Abstract
New findings have emerged about the mechanism of oncogene-induced senescence, and its involvement in a highly prevalent disorder, pituitary adenomas. These observations point to senescence as a target for effective therapy for both tumor silencing and growth restraint towards development of pituitary malignancy.
Cellular senescence involves more than dysfunctional telomeres, and can be triggered by various cellular stresses. Twenty years ago it was observed that an activated mutant of the RAS gene induced arrest of untransformed fibroblast growth rather than oncogenic transformation, and that this proliferative arrest exhibited features of replicative cellular senescence, including stable maintenance of cell-cycle arrest (1). Observations made in cultured cells led to the principle that oncogene-induced senescence (OIS) is largely irreversible and involves activation of a set of well-known tumor suppressors that are often inactivated in human cancer including p16INK4A, p1 INK4B, p21CIP1, p53 and pRB. Unraveling mechanisms for OIS has wide ramifications for our understandings of cancer risk from benign lesions, kinetics of malignant cell growth, and treatment (1).
New exciting insights into cytokine-mediated mechanisms for OIS are reported in a recent paper in Cell (2). This recent work shows that OIS is specifically associated with activation of a cytokine and chemokine response which may be protective for malignant transformation in keratinocytes. Because IL-6 can act as a potent inducer of growth arrest and/or differentiation and correlates with RASV12 -induced senescence in embryonic fibroblasts, the authors focused their attention on this cytokine as a candidate gene for mediating OIS. Kuilman et al., reasoned that if IL-6 mediates OIS, it should fulfill three criteria. First, its expression levels should mirror both OIS induction and bypass. Indeed, quantitative RT-PCR analysis showed that high IL-6 expression strongly correlated with OIS, and decreased IL-6-with OIS bypass. Second, IL-6 induction by oncogenic stress should occur independently of the cellular context. Indeed, RNA and protein analyses of diploid human fibroblasts and primary human melanocytes showed that IL-6 is upregulated by oncogenic signals, irrespective of cell type. Third, the induction of IL-6 by oncogenic stress should in fact be independent of p16INK4A, as p15 INK4B is also involved in IL-6 action.
IL-6 is required for both induction and maintenance of OIS, and acts in a cell-autonomous fashion to enable OIS. The results obtained by Kuilman et al., suggest that IL-6 acts in an autocrine manner to regulate OIS as this signaling cascade is blocked by si IL-6 mRNA and requires an intact IL-6R. Thus, the results suggest that a cell-autonomous pool of IL-6 produced by senescent cells and acting in an autocrine and paracrine fashion, mediates OIS. Although not precluding involvement of other signaling pathways, the results obtained by Kuilman et al., also demonstrate that C/EBPβ is a critical mediator of an interleukin pathway leading to human fibroblast OIS.
This model raises the question whether the role of IL-6 activated in response to oncogenic stress is unique among other interleukins, including IL-8. C/EBPβ is recruited to the IL-8 promoter in response to oncogenic stress, similarly to C/EBPβ binding to the IL-6 promoter. Because C/EBPβ and IL-6 apparently act in a positive feedback loop, this observation further implicates these interleukins for oncogenic signaling. Thus, the critical role of IL-6 in OIS is shared by IL-8, as knockdown of IL-8 lead to efficient cell bypass of OIS (2).
To further understand mechanisms for tumor-associated senescence, better-defined models of in vivo senescence are required.
The anterior pituitary gland commonly develops benign tumors which are frequently associated with high levels of trophic hormone production (3). Although, systematic assessment from autopsy and imaging studies have shown an incidence of clinically inapparent pituitary tumors of up to 20% of the population, clinically functional pituitary adenomas represent ~10% of intracranial neoplasms. Prolactin-secreting adenomas are the most common type (about 30%), GH secreting tumors account for about 20%, and some 10–15% are adenomas-secreting adrenocorticotrophin (ACTH), giving rise to hypercortisolism and Cushing’s disease. Despite the high incidence of adeno-hypophyseal adenomas, these monoclonal neoplasms very rarely undergo malignant transformation. Although these monoclonal adenomas are usually sharply demarcated benign lesions, they occasionally exhibit higher proliferation activity associated with invasive features and impinge upon local structures. However, true pituitary carcinomas are exceedingly rare and pituitary metastases have only been reported in isolated cases. Furthermore, despite the common occurrence of pituitary adenomas they are usually not associated with apparent hyperplasia, and provide an interesting model to further understand the protective role of OIS against malignant transformation.
