Abstract
During epididymal transit, sperm acquire the ability to initiate rapid forward progressive motility on release into the female reproductive tract or physiological media. Glycolysis is the primary source of the ATP necessary for this motility in the mouse, and several novel glycolytic enzymes have been identified that are localized to the principal piece region of the flagellum. One of these is the spermatogenic cell-specific type 1 hexokinase isozyme (HK1S), the only member of the hexokinase enzyme family detected in sperm. Hexokinase activity was found to be lower in immotile sperm immediately after removal from the cauda epididymis (quiescent) than in sperm incubated in physiological medium for 5 min and showing rapid forward progressive motility (activated). However, incubating sperm in medium containing diamide, an inhibitor of disulfide bond reduction, resulted in lower motility and HK activity than in controls. HK1S was present in dimer and monomer forms in extracts of quiescent sperm but mainly as a monomer in motile sperm. A dimer-size band detected in quiescent sperm with phosphotyrosine antibody was not detected in activated sperm, and the monomer-size band was enhanced. In addition, the general protein oxido-reductase thioredoxin-1 was able to catalyze the in vitro conversion of HK1S dimers to the monomeric form. These results strongly suggest that cleavage of disulfide bonds in HK1S dimers contributes to the increases in HK activity and motility that occur when mouse sperm become activated.
Keywords: disulfide bond reduction, epididymis, fibrous sheath, glycolysis, sperm, sperm activation, sperm capacitation, sperm maturation, sperm motility and transport
Sperm become motile when released from the epididymis into physiological media, and this is associated with a reduction of disulfide bonds in HK1S and an increase in HK activity.
INTRODUCTION
Sperm in the cauda epididymis are quiescent but become activated and display vigorous forward motility on release into the female reproductive tract or physiological media [1]. Although some of the molecular changes that occur with the later process of capacitation have been identified [1–3], little is known about the changes that occur during the short period when quiescent sperm become activated.
Several glycolytic enzymes present in mammalian sperm are products of novel genes or splice variants expressed only in spermatogenic cells and present in the principal piece of the flagellum [4–7]. Previous in vitro studies strongly had suggested that glycolysis contributes to the generation of ATP required for sperm motility [e.g., 8], but recent in vivo studies showed unequivocally that glycolysis is essential for mouse sperm function. Male mice with a targeted mutation of the spermatogenic cell-specific Gapdhs gene were infertile and their sperm were deficient in ATP and immotile [9].
We previously identified three spermatogenic cell-specific transcript variants for type 1 hexokinase (Hk1_v1, Hk1_v2, and Hk1_v3). The three transcript variants encode a novel spermatogenic cell-specific region (SSR) that replaces the amino-terminal porin binding domain (PBD) of the HK1 isoform present in somatic cells [10]. The Hk1_v2 and Hk1_v3 transcripts are responsible for the spermatogenic cell-specific type 1 hexokinase (HK1S) isozyme found in sperm [11]. HK1S is present mainly in the principal piece region of the sperm flagellum [12] and is tyrosine-phosphorylated when typical sperm collection procedures are used [12, 13]. Hexokinase is the first enzyme in the glycolytic pathway and a key regulator of glycolysis [14]. However, the relationship between HK1S and ATP production in the activation of sperm motility remains to be determined. We hypothesized that conversion of sperm from the quiescent to the activated state involves an increase in HK1S activity that leads to enhancement of glycolysis and increased production of ATP required for motility. The studies reported here demonstrate that reduction of disulfide bonds in HK1S is associated with an increase in hexokinase activity and the initiation of sperm motility, findings consistent with this hypothesis.
MATERIALS AND METHODS
Materials
Reagents were obtained from Sigma-Aldrich ( http//:www.sigma-aldrich.com ) unless otherwise indicated.
Animals
The CD-1 male mice used in this study were obtained from Charles River Laboratories ( http://www.criver.com ). All procedures involving mice were carried out according to U.S. Public Health Service guidelines and were approved in advance by the NIEHS Institutional Animal Care and Use Committee.
