbidity. Additional analyses, conducted with and without dichotomization of hs.CRP levels, confirmed the central role of waist circumference in the LGSI phenomenon, independent of gender and diagnosis of MS.
We conclude that in older individuals, MS is associated with LGSI, but the association is mainly supported by a strong independent correlation between waist circumference and high hs.CRP levels. In the absence of this specific MS component, it seems that the contribution of MS to LGSI would be modest at best.
Keywords: Metabolic syndrome, C-reactive protein, Systemic inflammation, Waist circumference, Elderly
1. Introduction
Metabolic syndrome (MS) is a very common clinical condition characterized by the clustering of cardiovascular risk factors related to insulin-resistance, including central obesity, impaired glucose tolerance, hypertension, and dyslipidemia [1,2]. MS is a risk factor for type 2 diabetes and coronary heart disease (CHD) [3-5], and is becoming increasingly important because of the worldwide epidemic of overweight and obesity. As suggested by epidemiological studies, MS is a typical condition of middle-aged and older people; indeed, in Western Countries, the prevalence of MS progressively increases with age ranging from 3 to 6% among subjects younger than 30 years to 25-40% among subjects aged over 70 years [6,7].
MS and its components have been consistently associated with the presence of “low grade” systemic inflammation (LGSI) [8,9]. Inflammation is recognized as one of the central features of atherosclerosis (ATS), and plays a key role in plaque rupture and therefore in most episodes of acute coronary events [10]. The acute phase reactant C-reactive protein (CRP) is an extremely sensitive marker of systemic inflammation. Elevated concentrations of CRP, although still in the normal range, not only are independent predictors of future CHD in adults and older individuals [11,12], but have been also associated with different features of MS [13,14]. The specific pathway/s linking elevated CRP levels to MS have not been definitively clarified. Different but not mutually exclusive mechanisms have been proposed including chronic over-nutrition [15], presence of sub-clinical atherosclerosis or cardiovascular disease related to MS [16], a direct effect of insulin-resistance [17], and body fat accumulation or obesity [18]. Schrager et al. recently suggested a direct effect of waist circumference on systemic inflammation [19]; nevertheless, the interaction between waist circumference, MS, and LGSI was not analyzed thoroughly in that study.
The aging process has been also associated with a progressive and significant increase in CRP plasma levels [20-22]; indeed, it is well known that, unlike middle-aged people, older individuals are often affected by LGSI. Interestingly, the association between aging and LGSI is not completely explained by comorbidity [22]; therefore, a primary dysregulation of the inflammatory mechanism cannot be ruled out in the elderly. This phenomenon, together with the presence of comorbidity, might modify the strength of the association between MS and LGSI, as well as the potential interaction between single MS components and LGSI.
A more accurate understanding of the relationship between MS (and its components) and LGSI in older individuals would be useful in order to better focus medical interventions aimed to reduce LGSI in the elderly.
In present study we investigated the relationship between CRP plasma levels, as a marker of LGSI, MS, and its single components in a large sample of community dwelling older Italian individuals enrolled in the InChianti study.
2. Materials and methods
This study is part of the “Invecchiare in Chianti” (Aging in the Chianti area, InCHIANTI) study, a prospective population-based study of older persons, designed by the Laboratory of Clinical Epidemiology of the Italian National Research Council of Aging (INRCA, Florence, Italy). The study included participants randomly selected from the residents in two towns of the Chianti area (Greve in Chianti and Bagno a Ripoli, Tuscany, Italy) [23]. A detailed description of the sampling procedure and data collection method has been previously published [23]. Briefly, in August 1998, 1270 persons ≥65-year-old were randomly selected from the population registries. In addition, men and women were sampled randomly from the age strata 20-29, 30-39, 40-49, 50-59 and 60-64 years, and sequentially invited to participate in the study until at least 30 men and 30 women for each decade between 20 and 59 years and 10 men and 10 women aged 60-64 years had been enrolled. Additional subjects (n, 29) were randomly selected among those who were ≥90-year-old, to obtain a sample of 30 men and 30 women in this age group. Of the initial 1299 subjects, 39 were not eligible (they had died or left the area). Overall, 1453 subjects were recruited. The participation rate was 69.4% in those aged < 65 years and 91.6% in those aged ≥65 years. Of these, 634 men (<65 years, n = 142; ≥65 years, n = 492) and 802 women (<65 years, n =154; ≥65 years, n = 648) with an age range of 21-103 years, underwent a complete dietary interview and were included in the final analytical sample. Clinical visits and assessments were performed in the study clinic and were preceded by an interview conducted at the participants’ homes. Trained interviewers administered two structured questionnaires. One collected data on dietary intakes and the other included many questions on household composition, social networks, economical status, education, and general information on health and functional status. The Italian National Research Council on Aging (INRCA) Ethical Committee ratified the entire study protocol.
In the present study, 1044 individuals aged over 65 years in which metabolic parameters and hs.CRP had been measured at baseline were included. One hundred twelve participants were excluded as they had refused to provide blood sample for analysis at baseline. They were characterized by older age (mean 79.9, S.D. 8.6 years), while no differences in socio-demographic characteristics emerged.
