Abstract
Conventional approaches for fat and water discrimination based on chemical shift fat suppression have reduced ability to characterize fatty infiltration due to poor contrast of microscopic fat. The multi-echo Dixon approach to water and fat separation has advantages over chemical-shift fat suppression: (1) water and fat images acquired in a single breath-hold, avoiding mis-registration, (2) fat has positive contrast, (3) compatible with pre-contrast and late enhancement imaging, (4) less susceptible to partial volume effects, (5) robust in the presence of background field variation, and (6) for the bandwidth implemented, chemical-shift artifact is decreased.
The proposed technique was applied successfully in all 28 patients studied: 10 studies with indication of CAD of which 4 cases with chronic MI exhibited fatty infiltration; 13 studies to rule out arrhythmogenic right ventricular cardiomyopathy (ARVC) of which there were 3 cases with fibro-fatty infiltration, 2 confirmed with ARVC; 5 cases of cardiac masses (2 lipomas). Precontrast CNR of intramyocardial fat was greatly improved, 240% relative to conventional fat suppression. For the parameters implemented, the SNR was decreased by 30% relative to conventional late enhancement. The multi-echo Dixon method for fat and water separation provides a sensitive means of detecting intramyocardial fat with positive signal contrast.
Keywords: MRI, heart, fat, chronic myocardial infarction, Arrhythmogenic Right Ventricular Dysplasia (ARVD), Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC), lipoma, phase sensitive inversion recovery (PSIR)
Introduction
The ability of MRI to discriminate between water and fat is important in tissue characterization. It has been shown that fibrofatty infiltration of the myocardium is associated with sudden death [1] and, therefore, non-invasive detection could have high prognostic value. Conventional approaches to fat and water discrimination based on fat suppression are commonly used to characterize masses but have reduced ability to characterize myocardial fatty infiltration due to the poor contrast of microscopic fat and partial volume effects. Multi-echo Dixon methods [2–10] for fat and water separation provide a sensitive means of detecting small concentrations of fat with improved contrast. In this study, these methods have been applied to the detection of fibro-fatty infiltration observed in chronic myocardial infarction (MI) as well cases of suspected arrhythmogenic right ventricular cardiomyopathy (ARVC). Fat and water separation has been implemented both pre-contrast as well as applied to late enhancement using a multi-echo PSIR-GRE sequence.
Approaches to characterizing tissue fat content include [9,10] (1) use of chemical shift saturation to suppress fat, (2) use of T1-weighted imaging to detect T1 shortening of fat which may be observed as bright signal intensity, and (3) use of multi-echo Dixon methods to reconstruct water and fat separated images [2–10]. In fat suppression imaging, regions with fat will appear dark, and the absence of signal relative to non-fat suppressed images will indicate presence of fat. A disadvantage of this approach is that it requires acquiring both fat suppressed and non-suppressed images. Furthermore, the performance of fat suppression depends on the field homogeneity. Importantly, for application in characterizing fatty infiltration in which voxels may have a low concentration of fat, the decrease in signal intensity (or negative contrast) is often difficult to discern from other signal intensity fluctuations. For this reason, the diagnosis of intramyocardial fat using conventional fat suppression can be subjective.
T1-weighted imaging may be used to detect fat [9,10] and T1-weighted inversion recovery (IR) has been used to detect fatty infiltration in chronic MI [11] prior to contrast administration. In this approach, an IR-cine SSFP sequence was used to acquire images at multiple inversion times. Regions with significant fat content were observed to have a shorter null time. Since this technique is performed prior to contrast, it may be difficult to precisely correlate with late enhancement images acquired at different cardiac phase and separate breath-holds. Further, additional pre-contrast breath-hold images must be acquired prior to having the late enhancement images which in some cases may be the first recognition of MI.
Multi-echo methods for fat and water separation are based on the difference in resonance frequencies between water and fat. Dixon’s original method for water and fat separation [2] acquires two images with different echo times chosen such that the water and fat are in-phase and opposed-phase, respectively, and may be combined to obtain separate water and fat images. This simple method assumes that the water is exactly on resonance which limits the performance of water and fat separation in the presence of B0-field inhomogeneity. A three-point method [3] was introduced to allow estimation of the background field at each voxel. In this method, the echo times are also set such that the water and fat are in-phase and opposed-phase in order to simplify estimation of the background fieldmap. However, phase ambiguities arising from large field variation can still lead to incorrect assignment of water and fat pixels. Phase unwrapping [4] has been proposed to deal with this issue, however, phase unwrapping methods are often not robust in cardiac imaging applications due to high field inhomogeneity [12] as well as relatively low SNR.
Recent methods [5–8] for multi-echo Dixon water and fat separation jointly estimate the fieldmap, water, and fat images. These methods apply spatial constraints in the process of fieldmap estimation which is generally more robust than phase unwrapping. These methods may also be used with arbitrary echo times which allows for more flexible sequence and protocol design. One method to solve this nonlinear estimation problem, termed iterative decomposition with echo asymmetry and least-squares (IDEAL) [5,6], consists of repeated linearizations of the original nonlinear problem, alternatively estimating the water and fat signals and the field map. A second method is the variable projection (VARPRO) method [8] which formulates the solution as a separable non-linear least squares problem. VARPRO attains the globally optimum maximum-likelihood (ML) solution and the implementation is very robust for the proposed cardiac application with large background field variation and low signal-to-noise ratio. VARPRO was found to be more robust [8] in terms of correct classification of water and fat than our implementation of IDEAL and was, therefore, used instead throughout the study.
The proposed use of multi-echo Dixon water-fat separation to chacterize intramyocardial fat has a number of potential benefits. The multi-echo Dixon method combined with fieldmap estimation [5–8] produces excellent discrimination between water and fat. The fat separated image provides positive contrast (containing only signal from lipids) which improves the diagnostic confidence. The proposed water and fat separation method may be combined with late enhancement to provide positive correlation between fibrosis and fat, which both appear bright post-contrast. The water and fat images are spatially registered since they are reconstructed from the same multi-echo dataset. Furthermore, chemical shift artifacts may be eliminated in reconstruction.