Preparation of Lysates from Quiescent, Activated, and Capacitated Sperm
Quiescent sperm lysates were prepared by collecting sperm directly from the caput or cauda epididymis into Eppendorf tubes without dilution and adding 50–100 μl of 20 mM PBS (pH 7.2) containing 0.1% Triton X-100 (PBSTX). After 20 min on ice, the samples were centrifuged at 4500 × g for 5 min at 4°C and the lysate and pellet fractions collected. For one study, quiescent sperm were lysed in PBSTX containing 1 mM diamide. Activated sperm lysates were prepared by making small cuts in the cauda epididymis and allowing sperm to disperse into 2 ml of M2 medium (Sigma) for 5 min at room temperature (RT). The sperm were washed twice in PBS by centrifugation at 7600 × g for 2 min at RT, 50–100 μl PBSTX were added, and lysates and pellets were collected as described previously. In an experiment to determine possible effects of epididymal fluid, sperm were collected directly into Eppendorf tubes without dilution, transferred into capillary tubes, and centrifuged in a Micro-Hematocrit II centrifuge (Becton-Dickinson; http://www.bd.com ) at full speed for 3 min at RT. The tube was scored and separated at the sperm/fluid interface; sperm were transferred into an Eppendorf tube and further processed as described.
To prepare capacitated sperm samples [15], small cuts were made in the cauda epididymis, and sperm were allowed to disperse into 2 ml PBS for 5 min at 37°C. After a brief low-speed centrifugation (100 × g for 15 sec at RT) to remove debris, the sperm was centrifuged at 800 × g for 8 min at 4°C and resuspended in M16 medium (Sigma), and aliquots were either processed as described in the following or incubated at 37°C in 5% CO2/95% air for 2 h. Sperm were lysed by adding 50–100 μl of PBSTX containing 0.2 mM sodium orthovanadate and 0.1 mM phenylmethylsulphonyl fluoride (PMSF).
The protein concentration of the sperm lysates were determined by spectrophotometry using Bradford reagent (Bio-Rad Laboratories; http://www.bio-rad.com ).
For immunoblotting, samples were suspended in 2× sample buffer (4% SDS, 100 mM Tris-HCl (pH 6.8), 20% glycerol, and 0.001% bromophenol blue) under either nonreducing or reducing (addition of 2% β-mercaptoethanol [BME]) conditions.
Labeling with Monobromobimane
Sperm were labeled with monobromobimane (mBBr; Invitrogen; http://www.invitrogen.com ) following a procedure described previously [16, 17]. Sperm from caput or cauda epididymis were incubated in PBS with or without 2.5 mM N-ethylmaleimide (NEM) for 30 min at 37°C, washed two times with PBS, and incubated in PBS with or without 1 mM dithiothreitol (DTT) for 10 min at RT. After being washed two times in PBS, sperm were incubated in PBS containing 3 mM mBBr for 10 min at RT, washed twice with PBS, and observed by fluorescence microscopy. The purpose of NEM was to protect disulfide linkages from reduction, of DTT was to reduce disulfide linkages and of mBBr was to detect free thiol groups. Images of each sample were captured using the same exposure time with a Zeiss Axioplan microscope equipped for epifluorescence illumination (filters for peak excitation at 365 nm, cutoff at 395 nm, and emission above 420 nm), a QImaging QICAM digital camera, and QCapture 2 software ( http//:www.qimaging.com ).
Hexokinase Assay
Quiescent and activated sperm were treated with PBSTX as described previously, sonicated, and centrifuged at 4500 × g for 5 min at 4°C. The lysates were collected and the pellets resuspended in an equal volume of PBSTX. The hexokinase activity assay was adapted from a procedure described previously [18, 19], with reduction of disulfide bonds in insulin used as a positive control. Fifty microliters of sample containing 0.8–2.5 μg protein were added to 950 μl assay solution containing 20 mM Tris-HCl (pH 7.5), 20 mM MgCl2, 4 mM EDTA, one unit/ml G6PDH, 10 mM glucose, 0.6 mM βNADP+, and 0.1% Triton X-100. After preincubation for 3 min at 30°C, hexokinase (HK) activity was initiated by adding 4 mM ATP, and NADPH production was measured at 340 nm by spectrophotometry after 12 min. Glucose was excluded from the control assay. Sample protein concentration was determined by spectrophotometry using Bradford reagent.
Treatment with Inhibitors
To inhibit phosphatases, sperm from the cauda epididymis were incubated in 1 ml of M2 medium containing 1 or 10 μM of okadaic acid (OA) (EMD Biosciences; http://www.emdbiosciences.com ) dissolved in 70% ethanol or in M2 medium containing an equivalent amount of ethanol alone as vehicle control. To inhibit reduction of disulfide bonds, sperm were incubated in 1 ml of M2 medium containing 0 (control), 0.1, 1, 5, or 10 μl of 1 M diamide in PBS as described [20]. Addition of diamide did not change the pH of the medium. These samples were separated by SDS-PAGE and immunoblotted as described in the following.