Metabolic syndrome was defined by the criteria of the NCEP-ATP III-AHA/NHLBI statement published in 2005 [24]. The diagnosis of MS was made in the presence of at least 3 of the following criteria:
Waist circumference ≥102 cm in men or ≥88 cm in women.
Triglycerides ≥150 mg/dL or treatment for hypertriglyceridemia.
HDL-C < 40 mg/dL in men or <50 mg/mL in women or treatment for low HDL-C.
Blood pressure ≥130/85 mmHg or treatment for hypertension.
Fasting glucose ≥100 mg/dL or treatment for hyperglycemia.
2.1. Clinical chemistry parameters
All parameters were measured on fresh serum drawn after 12 h overnight fasting, after the patient has been sedentary in sitting or supine position for 15 min. There were no evidences of acute illness at the time of enrollment and blood drawing. Commercial enzymatic tests (Roche Diagnostics) were used for determining serum total cholesterol (TC), triglycerides (TG), and HDL-C concentrations.
Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald’s formula: LDL-C: TC-(TG/5)-HDL-C. Fasting insulin was determined using a commercial double-antibody, solid-phase radioimmunoassay (Sorin Biomedica, Milan, Italy). Fasting blood glucose was determined by an enzymatic colorimetric assay using a modified glucose oxidase-peroxidase method (Roche Diagnostics, GmbH, Mannheim, Germany) and a Roche-Hitachi 917 analyzer.
2.2. High sensitivity CRP (hs.CRP) determination
Sampled blood was transferred into the tube avoiding hemolysis. After having been aliquoted, serum was frozen and stored at -80 °C until the tests were performed. Determination of CRP level was based on a high sensitivity enzyme-linked immunosorbent assay, using purified protein and polyclonal anti-CRP antibodies. The minimum detectable concentration was 0.03 mg/L. The average of two measures performed on each sample was used in the analysis. Hs-CRP plasma levels were defined high when >3 mg/L [25].
2.3. Covariates
2.3.1. Anthropometry
Weight and height were measured by using standard techniques. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). Waist circumference was measured to the nearest 0.5 cm by using a non-elastic plastic tape, at the level of the smallest area of the waist between the lower rib margin and the iliac crest.
2.3.2. Calf fat area
A right leg peripheral quantitative computed tomography (CT) scan was performed in 909 participants by a recent generation device (XCT 2000; Stratec, Pforzheim, Germany) to evaluate the total, muscular, and fat cross-sectional areas of the calf. Data were derived from standard 2.5 mm thick transverse scans obtained at 66% of the tibial length starting from the tibio-tarsal joint [26]. The fat area (in cm2) was calculated using the BonAlyse software version 3.1 (BonAlyse Ltd., Jyväskylä, Finland). Different tissues in the analyses were separated according to different density thresholds, using the “soft tissue” algorithm: a density value of 15 mg/mm3 was used to separate fat from muscle tissue, and 180 mg/mm3 to separate muscle from bone tissue. For statistical analysis, fat area was normalized by using the following formula: fat area × (mean population tibia lenght2/individual tibia lenght2).
2.3.3. Dietary variables
Data on dietary intake (i.e. energy intake and alcohol intake), were collected by the food-frequency questionnaire created for the European Prospective Investigation into Cancer and nutrition (EPIC) study [27]. Although the EPIC questionnaire was originally validated in middle-aged persons, we have previously shown that it provides good estimates of dietary intake when administered to older persons [28]. Participants were asked to specify how frequently (weekly, monthly, yearly) each specific food and beverage was consumed in the last year. To estimate quantities, colored photographs were shown with different sizes of portions for the main dishes. Specific software created for the EPIC study transformed data on food consumption into daily intake of energy, macronutrients, and micronutrients.
2.3.4. Ankle-brachial index
The ABI was measured using a hand-held Doppler stethoscope (Parks model 41-A, Aloha, OR). Systolic pressures were measured twice in the right brachial artery and twice in each posterior tibial artery [29]. The highest pressure in each measurement set was used to calculate the ABI, by dividing the lower of the right and left posterior tibial pressures by the brachial artery pressure. ABI was considered pathological when <0.9.
2.3.5. Comorbidity
The presence of specific diseases was established by standardized criteria combining information obtained from self-reported history, medical records, and from clinical examination. The following diseases were assessed: angina and acute myocardial infarction (CHD), peripheral arterial disease, stroke and transient ischemic attack, hypertension, arthritis, osteoporosis, chronic pulmonary obstructive disease, cancer, and dementia.
Diabetes mellitus was defined as the presence of at least one of the following conditions: previous physician’s diagnosis, current treatment with insulin or oral hypoglycemic, self-report of diabetes mellitus, and fasting blood glucose ≥ 126 mg/dL.
2.3.6. Smoking habit
All participants were asked about smoking habits and pack-years (measure that combines intensity and duration) was calculated as packs smoked per day × years of smoking.