It was hypothesized that the multi-echo Dixon method of fat-water imaging could be used to detect intramyocardial fat. Multi-echo GRE fat-water imaging was performed on 28 patients with either known or suspected coronary artery disease, or with suspicion of intramyocardial fat.
Methods
Imaging
A multi-echo GRE sequence was implemented with fat and water separation using the VARPRO multi-point Dixon reconstruction method [8]. The imaging sequence was ECG triggered, with 2 R-R intervals between inversions, and used an echo-train readout with 4 echoes with flyback gradients for monopolar readout. The echo-train readout was used to increase the acquisition efficiency and thereby maintain acceptable breath-hold duration. Typical parameters for imaging with the MAGNETOM Espree (Siemens AG Medical Solutions, Erlangen, Germany) 1.5T short, wide bore scanner (33 mT/m, slew rate (SR)=100 T/m/s, 70 cm bore diameter, 1.25 m magnet length) were: bandwidth=977 Hz/pixel, TE= 1.64, 4.17, 6.7, and 9.23 ms, TR=11.2 ms, flip angle=20–25°, image matrix=256×126, views-per-segment=19, breath-hold duration=16 heartbeats including 2 initial heartbeats discarded for transition to steady state. PE oversampling was used in cases where the matrix size was rounded up due to segmentation.
The multi-echo GRE sequence incorporated an optional inversion recovery (IR) preparation for late enhancement imaging following contrast administration. Late enhancement imaging used phase sensitive reconstruction [13] for TI insensitivity and to eliminate artifacts arising from magnitude detection. The IR preparation is not used pre-contrast. The PSIR multi-echo GRE sequence acquires a proton density (PD) reference on alternate heartbeats which was used to jointly estimate a fieldmap and fat and water separation matrix that is applied to the inversion-recovery (IR) images. Sensitivity maps estimated from the complex PD images were used for B1-weighted combining with optimal noise weighting of multi-coil IR and PD fat and water images [13]. The PD images were also used for estimating the background phase for phase sensitive reconstruction and for correcting surface coil intensity variation as previously described [13]. The PSIR late enhancement sequence is essentially the same as the conventional PSIR late enhancement sequence [13] with modification for multi-echo readout.
The fat is shifted slightly in the readout direction relative to the water due to the difference in resonance frequencies [9,10]. With the bandwidth used in this study the chemical shift is approx 215 Hz / 977 Hz/pixel = 0.22 pixels. The sub-pixel shift is generally negligible at such large bandwidths but is easily corrected in the reconstruction. One approach [14] to correcting for chemical shift in multi-echo fat water separation is to correct for chemical shift by performing the fat-water separation in k-space after the background phase of each echo image due to field inhomogeneity has been removed.
Images were reconstructed off-line using MATLAB®. The VARPRO method [8] was used to robustly estimate the fieldmap in the presence of field inhomogeneity.
SNR Measurements
The SNR for various protocols was measured and compared. Comparison is made between (a) multi-echo Dixon water and fat separated late enhancement images and the conventional single echo late enhancement approach, (b) multi-echo Dixon water and fat separated images acquired pre- and post-contrast enhancement, and (c) multi-echo Dixon water and fat separated images acquired pre-contrast and conventional TSE with and without chemical shift fat suppression. SNR measurements were made in both phantoms and in-vivo. SNR scaled images [15] were reconstructed based on noise estimates derived from pre-scan noise acquisition.
The SNR of the water and fat separated late enhancement images will be reduced relative to the conventional single echo late enhancement approach due to the increase in signal bandwidth. The SNR loss due to the increased bandwidth is offset to some extent by the effective signal averaging gain from combining of multiple echo images in the process of separating water and fat images. The effective number of signal averages (NSA) for the parameters used was calculated from the Cramer-Rao lower bound (CRLB) as a function of the ratio of water to fat within a voxel [16]. The VARPRO method has been shown to meet the CRLB over a wide range of parameters [8]. The CRLB was computed for 4 echoes using the TE values of the protocol used in this study.
The pre-contrast protocol has higher SNR for the fat signal than the late enhancement protocol due to the incomplete T1-recovery following IR preparation. The water signal is approximately nulled for the late enhancement images. SNR comparison between pre-contrast and late enhancement using the measurement of the fat signal from epicardial fat in multi-echo Dixon fat separated images. SNR comparison was also made using measurements of oil phantom images using an inversion time (TI) = 300 ms which is typical for late enhancement. The SNR was measured and compared for both conventional single echo turbo-FLASH and multi-echo Dixon water and fat separated approaches using an oil phantom. The turbo-FLASH sequence used the same readout flip angle and matrix size, and had a bandwidth of 140 Hz/pixel (TE/TR= 3.2/7.6 ms).
SNR comparison of multi-echo Dixon water and fat separated images with conventional TSE were made using oil and water phantoms. In-vivo comparison of pre-contrast SNR of the fat separated images was made with the CNR of the fat region for pre-contrast TSE with and without chemical shift fat suppression. For the CNR measurement which is limited by the local signal inhomogeneity (see Discussion), the CNR was estimated as the signal difference between fat intensity with and without fat suppression divided by the standard deviation of a small region adjacent to the fat region in the fat suppressed image. The TSE protocol used a bandwidth of 780 Hz/pixel, echo-train length = 21, 180° readout flip angle. Parallel imaging with acceleration rate 2 was used for in-vivo imaging with TSE, however parallel imaging was not used for phantom measurements to simplify SNR measurements, therefore the phantom measurements will have approximately √2 higher relative SNR than for in-vivo.