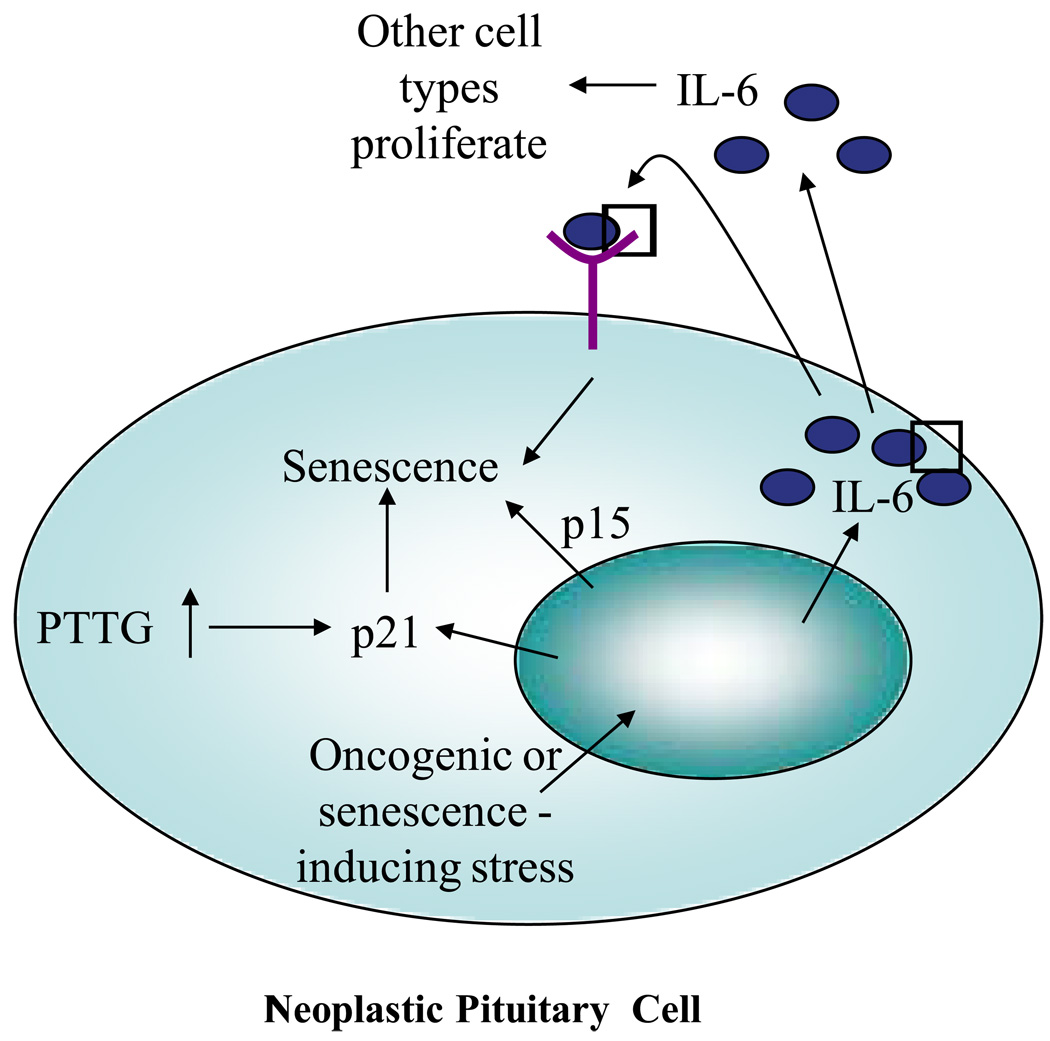
Pituitary tumor-transforming gene (PTTG) is a protein initially isolated from pituitary tumor cells and behaves as a mammalian securin homologue facilitating sister chromatid separation during methaphase (4). Pituitary-directed transgenic PTTG overexpression leads to development of focal hormone-secreting pituitary adenomas (5), and PTTG is also abundantly expressed in human pituitary adenomas (4). PTTG depletion results in pituitary hypoplasia, genomic instability, aneuploidy and activation of DNA-damage signaling pathways (6). PTTG deletion, as observed in PTTG-null mice, also results in pituitary-specific senescent features, including increased levels of p53 and Cdk inhibitors, p19 and p21, overexpression of cyclin D1, apoptosis block, and elevated senescence-associated β-galactosidase expression. However, PTTG-dependent pituitary senescence is not associated with telomere shortening, supporting the presence of premature pituitary cell senescence. In the Rb+/− mouse, PTTG depletion selectively rescues pituitary but not thyroid, tumor development and also enhances senescence in mouse embryonic fibroblasts (MEF). In contrast, deletion of p21 from Rb+/− Pttg−/− mice showed increased pituitary cell proliferation and decreased number of senescent MEFs (7, 8). Interestingly, intranuclear p21 was shown to accumulate in aneuploid Pttg-null pituitary GH-secreting cells. Pituitary proliferation rates were restored, with accelerated MEF S phase entry and enhanced transformation rates in the triply Rb+/−Pttg−/−p21−/− mutant mice. Human GH-producing pituitary adenomas shown to overexpress PTTG, also exhibit aneuploidy and senescence as evidenced by increased p21, kinase mutated in ataxia-telangiectasia (ATM) and senescence-associated –β-galactosidase levels. In contrast, p21 was undetectable in the human pituitary carcinomas tested. Thus, intracellular dysequilibrium of PTTG, as evidenced in both pituitary tumors and in PTTG-depleted pituitary glands, leads to aneuploidy, DNA damage and activation of senescence pathways. These results suggest that aneuploid pituitary cell p21 may constrain pituitary tumor growth, thus providing a proposed mechanism for the very low observed incidence of pituitary carcinomas.
Thus, pituitary adenomas constitute faithful vivo models of senescence. Mechanisms underlying pituitary senescence are not completely clear. One mechanism could be activation of DNA damage and p53/p21 senescence induced by the securin properties of PTTG. PTTG also behaves as protooncogene, and high PTTG as well as other oncogene levels can trigger oncogene-induced senescence. It is likely that in the pituitary, OIS may be mediated by IL-6.