2.3.7. Drug treatments
Drugs were coded according to the Anatomical Therapeutic and Chemical codes. The medications considered in the present study were: insulin, hypoglycemic, and statins.
2.4. Statistical analysis
Continuous variables were expressed as mean (S.D.) or median (interquartile range). Means were compared by ANOVA, while medians were compared by non-parametric tests (Mann-Whitney). Correlations between continuous variables were tested by multivariate linear regression analysis. Prevalences were compared by the χ2-test. Due to the skewed distribution, hs.CRP, insulin, TG, HOMA, ABI, and calf fat area values were log-transformed (natural logarithm—Ln) in order to approximate a normal distribution before entering regression analysis.
In order to select the variables independently associated with the risk of having increased hs.CRP plasma levels (>3 mg/L) in subjects with MS, we calculated the Odds Ratio by using a multivariate stepwise logistic regression analysis. The variables entered into the logistic models were those associated with hs.CRP at univariate analysis including: age, gender, waist circumference, smoking, alcohol intake, CHD, stroke, ABI, Homeostasis Model Assessment (HOMA), statin, oral hypoglycemic drugs and insulin treatment plus comorbidity (dementia, arthritis, osteoporosis, chronic pulmonary obstructive disease, and cancer).
The Likelihood Ratio Test (LRT) was used in order to compare the goodness-of-fit of different logistic models in predicting high hs.CRP plasma levels according to each metabolic syndrome component (age and gender adjusted).
The risk (OR) of high hs.CRP levels shown in Fig. 1 was calculated by logistic regression analysis, age and gender adjusted. For each MS criteria, a new variable was created by including the diagnosis of MS plus the absence (-) or the presence (+) of the single criteria.
Fig. 1.
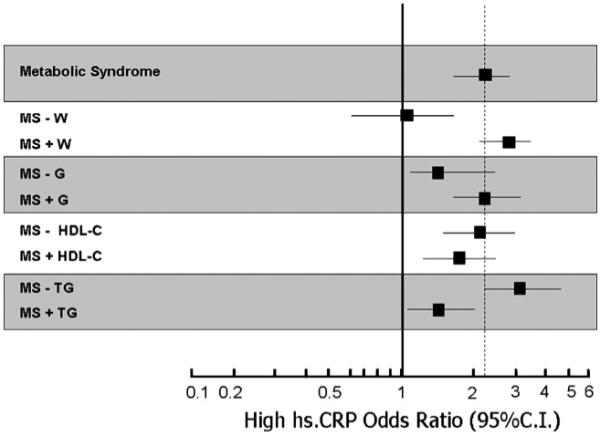
Age and gender adjusted Odds Ratio (95% CI) for high hs.CRP plasma levels in community dwelling older subjects from the InChianti study with MS, according to absence (-) or presence (+) of single MS components.
Systat for Windows, version 5.0, and SPSS for Windows, version 7.0 (SPSS Inc., Chicago, IL) statistical packages were used.
3. Results
The prevalence of MS in the whole sample was 31%; it was lower in men (24.5%) compared to women (36%) (χ2, p < 0.001). Overall, the prevalence of high hs.CRP levels was 45.4%; in particular, it was 41.3% in subjects without MS, and 54.5% in subjects with MS (χ2, p < 0.001).
MS was associated with high hs.CRP levels (OR: 2.30, 95% CI: 1.75-2.99) after adjustment for age and gender. After adjustment for comorbidity (CHD, stroke, dementia, arthritis, osteoporosis, chronic pulmonary obstructive disease, and cancer) the association was attenuated but remained clinically and statistically significant (OR: 1.93, 95% CI: 1.46-2.55). The median values of hs.CRP progressively increased from 2.40 mg/L in subjects without MS, to 3.24, 3.45, and 5.07 mg/L in individuals with 3, 4, and 5 criteria for MS, respectively (p < 0.05).
The main characteristics of the whole sample according to absence (MS-) or presence (MS+) of MS are shown in Table 1. In subjects with MS, the most frequent ATP-III MS component was hypertension, followed by increased waist circumference, hypertriglyceridemia, hyperglycemia, and low HDL-C levels.
Table 1.