Patient Studies
Imaging using multi-echo Dixon water and fat separation was performed on 28 patients with either known or suspected coronary artery disease (CAD), suspicion of cardiac tumors or masses, or to rule out ARVC under clinical research protocols approved by the Institutional Review Boards of the National Heart, Lung, and Blood Institute and Suburban Hospital, with written informed consent. In patient studies with known or suspected CAD (N=10), late enhancement using the conventional single echo turbo-FLASH sequence was performed, and multi-echo Dixon water and fat separated images were acquired post-contrast for all cases which exhibited MI. In several cases with known prior MI, multi-echo Dixon water and fat separated images were acquired pre-contrast (N=5) as well as post-contrast. Although fatty infiltration was not expected in cases of acute MI, water and fat separated imaging was performed to serve as a negative control. In studies with suspicion of cardiac masses (N=5), pre-contrast dark-blood prepared turbo-spin echo (TSE) imaging was performed with and without chemical shift fat saturation to assess tissue characteristics. In these cases, multi-echo Dixon water and fat separated images were acquired pre-contrast for all cases. In studies to rule out ARVC (N=13), the standard protocol used pre-contrast dark-blood prepared TSE imaging with and without chemical shift fat saturation to assess fatty infiltration and late enhancement to assess fibrosis. In these cases, multi-echo Dixon water and fat separated images were acquired pre-contrast for all cases.
Gadolinium late enhancement imaging was performed typically 10–20 minutes post contrast agent administration (0.15 mmol/kg dose).
Results
Phantom measurement & SNR calculation
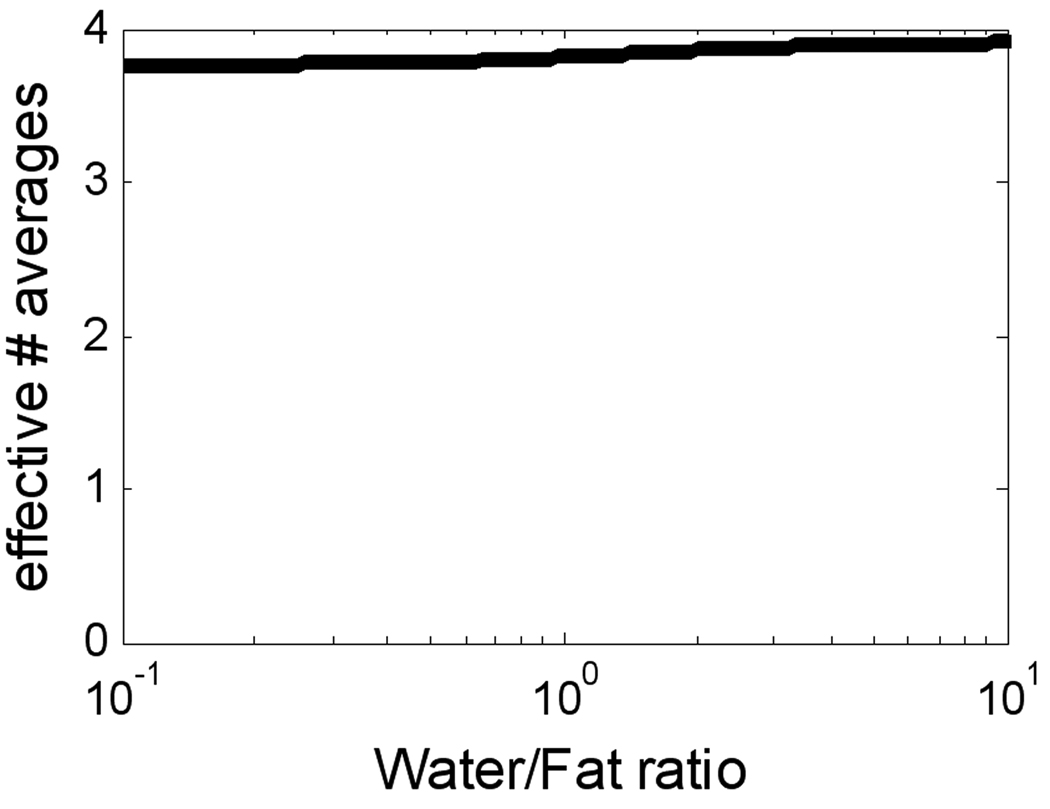
The effective number of signal averages for the specific 4-echo protocol that was used was calculated using the CRLB to be > 3.8 over a wide range of water/fat ratios (Fig. 1). The measured SNR loss of the water and fat separated late enhancement (phantom) image was approximately 30% relative to the single echo conventional late enhancement sequence, in close agreement with the predicted loss for these specific protocols (see Discussion). For the oil phantom, the measured SNR for the fat separated image using the multi-echo Dixon PSIR late enhancement protocol was 108.4 vs. 138.2 using the conventional PSIR late enhancement protocol.
Figure 1.
Effective number of signal averages for water and fat separation using 4-echo protocol used in study.
The SNR of the multi-echo Dixon fat separated image using the pre-contrast protocol was 192 (oil phantom) compared with the corresponding conventional TSE image, 396 (oil phantom). The multi-echo Dixon fat separated image has approximately ½ of the SNR of the TSE image (without parallel imaging).
The SNR of the multi-echo Dixon fat separated image was compared between pre-contrast protocol (192) vs late enhancement protocol (108.3). The pre-contrast protocol yielded approximately 1.8 times the SNR as the late enhancement protocol which uses inversion recovery.