IL-6 (and IL-6R) and other members of the gp130 cytokine family such as LIF, IL-11 and CNTF, are produced in the pituitary gland (9,10), and regulate synthesis of anterior pituitary hormones, consistent with a paracrine or autocrine model. IL-6 directly regulates pituitary cell growth (10, 11). Intriguingly, although IL-6 stimulates DNA synthesis and cell number in the GH3 pituitary cell line, similar concentrations of IL-6 actually inhibit growth of normal rat pituitary cells (12). In several tumor types (ACTH, PRL-, GH-secreting and non-functioning adenomas), IL-6 exhibited either inhibitory or stimulatory effects not associated with tumor type or size. Using blocking antibodies, as a functional assay for IL-6 intrapituitary action, intrinsic adenoma IL-6 was shown to regulate c–fos expression. Thus, in the normal pituitary, both paracrine and autocrine-derived IL-6 (9–11) inhibit pituitary trophic growth. In contrast, in rapidly replicating GH3 cells, paracrine IL-6 further induces DNA synthesis and cell proliferation, suggesting that paracrine IL-6 is required for senescence bypass.
Considering the modest adult pituitary gland cell proliferation, pituitary cell growth regulation by IL-6 underlies the role of cytokines as factors controlling pituitary cell division. The new findings of the IL-6 role in OIS, suggest the involvement of endogenous IL-6 in development of pituitary adenoma OIS, which, as well as its induction by aberrant intracellular PTTG levels, may explain the benign nature of these abundant tumors.
Oncogene-associated senescent response requires time to develop, allowing an initial proliferative phase, but eventually resulting in a benign tumor with stable growth arrest, which is indeed the natural history of pituitary adenoma growth. A response to oncogenic stress that restrains proliferation (and the resultant oncogenic threat) but allows the cell to remain viable and perform its physiological function thus favors vital functioning of the pituitary gland for homeostasis control.
It is not clear whether intrinsic pituitary cell IL-6 expresssion is oncogene-regulated. However, endogenous IL-6 may underlie the slow proliferation rate and benign nature of pituitary tumors. It is plausible that paracrine IL-6 effects may allow initial pituitary cell growth, while autocrine IL-6 in the same tumor triggers senescence and restrains aggressive growth and malignant transformation. Further experiments are required to determine involvement of CEBP/β and other pituitary cytokines including IL-11 or LIF in pituitary senescence.