Principal characteristics of 1044 community dwelling older individuals without (n, 721) or with (n, 323) metabolic syndrome (MS) from the InChianti study
| Variable | MS-(n, 721) | MS+ (n, 323) | p |
|---|---|---|---|
| Age (years) | 76.3±7.9 | 75±6.8 | 0.005 |
| Gender (F%) | 53.4 | 65.3 | 0.001 |
| hs.CRP (mg/L) | 2.40 (1.20-5.45) | 3.38 (1.81-6.46) | 0.001 |
| ATP III MS CRITERIA (%) | |||
| Increased waist | 26.6 | 79.9 | 0.001 |
| High triglycerides | 9.6 | 60.1 | 0.001 |
| Low HDL-C | 9.0 | 54.5 | 0.001 |
| Hypertension | 90.5 | 98.5 | 0.001 |
| Hyperglycemia | 12.6 | 57.0 | 0.001 |
| Glucose (mg/dL) | 89.4±18 | 109.9±35 | 0.001 |
| Insulin (U/L) | 9.45 (6.65-13.2) | 11.6 (8.5-16.6) | 0.001 |
| HOMA | 2.05 (1.42-2.95) | 3.02 (2.02-4.51) | 0.001 |
| Tot. Chol. (mg/dL) | 215±39 | 220±40 | 0.04 |
| Trigl. (mg/dL) | 106±46 | 176±87 | 0.001 |
| LDL-C (mg/dL) | 134.6±33.8 | 138.4±35.5 | 0.08 |
| HDL-C (mg/dL) | 59±15 | 47±11 | 0.001 |
| Waist (cm) | 89±9.2 | 99±9.4 | 0.001 |
| BMI (kg/m2) | 26.2±3.46 | 29.9±4.19 | 0.001 |
| Calf fat area (mm2) | 1504 (905-2577) | 1938 (1232-2925) | 0.001 |
| SBP (mm/Hg) | 148.5±19.5 | 155±19 | 0.001 |
| DBP (mm/Hg) | 83.5±8.3 | 85±8.4 | 0.01 |
| Energy (Kcal/day) | 1902±569 | 1865±545 | 0.36 |
| Alcohol (g/day) | 7.64 (0-26.6) | 5.73 (0-14.5) | 0.02 |
| Diabetes (%) | 8.1 | 21.6 | 0.001 |
| CHD (%) | 6.6 | 10.0 | 0.04 |
| Stroke (%) | 6.6 | 6.9 | 0.48 |
| ABI <0.9 (%) | 8.3 | 17.0 | 0.001 |
| Smoking (%) | |||
| Never | 57.9 | 63.5 | |
| Former | 30.5 | 27.3 | |
| Current | 11.6 | 8.9 | 0.07 |
BMI, body mass index; ABI, ankle brachial index; CHD, coronary heart disease. For CRP, insulin, HOMA, ABI: median (interquartile range).
The main characteristics of the individuals with MS, stratified by normal (-) vs. high (+) hs.CRP plasma levels are reported in Table 2. Among the MS criteria, the prevalence of increased waist circumference was significantly higher, while the prevalence of hypertriglyceridemia was lower in individuals with high hs.CRP.
Table 2.
Principal characteristics of 323 community dwelling older individuals from the InChianti study affected by metabolic syndrome and stratified by absence (CRP-) or presence (CRP+) of elevated (>3 pg/mL) hs.CRP plasma levels
| Variable | CRP- (n, 147) | CRP+ (n, 176) | p |
|---|---|---|---|
| Age (years) | 75±6.7 | 75±6.9 | 0.59 |
| Gender (F%) | 70.7 | 60.8 | 0.03 |
| Hs.CRP (mg/L) | 1.63 (1.05-2.26) | 6.18 (4.29-12.4) | 0.001 |
| ATP III MS CRITERIA (%) | |||
| Increased waist | 74.8 | 84.1 | 0.02 |
| High triglycerides | 68.7 | 52.8 | 0.003 |
| Low HDL-C | 56.5 | 52.8 | 0.29 |
| Hypertension | 98.0 | 98.9 | 0.41 |
| Hyperglycemia | 52.4 | 60.8 | 0.08 |
| Glucose (mg/dL) | 106±29 | 113±28 | 0.07 |
| Insulin (U/L) | 10.8 (8.51-14.9) | 12.47 (8.48-18) | 0.03 |
| HOMA | 2.81 (1.93-3.79) | 3.19 (2.13-5.01) | 0.01 |
| Tot. chol. (mg/dL) | 224±42 | 217±38 | 0.13 |
| Trigl. (mg/dL) | 182±83 | 171±90 | 0.26 |
| LDL-C (mg/dL) | 140±38 | 137±32 | 0.43 |
| HDL-C (mg/dL) | 48±11 | 46±12 | 0.25 |
| Waist (cm) | 97±9.5 | 100±9 | 0.001 |
| BMI (kg/m2) | 29.0±3.8 | 30.6±4.3 | 0.001 |
| Calf fat area (mm2) | 1949 (1323-3024) | 1847 (1056-2900) | 0.49 |
| SBP (mm/Hg) | 155±20 | 156±17.7 | 0.68 |
| DBP (mm/Hg) | 85.1±7.9 | 85.4±8.8 | 0.73 |
| Energy (Kcal/day) | 1872±593 | 1859±503 | 0.82 |
| Alcohol (g/day) | 5.73 (0-13.6) | 6.04 (0-19.3) | 0.27 |
| Diabetes | 22.3 | 20.9 | 0.49 |
| CHD (%) | 7.5 | 12.1 | 0.12 |
| Stroke (%) | 5.5 | 8.0 | 0.25 |
| ABI <0.9 (%) | 11.6 | 21.6 | 0.01 |
| Smoking (%) | |||
| Never | 69.4 | 58.5 | |
| Former | 25.2 | 29.5 | |
| Current | 5.4 | 11.9 | 0.06 |
BMI, body mass index; ABI, ankle brachial index; CHD, coronary heart disease. For CRP, insulin, HOMA, ABI: median (interquartile range).