Patient Images
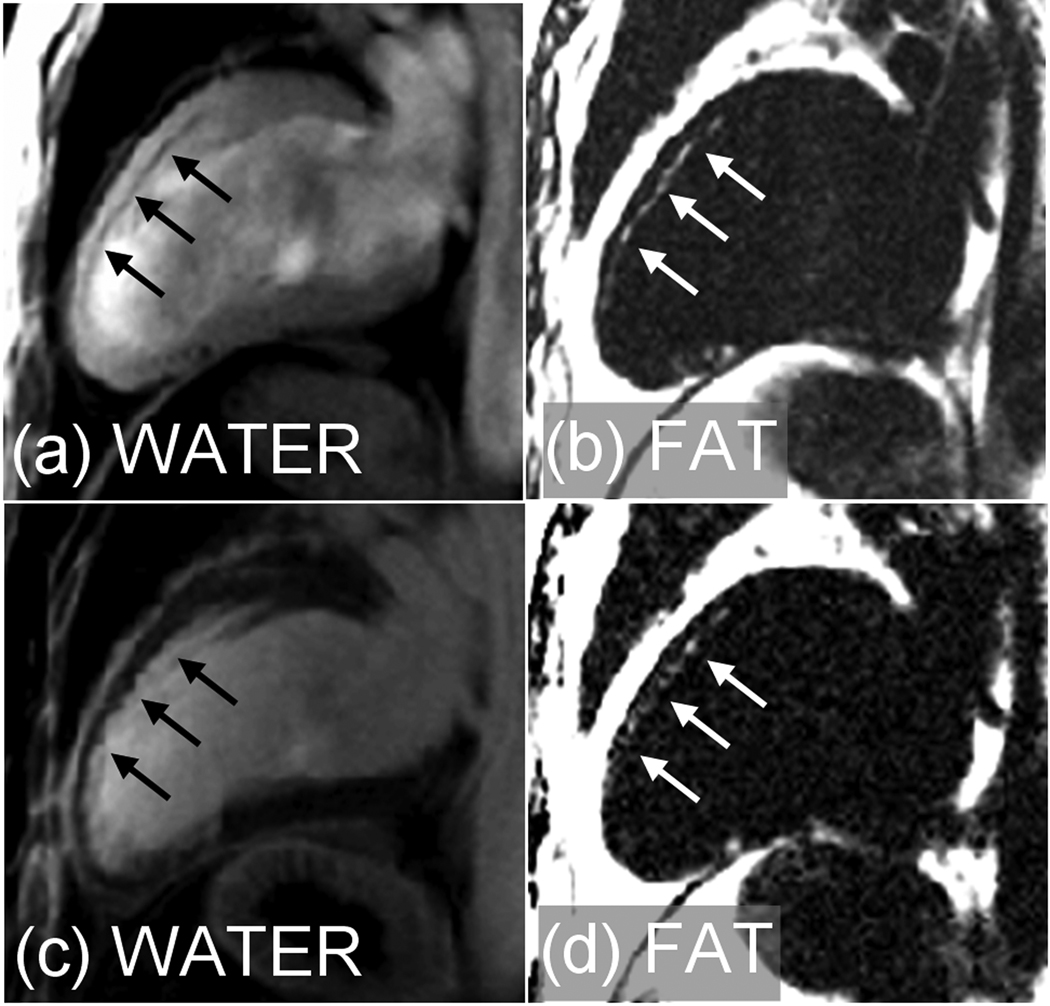
The technique was applied successfully in all 28 patients. Of the 10 studies with indication of CAD, there were 8 cases with MI (1 acute/7 chronic) of which 4 cases with chronic MI exhibited fatty infiltration. Water and fat separated images for a case with fatty infiltration in chronic anteroseptal MI are shown in Fig. 2 for both pre-contrast (a),(b) and PSIR late enhancement (c),(d). The phase encode (PE) direction is horizontal in all the figures. The measured SNR of the fatty infiltration was 4.0±1.4 (m±SD, N=4) measured by PSIR late enhancement. There were 5 chronic MI cases in which both pre- and post-contrast water and fat separated images were acquired. The SNR of epicardial fat was measured since only 2 of these 5 cases had fatty infiltration in the MI. The measured SNR of the epicardial fat signal was 71.7±29.5 pre-contrast vs 27.6±11.1 post-contrast, N=5 cases (m±SD). Water and fat separated PSIR late enhancement images (Fig. 3) are shown for 1 additional patients with chronic MI with fatty infiltration. The overall infarct image quality is quite good and comparable to conventional late enhancement without water and fat separation, with the water separated image yielding the same diagnostic quality as the conventional late enhancement image in all of the cases, despite slight reduction in SNR.
Figure 2.
Water and fat separated images using multi-echo Dixon method for patient with chronic MI showing fatty infiltration. (a),(b) pre-contrast and (c),(d) PSIR late enhancement.
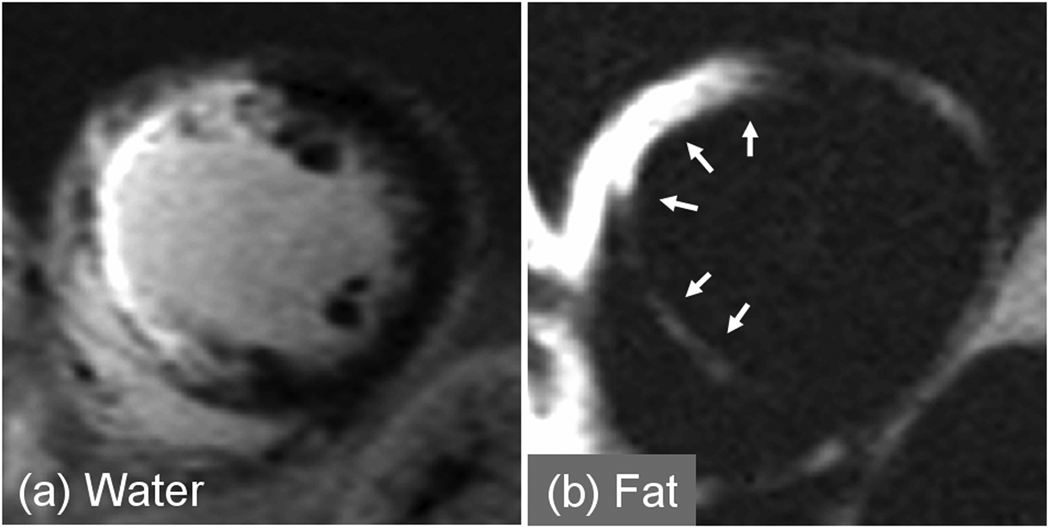
Figure 3.
PSIR water and fat separated late enhancement images acquired in a single breath-hold using multi-echo Dixon method for patient with chronic MI showing fibro-fatty infiltration (a) water, and (b) fat.