In Vitro Thioredoxin Assay
The reduction of HK1S in vitro was assayed as described [21] using human recombinant thioredoxin-1 (TXN1). Sperm lysate was added to 100 μM phosphate buffer (pH 7.0) containing 10 μM human recombinant TXN1 (T8690, Sigma), 0.1 mM DTT was added to initiate the reaction, and samples were collected after incubation at 30°C for 10 min. The samples were suspended in 4× sample buffer (8% SDS, 200 mM Tris-HCl, pH 6.8, 40% glycerol, and 0.002% bromophenol blue) without BME, subjected to PAGE under nonreducing conditions, and immunoblotted as described in the following.
Immunoprecipitation
Quiescent sperm were suspended in RIPA buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% deoxycholic acid, 0.5% Triton X-100, and one tablet of protease inhibitor cocktail per 10 ml of RIPA buffer [Roche Applied Science; http://www.roche-applied-science.com ]). Supernatants were collected by centrifugation at 15 800 × g for 30 min at 4°C, 5–10 μg of antibody to phosphotyrosine (P-Tyr) (Millipore, clone 4G10; http://www.millipore.com ) or HK1 (Millipore, MAB1532) was added, and the samples were kept at 4°C overnight. Immunopure immobilized protein G beads (Pierce Biotechnology; http://www.piercenet.com ) were added, incubated for 2 h at 4°C, collected by centrifugation, and washed four times with PBS. Proteins were released from the beads by heating at 95°C for 5 min in 2× sample buffer containing BME, and immunoblotting was performed as described in the following.
Immunoblotting
Samples were separated by SDS-PAGE on 4%–20% gradient Ready Gels or 10% Ready Gels (Bio-Rad Laboratories), and the proteins were transferred from the gels to Immobilon-P PVDF membranes (Millipore). The membrane was probed with antibodies to HK1 (Millipore, MAB1532; Santa Cruz clone H-95; http://www.scbt.com ), P-Tyr (4G10; Millipore), SSR (the spermatogenic-cell specific region of HK1S) [10], or α-tubulin (T5168; Sigma-Aldrich), and the antigens were detected using ECL reagents (GE Lifesciences, RPN2106; http://www.gelifesciences.com ) as recommended by the supplier. The membrane was stained with Coomassie Brilliant Blue R-250 (CBB) Staining Solution (Bio-Rad Laboratories) to detect proteins.
Two-Dimensional PAGE
Lysates of diamide-treated or untreated quiescent sperm were separated in the first dimension under nonreducing conditions on 4%–20% gradient Ready Gels. The protein-containing lane was cut from the gel, immersed in 2× sample buffer containing 2% BME for 10 min at RT, and placed in the sample well of a 10% gel, and proteins were separated in the second dimension under reducing conditions. Proteins were transferred from the first and second gels to a PVDF membrane, the membrane was immunostained with antibodies to SSR and P-Tyr, and the antigens were detected using ECL reagents as described previously.
Band Intensity Analysis
The intensity of bands was measured using Image J software (NIH, version 1.38; http://rsb.info.nih.gov ).
Sperm Motility Measurements
Quantitative parameters of sperm motility were determined by computer-assisted sperm analysis (CASA). Sperm from the cauda or caput epididymis were incubated in M2 medium at RT for 5 min and loaded into assay chambers. Sperm tracks (1.5 sec, 30 frames) were captured at 60 Hz and analyzed using HTM-IVOS Sperm Analyzer software (Hamilton Thorne Biosciences, version 12.2L; http://www.hamiltonthorne.com ).
ATP Assay
Quiescent sperm collected in Eppendorf tubes were suspended in 50 μl of PBSTX. Activated sperm were suspended in 50 μl of PBSTX after being incubated in M2 medium for 5 min at RT with or without inhibitors (described previously) and washed in PBS twice by centrifugation at 900 × g for 3 min. The sperm samples were then suspended in 450 μl of preheated extraction buffer (4 mM EDTA/0.1 mM Tris-HCl, pH 7.75) and incubated at 100°C for 2 min. After centrifugation at 20 000 × g for 5 min, the supernatant was collected and ATP measured in duplicate 50-μl aliquots of the supernatant using the ATP Bioluminescence Assay Kit CLS II (Roche Applied Science; http://www.roche-applied-science.com ). Protein concentrations of the soluble sperm fractions were measured by spectrophotometry using Bradford reagent.