In subjects with MS, multivariate logistic regression analysis demonstrated that waist circumference (III vs. I tertile OR: 3.00; 95% CI: 1.26-7.17), and HOMA score (OR: 1.73; 95% CI: 1.15-2.60) were significantly associated with elevated hs.CRP levels (>3 mg/L); the association was strong and independent of potential confounders including age, gender, smoking, alcohol intake, CHD, stroke, ABI, statin, oral hypoglycaemic drugs and insulin treatment. Further adjustment for comorbidity (including dementia, arthritis, osteoporosis, chronic pulmonary obstructive disease, and cancer) did not significantly change the results (waist circumference III vs. I tertile OR: 2.60, 95% CI: 1.79-3.77; HOMA score: OR: 1.15; 95% CI: 1.04-1.24).
To further explore the role of single MS criteria in determining the likelihood of having high hs.CRP levels, we compared the predictive value of a logistic model (age and gender adjusted) including one single MS criteria (model A), with the predictive value of a model including the same MS criteria plus MS without that specific criterion (model B). For this analysis MS was defined as the presence of 3 out of the 4 remaining criteria. The same analysis was repeated for each of the five components. Goodness-of-fit comparison was performed by using the likelihood ratio test (LRT). As shown in Table 3, for glucose, HDL-C, and triglycerides the logistic model including MS (model B) had a significantly better goodness-of-fit compared with model A. Conversely, for waist circumference there was no difference between the two models (p for LRT: 0.43). As regard to hypertension, over 98% of individuals with MS were affected by hypertension according to the ATP III definition, and therefore this specific analysis was not feasible.
Table 3.
Likelihood ratio tests (age and gender adjusted) comparing the goodness-of-fit of different logistic models in predicting high hs.CRP plasma levels according to each metabolic syndrome component
| Model A |
Model B |
Log likelihood | p | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Waist | 2.37 | (1.77-3.15) | -649.29 | |||
| Waist | 2.43 | (1.81-3.26) | -648.99 | 0.43 | ||
| MS without waist | 1.23 | (0.73-2.09) | ||||
| Glucose | 1.32 | (0.99-1.76) | -665.47 | |||
| Glucose | 1.43 | (1.07-1.93) | -663.11 | 0.02 | ||
| MS without glucose | 1.51 | (1.04-2.20) | ||||
| HDL-C | 1.62 | (1.19-2.20) | -662.57 | |||
| HDL-C | 1.91 | (1.39-2.63) | -653.50 | 0.001 | ||
| MS without HDL-C | 2.21 | (1.53-3.20) | ||||
| Triglycerides | 1.09 | (0.81-1.46) | -665.81 | |||
| Triglycerides | 1.32 | (0.98-1.79) | -667.16 | 0.001 | ||
| MS without triglycerides | 2.99 | (2.00-4.47) | ||||
| Hypertension | 1.54 | (0.92-2.58) | -665.81 | |||
| Hypertension | 1.59 | (0.93-2.70) | -665.90 | 0.66 | ||
| MS without hypertension | 1.51 | (0.023-9.82) | ||||
In Fig. 1 are reported the Odds Ratio and 95% CI (age and gender adjusted) for elevated hs-CRP plasma levels in subjects with MS compared with those without MS, according to the absence (-) or presence (+) of each MS components. In the analyses done for MS without a selected component the reference group was the entire non-MS group, regardless the presence or absence of the selected criterion. After forcing into MS or removing from MS each single component, MS was still associated with increased risk of high hs.CRP levels, with the only exception of MS without increased waist circumference (OR: 1.02, 95% CI: 0.61-1.71). Intriguingly, the absence and presence of TG among MS criteria was associated with an increase and a decrease in the risk of LGSI, respectively. The most probable explanation for this unexpected finding lies in the different prevalence of increased waist circumference in the two groups (TG-: 95% vs. TG+: 69%).
The association between waist circumference and hs.CRP was further confirmed in the analysis performed with continuous value of hs.CRP as dependent variable. Multivariate linear regression analysis (adjusted for age and gender) showed that the association between MS and hs.CRP (β: 1.1; p < 0.001) was still significant after further adjustment for each single MS component (HDL-C, TG, fasting glucose and hypertension; all p < 0.01), but not after adjustment for waist circumference (β: 1.01, p < 0.08).