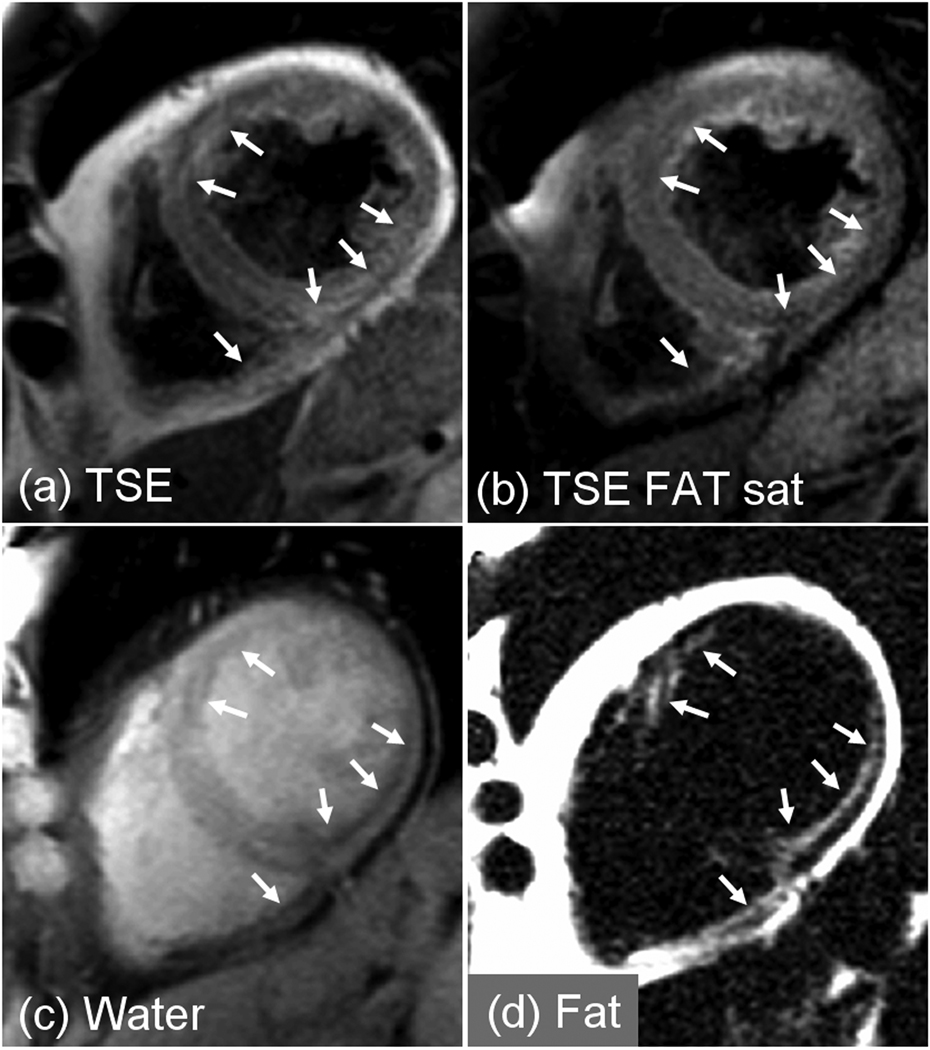
There were 13 studies to rule out ARVC with patients typically referred due to family history or arrhythmias. There were 4 cases of atypical late enhancement of which 3 cases had intramyocardial fatty infiltration (1 case confirmed by biopsy of the septum). In 1 case (Fig. 4), the patient was diagnosed with myocardial lipodystrophy since the case did not meet Task Force criteria for the diagnosis of ARVC. A 2nd case (Fig. 5) with fibrofatty infiltration (confirmed by needle biopsy) met the diagnostic Task Force criteria for ARVC (>2 major criteria of different categories). A 3rd case was confirmed to have ARVC, meeting 1 major and 2 minor Task Force criteria. A 4th ARVC rule out study met only a single major criteria for ARVC and although intramyocardial fat was present in the apical septal region, apical fat is a frequent finding in normal hearts [1]. The remaining 9 cases did not meet the criteria for ARVC.
Figure 4.
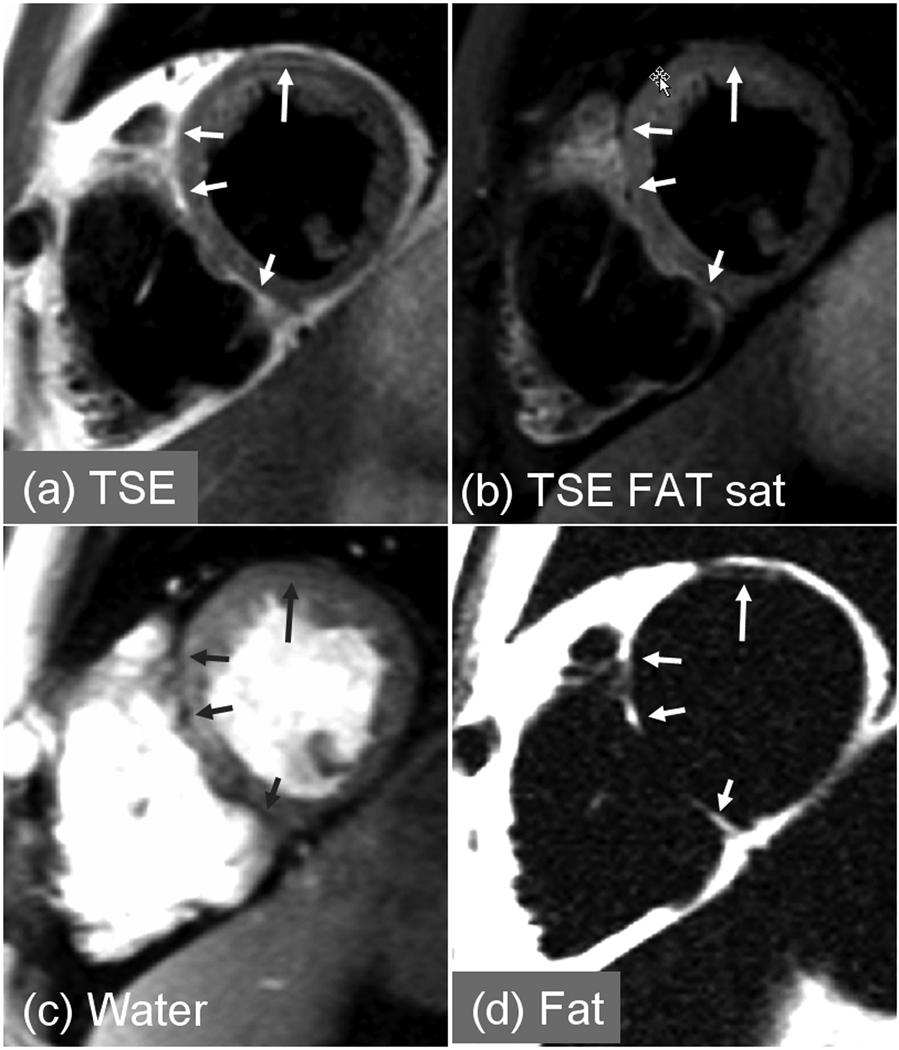
Patient with myocardial lipodystrophy. Intramyocardial fat clearly evident in the fat separated image using multi-echo Dixon method (d) which is difficult to discern in conventional fat suppressed image (b).