The findings of Kuilman et al., and the involvement of cytokines in a highly prevalent natural disease model, shapes our thinking towards the use of this pathway as a target for effective therapy for tumor silencing and prevention of adenoma progression towards malignancy.
Activated oncoproteins or loss of tumor suppressor proteins (oncogenic stress) trigger oncogene-induced cellular senescence (OIS). However, other factors may affect pituitary tumors so as to induce OIS. In Kuilman et al.’s model (2) in melanocytic nevi, when OIS occurs there is an upregulation of p15INK4B, p16INK4A, p21CIP and CDK inhibitors. IL-6 is also upregulated in OIS and the IL-6/IL6-R pathway is essential for mediating OIS, inducing and maintaining senescence in an autocrine fashion, but acting pro-mitogenically in a paracrine fashion. Since IL-6 also regulates normal and tumoral pituitary cell growth, this process may also occur in incipient neoplastic pituitary cells. In Kuilman’s findings, p15INK4B was upregulated and its induction linked to IL-6 and CEBP/β, indicating regulation by oncogenic stress. Deranged intracellular PTTG levels, results in pituitary-specific senescent features, including increased p21 levels and elevated senescence-associated β-galactosidase expression.
References
- 1.Mooi WJ, Peeper DS. Oncogene-induced cell senescence--halting on the road to cancer. N Engl J Med. 2006;355:1037–1046. doi: 10.1056/NEJMra062285. [DOI] [PubMed] [Google Scholar]
- 2.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Melmed S. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest. 2003;112:1603–1618. doi: 10.1172/JCI20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med. 1999;5:1317–1321. doi: 10.1038/15275. [DOI] [PubMed] [Google Scholar]
- 5.Abbud RA, Takumi I, Barker EM, Ren SG, Chen DY, Wawrowsky K, Melmed S. Early multipotential pituitary focal hyperplasia in the alpha-subunit of glycoprotein hormone-driven pituitary tumor-transforming gene transgenic mice. Mol Endocrinol. 2005;19:1383–1391. doi: 10.1210/me.2004-0403. [DOI] [PubMed] [Google Scholar]
- 6.Vlotides T, Eigler T, Melmed S. Pituitary tumor-transforming gene: physiology and implications for tumorigenesis. Endocr Rev. 2007;28:165–186. doi: 10.1210/er.2006-0042. [DOI] [PubMed] [Google Scholar]
- 7.Chesnokova V, Zonis S, Rubinek T, Yu R, Ben-Shlomo A, Kovacs K, Wawrowsky K, Melmed S. Senescence mediates pituitary hypoplasia and restrains pituitary tumor growth. Cancer Res. 2007;67:10564–10572. doi: 10.1158/0008-5472.CAN-07-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesnokova V, Zonis S, Kovacs K, Ben-Shlomo A, Wawrowsky K, Bannykh S, Melmed S. p21(Cip1) restrains pituitary tumor growth. Proc Natl Acad Sci USA. 2008;105:17498–17503. doi: 10.1073/pnas.0804810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones TH, Daniels M, James RA, Justice SK, McCorkle R, Price A, Kendall-Taylor P, Weetman AP. Production of bioactive and immunoreactive interleukin-6 (IL-6) and expression of IL-6 messenger ribonucleic acid by human pituitary adenomas. J Clin Endocrinol Metab. 1994;78:180–187. doi: 10.1210/jcem.78.1.8288702. [DOI] [PubMed] [Google Scholar]
- 10.Arzt E, Páez Pereda M, Perez Castro C, Pagotto U, Renner U, Stalla GK. Pathophysiological role of the cytokine network in the anterior pituitary gland. Frontiers in Neuroendocrinology. 1999;20:71–95. doi: 10.1006/frne.1998.0176. [DOI] [PubMed] [Google Scholar]
- 11.Arzt E. gp130 cytokine signaling in the pituitary gland: a paradigm for cytokine-neuro-endocrine pathways. J Clin Invest. 2001;108:1729–1733. doi: 10.1172/JCI14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arzt E, Buric R, Stelzer G, Stalla J, Sauer J, Renner U, Stalla GK. Interleukin involvement in anterior pituitary cell growth regulation: effects of IL-2 and IL-6. Endocrinology. 1993;132:459–467. doi: 10.1210/endo.132.1.8419142. [DOI] [PubMed] [Google Scholar]