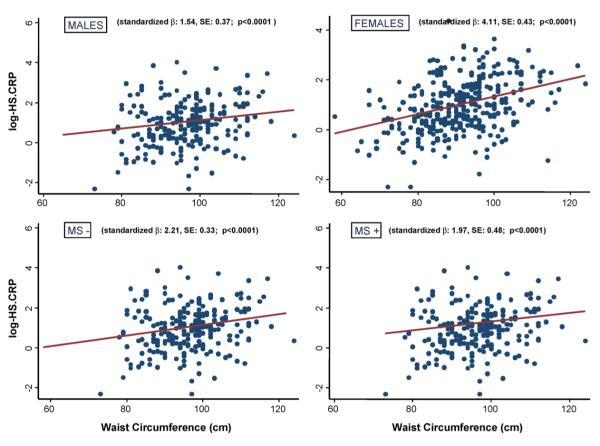
To further investigate the interaction among MS components, the association between hs.CRP levels and all possible combinations of MS components was tested in regression analysis adjusted for age and gender. Only three combinations (namely: (1) hypertriglyceridemia + hypertension + hyperglycemia + increased waist; (2) hypertension + hyperglycemia + increased waist; (3) low-HDL + hypertension + increased waist) were significantly associated with hs.CRP levels (all p < 0.001). As in our sample virtually all individuals with MS were affected by hypertension, waist circumference was actually the only MS criteria shared by these three MS phenotypes. Finally, the strength of the association between waist circumference and hs.CRP levels was separately tested in males (β: 1.54, S.E.: 0.37, p < 0.0001), in females (β: 4.11, S.E.: 0.43, p < 0.0001; age adjusted), in subjects without MS (β: 2.21, S.E.: 0.33, p < 0.0001) and with MS (β: 1.97, S.E.: 0.48, p < 0.0001; age and gender adjusted) (Fig. 2). The association between waist circumference and hs.CRP remained substantially unchanged (all p < 0.0001) after including the other four components of MS and BMI into the regression models (data not shown). Similarly, the exclusion of the subjects with hs.CRP levels >10 mg/L from the analysis did not modify the relationship. These results confirmed that the association between waist circumference and LGSI was independent of gender, MS, and BMI, being stronger in females compared with males, and in subjects without compared to those with MS.
Fig. 2.
Regression between waist circumference and hs.CRP plasma levels in community dwelling older subjects from the InChianti study, divided by gender (Males: n, 458 and Females: n, 586), and by absence (MS-: n, 721) or presence (MS+: n, 323) of metabolic syndrome by the ATP III criteria.
4. Discussion
We investigated the association of MS and its components with the presence of LGSI (defined as hs.CRP levels >3 mg/L) by using data from a large sample of community dwelling older Italian individuals from the InChianti study.
As previously reported [11,12], the diagnosis of MS was associated with increased levels of hs.CRP; nevertheless, in our sample MS was associated with only a 13.2% increase in the prevalence of high hs.CRP (41.3% in MS- vs. 54.5% in MS+) suggesting the possibility that other variables, including the single components of MS and comorbidity, might be associated to LGSI independent of the effect of MS.
We found that waist circumference was significantly and independently associated to elevated hs.CRP. In addition, we demonstrated that among individuals with MS, the association of waist circumference with hs.CRP levels was independent of the effect of important potential confounders including age, vascular disease and atherosclerosis (CHD, stroke, and ABI), insulin-resistance (HOMA score), and comorbidity. The risk of having high hs.CRP increased from the first to the third tertile of waist circumference, indicating a possible dose-effect relationship.
Moreover, as demonstrated in Table 3 and in Fig. 1, our results suggest that increased waist circumference was the most important correlate of increased hs.CRP levels. Indeed, among individuals with elevated waist circumference, the presence of other MS criteria only marginally increased the risk of having LGSI. Our results confirm data from previous recent studies indicating that also in adult older individuals with MS central obesity is the major contributing feature of increased CRP plasma levels [30,31].
Interestingly, waist circumference and HOMA score were independently correlated with hs.CRP plasma levels in these subjects, suggesting that insulin-resistance and abdominal obesity might independently contribute to LGSI. On the other hand, from our cross-sectional analysis we cannot infer whether insulin-resistance might cause inflammation or vice versa inflammation might determine resistance to insulin.
It has been shown that waist circumference is closely correlated with the level of abdominal visceral adipose tissue and related metabolic variables, in both sexes [32-34]. It is a simple measurement that estimates the extension of abdominal obesity, which is closely linked to the deposition of visceral fat. The shared variance between waist circumference and visceral adipose tissue has been calculated as high as 75% [32,35], suggesting that the measurement of waist circumference may be useful for the clinical assessment of visceral fat accumulation. Moreover, more than 90% of the variation in waist circumference is explained by differences in total body and visceral fat accumulation [32]. Interestingly, despite sub-cutaneous cross-sectional fat area in the calf (measured by CT scan) was increased in subjects with MS, it was not associated with elevated hs.CRP levels, both in MS+ and MS- individuals. This observation supports the notion that also in older subjects LGSI is specifically related to visceral fat, while the role of peripheral muscle fat accumulation might be negligible.