Figure 5.
Patient with ARVC showing intramyocardial fat clearly evident in the fat separated image (d) which is difficult to discern in conventional fat suppressed image (b).
In these ARVC rule study cases with fibrofatty infiltration, the multi-echo Dixon water and fat separated images acquired in a single breath-hold, are compared with the conventional approach using dark-blood prepared TSE acquired with and without chemical shift fat saturation in 2 separate breath-holds. In the 1st patient with fibro-fatty infiltration (Fig. 4) intramyocardial fat is clearly evident in the fat separated image (Fig. 4d) but difficult to discern in the conventional fat suppressed dark-blood TSE image (Fig. 4b). Fat is observed (see arrows) in endocardial regions of both LV and RV myocardium. In the second patient with confirmed ARVC (Fig. 5) intramyocardial fat is evident in the septum and anterior sector (longer arrow) of LV myocardium which is more readily discerned in the fat separated image (Fig. 5d) which has positive contrast, than by comparison of TSE images acquired with and without chemical shift fat suppression (Fig. 5(a),(b)).
In the above 3 cases in which fibrofatty infiltration was detected using the proposed multi-echo Dixon water and fat separation method, the measured CNR of the fat signal was 14.0±3.8 (mean±SD, N=3). In these same cases fatty infiltration could be detected in 2 of the 3 cases using the conventional TSE with and without chemical shift fat suppression, the measured CNR was 5.8±3.1 (N=2). Thus, the CNR of the multi-echo Dixon method was 2.4 times higher than conventional TSE in these cases. The TSE images for the 3rd case were acquired at a different slice orientation where fat was not detected.
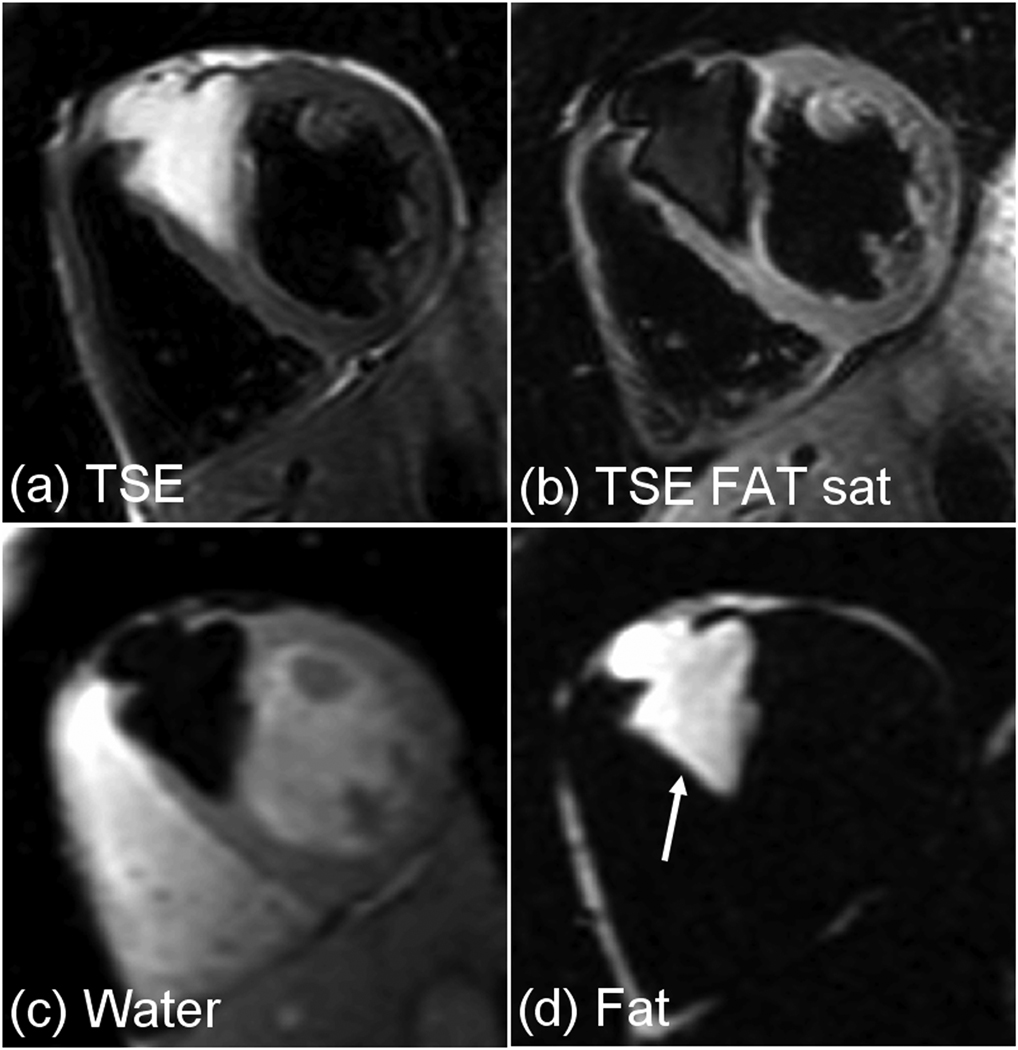
There were 5 patients referred for suspected cardiac masses. One patient had a large lipoma (Fig. 6) and another had a region with lipomatous hypertrophy of the interatrial septum. While fat suppression of the epicardial fat and the lipoma mass (Fig. 6b) was excellent, the water and fat separated images (Fig. 6(c) and (d)) had even greater contrast.