It has been shown that adipose tissue has an important role in releasing cytokines such as interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) [36]; in addition, a macrophage infiltration of adipose tissue has been demonstrated in obese subjects, which might contribute to the systemic inflammation reported in abdominal obesity [37]. Circulating CRP levels are also increased in obese individuals, and have been correlated to amount of visceral fat as assessed by waits circumference [38,39]. Liver is believed to be the major source of CRP; CRP synthesis is mainly stimulated by IL-6 and other cytokines, about 30% of circulating IL-6 originating from adipose tissue in normal individuals [40]. Recent data suggest that adipose tissue might also be a source of CRP [41], and it has been suggested by Dhillon and Colleagues (unpublished observations) that in humans the total amount of CRP produced by the fat tissue might be significant, as it is a much larger organ compared to the liver.
As proposed by Depres & Lemieux in the “overflow-ectopic fat” model [36], when extra energy (resulting from excess intake and sedentary life style) is not channeled to insulin-sensitive subcutaneous adipose tissue, it would be deposited at undesiderable sites such as liver, heart, and skeletal muscle, and visceral fat. The consequence of this “ectopic” fat deposition would include visceral adiposity, insulin-resistance, dyslipidemia, and inflammatory profile, all defining features of the MS. It is of interest to observe that the aging process is “usually” associated with similar changes in body composition. After 30 years of age, fat-free mass (FFM) decreases whereas fat mass (FM) progressively increases. Moreover, a significant redistribution of FM and FFM during aging has been described, with a greater relative increase in intra-abdominal visceral fat compared to sub-cutaneous/total body fat [42]. An increase in intramuscular and intra-hepatic fat in older persons has been also reported, and it has been associated with insulin-resistance [43].
On the whole, compared to adults, older individuals are characterized by an increase in the proportion of visceral adipose tissue [42,44,45], even when the total amount of fat is the same [45].
Despite the fact that fat accumulation and abdominal redistribution are common findings in the elderly, the strong independent association between waist circumference and hs.CRP levels found in our older individuals suggests that, even in advanced age, visceral accumulation of adipose tissue might become “pathological” when exceeding a certain threshold. From this point of view, the cut-off defined by ATP III (88 cm in women and 102 cm in men) seems to be useful in identifying those individuals in which the accumulation of visceral fat becomes “pathological” as associated with LGSI. From a medical and public health point of view, this is an important issue since increased CRP levels predict, in older age, not only CHD mortality [7], but also total mortality, and a number of important geriatric outcomes [46].
Finally, the most important limitation of our study must be acknowledged. We have used waist circumference as the only available measure of abdominal fat deposition; although it is considered a good indicator of visceral fat deposition [28,33], it is anyhow an indirect measurement, and a more careful method (e.g. abdomen CT scan) would be preferable.
In conclusion, the findings of our study suggest that, in community dwelling older individuals MS is associated with LGSI. Nevertheless, this association is mainly accounted for by the presence of increased waist circumference; indeed, in the absence of this MS component, the contribution of MS to LGSI seems to be modest.
We suggest that among the “usual” age-associated modifications in body composition, an over-deposition of abdominal fat, indicated by increased waist circumference, might be deleterious due to the increased risk for LGSI. Our findings do not question the existence of MS nor its usefulness in identifying a cluster of vascular risk factors in the older population, but reconsider the actual contribute of MS to the phenomenon of LGSI in advanced age.
References
- [1].Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- [2].Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34:416–22. doi: 10.1007/BF00403180. [DOI] [PubMed] [Google Scholar]
- [3].Liese AD, Mayer-Davis EJ, Haffner SM. Development of the multiple metabolic syndrome: an epidemiologic perspective. Epidemiol Rev. 1998;20:157–72. doi: 10.1093/oxfordjournals.epirev.a017978. [DOI] [PubMed] [Google Scholar]
- [4].Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart Association. National Heart, Lung, and Blood Institute Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- [5].Strandberg TE, Tilvis RS. C reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arterioscler Thromb Vasc Biol. 2000;20:1057–60. doi: 10.1161/01.atv.20.4.1057. [DOI] [PubMed] [Google Scholar]
- [6].Ford ES, Wayne HG, Dietz WH. Prevalence of metabolic syndrome among U.S. adults: findings from the third national health and nutrition examination survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- [7].Miccoli R, Bianchi C, Odoguardi L, et al. Prevalence of the metabolic syndrome among Italian adults according to the ATP III definition. Nutr Metab Cardiovasc Dis. 2005;15:250–4. doi: 10.1016/j.numecd.2004.09.002. [DOI] [PubMed] [Google Scholar]
- [8].Pickup JC, Mattock MB, Chusney GD, et al. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–92. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- [9].Yudkin JS, Stehouwer CDA, Emeis JJ, et al. C-reactive protein in healthy subjects: association with obesity, insulin resistance, and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- [10].Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- [11].Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- [12].Strandberg TE, Tilvis RS. C-reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arterioscler Thromb Vasc Biol. 2000;20:1057–60. doi: 10.1161/01.atv.20.4.1057. [DOI] [PubMed] [Google Scholar]
- [13].Festa A, D’Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the insulin resistance atherosclerosis study (IRAS) Circulation. 2000;102:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- [14].Frohlich M, Imhof A, Berg G, et al. Association between C reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–9. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- [15].Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–8. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- [16].Tracy RP. Inflammation in cardiovascular disease: cart, horse, or both? Circulation. 1998;97:2000–2. doi: 10.1161/01.cir.97.20.2000. [DOI] [PubMed] [Google Scholar]
- [17].Campos SP, Baumann H. Insulin is a prominent modulator of the cytokine-stimulated expression of acute-phase plasma protein genes. Mol Cell Biol. 1992;12:1789–97. doi: 10.1128/mcb.12.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- [19].Schrager MA, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–25. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- [21].Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53:M20–6. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- [22].Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–9. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferrucci L, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- [24].Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- [25].Pearson TA, Bazzarre TL, Daniels SR, et al. American Heart Association guide for improving cardiovascular health at the community level: a statement for public health practitioners, healthcare providers, and health policy makers from the American Heart Association Expert Panel on Population and Prevention Science. Circulation. 2003;107(4):645–51. doi: 10.1161/01.cir.0000054482.38437.13. [DOI] [PubMed] [Google Scholar]
- [26].Rittweger J, Beller G, Ehrig J, et al. Bone-muscle strength indices for the human lower leg. Bone. 2000;27:319–26. doi: 10.1016/s8756-3282(00)00327-6. [DOI] [PubMed] [Google Scholar]
- [27].Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26:152–60. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- [28].Bartali B, Turrini A, Salvini S, et al. Dietary intake estimated using different methods in two Italian older populations. Arch Gerontol Geriatr. 2004;38:51–60. doi: 10.1016/s0167-4943(03)00084-0. [DOI] [PubMed] [Google Scholar]
- [29].Newman AB, Sutton-Tyrell K, Kuller LH. Lower-extremity arterial disease in older hypertensive adults. Arterioscler Thromb. 1993;13:555–62. doi: 10.1161/01.atv.13.4.555. [DOI] [PubMed] [Google Scholar]
- [30].Santos AC, Lopes C, Guimaraes JT, Barros H. Central obesity as a major determinant of increased high-sensitivity C-reactive protein in metabolic syndrome. Int J Obes. 2005;29:1452–6. doi: 10.1038/sj.ijo.0803035. [DOI] [PubMed] [Google Scholar]
- [31].Dupuy AM, Jaussent I, Lacroux A, Durant R, Cristol JP, Delcourt C. Waist circumference adds to the variance in plasma C-reactive protein levels in elderly patients with metabolic syndrome. Gerontology. 2007;53:329–39. doi: 10.1159/000103555. [DOI] [PubMed] [Google Scholar]
- [32].Pouliot M-C, Despres J-P, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- [33].Seidell JC, Oosterlee A, Deurenberg P, Hautavast JG, Ruijs JH. Abdominal fat depots measured by computed tomography: effects of degree of obesity, sex, and age. Eur J Clin Nutr. 1988;42:805–15. [PubMed] [Google Scholar]
- [34].Despres J-P, Prud’homme D, Pouliot MC, Tremblay A, Bouchard C. Estimation of deep abdominal adipose tissue accumulation from simple anthropometric measurements in men. Am J Clin Nutr. 1991;54:471–7. doi: 10.1093/ajcn/54.3.471. [DOI] [PubMed] [Google Scholar]
- [35].Ferland M, Despres J-P, Tremblay A, et al. Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. Br J Nutr. 1989;61:139–48. doi: 10.1079/bjn19890104. [DOI] [PubMed] [Google Scholar]
- [36].Depres J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- [37].Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and the risk of incident cardiovascular events: an 8-years follow-up of 14,719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- [39].Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- [40].Mohamed-Ali V, Goodrisk S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6 but not tumor necrosis factor-alpha, in vivo. J Clin Endocr Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- [41].Lau D, Yan H, Abdel-Hafez M, Kermouni A. Adipokines and the paracrine control of their production in obesity and diabetes. Int J Obes Relat Metab Disord. 2002;26:S111. Abstract. [Google Scholar]
- [42].Beaufrere B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr. 2000;54:S48–53. doi: 10.1038/sj.ejcn.1601025. [DOI] [PubMed] [Google Scholar]
- [43].Cree MG, Newcomer BR, Katsanos CS, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–71. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- [44].Horber FF, Ruber B, Thomi FA, Jensen EX, Haeger P. Effect of sex and age on bone mass, body composition, and fuel metabolism in humans. Nutrition. 1997;13:424–534. doi: 10.1016/s0899-9007(97)00031-2. [DOI] [PubMed] [Google Scholar]
- [45].Zamboni M, Armellini F, Harris T, et al. Effects of age on body fat distribution and cardiovascular risk factors in women. Am J Clin Nutr. 1997;66:111–5. doi: 10.1093/ajcn/66.1.111. [DOI] [PubMed] [Google Scholar]
- [46].Morley JE, Baumgartner RN. Cytokine-related aging process. J Geront Med Sci. 2004;59(9):M924–9. doi: 10.1093/gerona/59.9.m924. [DOI] [PubMed] [Google Scholar]