Figure 6.
Pre-contrast images for patient with large anteroseptal lipoma (a) TSE without fat saturation, and (b) TSE with fat saturation, acquired in 2 separate breath-holds, and (c) water , and (d) fat separated images acquired in a single breath-hold using a multi-echo Dixon approach.
Epicardial fat was readily distinguished from myocardium in all cases of water and fat separated images.
Discussion
The proposed approach can characterize intramyocardial fat before or after contrast administration. A benefit of using late enhancement with fat-water separation is the ability to display contrast enhanced myocardial fibrosis in the water image and fatty infiltration in the fat image, both acquired simultaneously. Initial experience indicates a much higher contrast and sensitivity than conventional fat suppression. The fat separated image has positive contrast against a noise background providing greater confidence in detection than conventional fat suppression which is often difficult to discriminate from fluctuations in the water signal. Consider the example of chronic MI shown in Fig. 3. In the septal region, the SNR of the MI water signal is approximately 38, and fat in this same region has an SNR of 5.3 corresponding to a water/fat signal ratio of 7.2 (i.e., <15% fat). The fat signal in the fat separated image is readily detectable against the noise background, however, if the myocardial signal fluctuation for a conventional fat suppressed image due to stagnant blood signal, Gibb’s ringing, or other effects was on the order of 10–20% then the detectability of the hypointense signal region due to fat would be greatly reduced. In the cases of Figure 4(b) and Figure 5(b) it would be difficult to make a diagnosis of intramyocardial fat with high confidence despite reasonably good fat saturation.
Dark-blood preparation commonly used with TSE imaging often leads to bright stagnant blood artifacts in the trabeculae and along the endocardial border as well as posterior wall signal loss due to cardiac motion which further complicates the image interpretation (Fig. 4). Therefore, although the dark blood prepared TSE has the potential to achieve high SNR, the loss in CNR due to signal inhomogeneity make this approach less reliable in clinical practice. The measured CNR of the myocardial infiltrated fat signal was 2.4 times better for the multi-echo fat separated image than using the dark blood TSE with chemical shift fat suppression. Furthermore, conventional fat suppression is highly dependent on the ability to shim the inhomogeneous background field.
The phase sensitive reconstruction is insensitive to TI which is particularly important when assessing atypical late enhancement with a patchy appearance. The proposed method has the additional benefit of using a single breath-hold to produce fat and water images, thereby improving the workflow and ensuring spatial registration. The VARPRO method provided robust fieldmap estimates.
Use of multi-echo Dixon fat-water separation with 3 echoes at non-optimum TE’s may be ill-conditioned and not achieve the full SNR gain (effective number of signal averages). Although optimum TE’s calculated for the case of 3-echoes [16] were not achievable using monopolar readout, the 4 echo implementation is very robust and achieved within 5% of the optimum expected SNR (4 effective averages) almost completely independent of the water/fat ratio (Fig. 1). Conventional late enhancement using a segmented turboFLASH sequence without water and fat separation uses a lower bandwidth with slightly shorter TR (e.g., 140 Hz/pixel and 8 ms). Using the parameters of this study, the SNR loss for the 4 echo water and fat separated approach compared to conventional is approximately or 32%. The actual SNR loss is slightly less due to the longer TR. The SNR loss may be reduced by using a lower bandwidth. Using the Siemens 1.5T MAGNETOM Avanto (45 mT/m, SR=200 T/m/s), the same TE’s and TR may be achieved using a bandwidth of 723 Hz/pixel. With this bandwidth, the SNR loss may be reduced to approximately 14%.
The SNR of pre-contrast multi-echo fat separated images was approximately 2.6 times that of post-contrast late enhancement using inversion recovery with the given protocol. The late enhancement fat signal depends on the inversion recovery time (TI). Despite the SNR loss, the added benefit of post-contrast late enhancement imaging is the positive correlation of fat with fibrous tissue in spatially registered images acquired simultaneously. The improved SNR of the pre-contrast images provides additional confidence in situations when both protocols may be acquired.
A benefit of the multi-echo Dixon method is the mitigation of the chemical shift artifact [9,10]. Conventional late enhancement imaging using 140 Hz/pixel has significant chemical shift artifact where the fat is displaced relative to the water in the readout direction by approximately 1.5 pixels. The multi-echo Dixon approach which uses a much larger bandwidth has a sub-pixel shift (0.2 pixel), and, if necessary, may be completely eliminated as described above in the Methods. Using the conventional approach, the epicardial fat may be displaced by as much as 30% of the diastolic wall thickness.
The presence of intramyocardial fat in diseases such as ARVC may form a substrate for reentrant ventricular arrhythmias leading to sudden death [1,17–20]. Analysis of autopsies has shown that fibrofatty infiltration into the myocardium was more predictive of sudden death than simply fatty infiltration [1]. However due to the subjectivity of interpreting the presence of intramyocardial fat using conventional fat suppression methods, MRI fibrofatty infiltration is not part of the current accepted Task Force criteria [17]. The proposed multi-echo Dixon method may be helpful in diagnosis of patients with ARVC due to the improved fat-myocardial contrast. The prognostic significance of fibrofatty infiltration in chronic MI is currently unknown but potentially of interest in predicting arrhythmias or prognosis. Pericardial fat accumulation may also be used as a predictor of coronary artery disease [21]. The fat separated images may be useful in quantifying the amount of pericardial fat.
Conclusion
The multi-echo Dixon method for fat and water separation provides a sensitive means of detecting intramyocardial fat with positive signal contrast, thereby achieving a high degree of confidence, whereas conventional fat suppression is often difficult to interpret due to fluctuations in the water signal. The proposed water and fat separation method is combined simultaneously with late enhancement imaging to provide positive correlation between fibrosis and fat. The proposed VARPRO approach to multi-echo Dixon water and fat separation is robust for clinical application to cardiac imaging. Using the proposed method, fibro-fatty infiltration has been observed in chronic MI as well as cases with ARVC. This technique could be used to assess the prognostic value of the presence and amount of myocardial fat infiltration.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Heart, Lung and Blood Institute; a Cooperative Research and Development Agreement between the National Heart, Lung and Blood Institute and Siemens Medical Solutions; and research grants: NIH P41-EB03631-16 and NIH-R01-CA098717.
References
- 1.Burke AP, Farb A, Tashko G, Virmani R. Arrhythmogenic Right Ventricular Cardiomyopathy and Fatty Replacement of the Right Ventricular Myocardium: Are They Different Diseases? Circulation. 1998 Apr 28;97(16):1571–1580. doi: 10.1161/01.cir.97.16.1571. [DOI] [PubMed] [Google Scholar]
- 2.Dixon W. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 3.Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magn Reson Med. 1991;18:371–383. doi: 10.1002/mrm.1910180211. [DOI] [PubMed] [Google Scholar]
- 4.Szumowski J, Coshow WR, Li F, Quinn SF. Phase unwrapping in the three-point Dixon method for fat suppression MR imaging. Radiology. 1994;192:555–561. doi: 10.1148/radiology.192.2.8029431. [DOI] [PubMed] [Google Scholar]
- 5.Reeder SB, Wen Z, Yu H, Pineda AR, Gold GE, Markl M, Pelc NJ. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn Reson Med. 2004;51:35–45. doi: 10.1002/mrm.10675. [DOI] [PubMed] [Google Scholar]
- 6.Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, Gold GE, Beaulieu CH, Pelc NJ. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast-spin echo imaging. Magn Reson Med. 2005 Sep;54:636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, Reeder SB, Shimakawa A, Brittain JH, Pelc NJ. Field map estimation with a region growing scheme for iterative 3-point water-fat decomposition. Magn Reson Med. 2005;54:1032–1039. doi: 10.1002/mrm.20654. [DOI] [PubMed] [Google Scholar]
- 8.Hernando D, Haldar JP, Sutton BP, Ma J, Kellman P, Liang Z-P. Joint Estimation of Water/Fat Images and Field Inhomogeneity Map. Magn Reson Med. doi: 10.1002/mrm.21522. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic resonance imaging: physical principles and sequence design. Vol. 1999. New York: John Wiley & Sons; pp. 421–449. [Google Scholar]
- 10.Stark DD, Bradley WG., Jr . Magnetic Resonance Imaging. St Louis: Mosby Inc; pp. 159–179. [Google Scholar]
- 11.Golfarb JW, Arnold S, Roth M, Han J. T1-Weighted Magnetic Resonance Imaging Shows Fatty Deposition After Myocardial Infarction. Magn Reson Med. 2007 May;57:828–834. doi: 10.1002/mrm.21207. [DOI] [PubMed] [Google Scholar]
- 12.Reeder SB, Faranesh AZ, Boxerman JL, McVeigh ER. In vivo measurement of T2* and field inhomogeneity maps in the human heart at 1.5 T. Magn Reson Med. 1998 Jun;39(6):988–998. doi: 10.1002/mrm.1910390617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase Sensitive Inversion Recovery for Detecting Myocardial Infarction using Gadolinium Delayed Hyperenhancement. Magn Reson Med. 2002 Feb;47(2):372–383. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu W, Reeder SB, Daniel BL, Hargreaves BA. Chemical Shift Correction in Bipolar Multi-Echo Sequences for Water and Fat Separation, ISMRM2007 abstract 1622 [Google Scholar]
- 15.Kellman P, McVeigh ER. Image Reconstruction in SNR Units: A General Method for SNR Measurement. Magn Reson Med. 2005 Dec;54:1439–1447. doi: 10.1002/mrm.20713. [Published erratum in Magn Reson Med 2007;58:211–212] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pineda AR, Reeder SB, Wen Z, Pelc NJ. Cramer–Rao Bounds for Three-Point Decomposition of Water and Fat. Magn Reson Med. 2005;54:625–635. doi: 10.1002/mrm.20623. [DOI] [PubMed] [Google Scholar]
- 17.Bluemke DA, Krupinski EA, Ovitt T, Gear K, Unger E, Axel L, Boxt LM, Casolo G, Ferrari VA, Funaki B, Globits S, Higgins CB, Julsrud P, Lipton M, Mawson J, Nygren A, Pennell DJ, Stillman A, White RD, Wichter T, Marcus F. MR Imaging of Arrhythmogenic Right Ventricular Cardiomyopathy: Morphologic Findings and Interobserver Reliability. Cardiology. 2003;99:153–162. doi: 10.1159/000070672. [DOI] [PubMed] [Google Scholar]
- 18.Tandri H, Castillo E, Ferrari VA, Nasir K, Dalal D, Bomma C, Calkins H, Bluemke DA. Magnetic Resonance Imaging of Arrhythmogenic Right Ventricular Dysplasia: Sensitivity, Specificity, and Observer Variability of Fat Detection Versus Functional Analysis of the Right Ventricle. J Am Coll Cardiol. 2006 Dec 5;48(11):2277–2284. doi: 10.1016/j.jacc.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 19.Tandri H, Saranathan M, Rodriguez ER, Martinez C, Bomma C, Nasir K, Rosen B, Lima JA, Calkins H, Bluemke DA. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005 Jan 4;45(1):98–103. doi: 10.1016/j.jacc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 20.Sen-Chowdhry S, Prasad SK, Syrris P, Wage R, Ward D, Merrifield R, Smith GC, Firmin DN, Pennell DJ, McKenna WJ. Cardiovascular magnetic resonance in arrhythmogenic right ventricular cardiomyopathy revisited: comparison with task force criteria and genotype. J Am Coll Cardiol. 2006 Nov 21;48(10):2132–2140. doi: 10.1016/j.jacc.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 21.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, Masuda Y. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001 Jul;157(1):203–209. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]