RESULTS
HK Activity and ATP Levels Are Lower in Quiescent Sperm Than in Activated Sperm
Glycolysis is the main source of ATP required for motility by sperm in mice [9, 22], and we hypothesized that glycolysis is less active in quiescent than in activated sperm. The only hexokinase detected in mouse sperm is HK1S [11], and total HK activity was 2-fold higher in total activated sperm than in total quiescent sperm (Fig. 1A). This was unchanged when epididymal fluid was removed from quiescent sperm samples by centrifugation (data not shown). However, the increase in HK activity was 4-fold higher in lysates of activated sperm than in lysates of quiescent sperm (Fig. 1A). The HK activity also was measured in quiescent sperm isolated from the caput epididymis and was lower than in quiescent sperm isolated from the cauda epididymis (Fig. 1B). In addition, the ATP levels also were significantly higher in activated than in quiescent sperm (Fig. 1C).
FIG. 1.
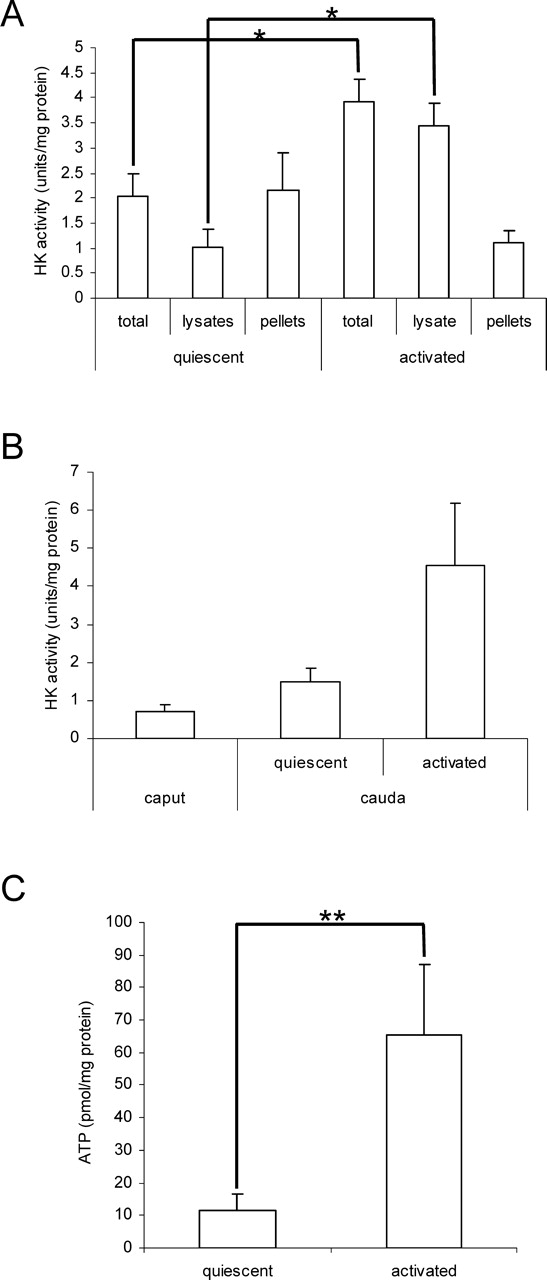
HK activities and ATP levels in quiescent and activated mouse sperm. A) Sperm were assayed immediately after removal from the cauda epididymis (quiescent) or after incubation for 5 min in M2 medium (activated). HK activity was determined in total sperm, PBSTX lysates, and pellets of PBSTX extracted sperm. Data are expressed as the mean ± SD; n = 3, (P < 0.01). B) HK activity was assayed in total sperm immediately after removal from the caput epididymis (quiescent) and in total sperm from the cauda epididymis (quiescent and activated). Data are expressed as the mean ± SD, n = 3 (caput) and n = 5 (cauda). C) ATP activity was assayed in total sperm from the cauda epididymis (quiescent and activated). Data are expressed as the mean ± SD, n = 3 (quiescent) and n = 5 (activated), (P < 0.05).
HK1S Is Mainly a Dimer in Quiescent Sperm and a Monomer after Activation
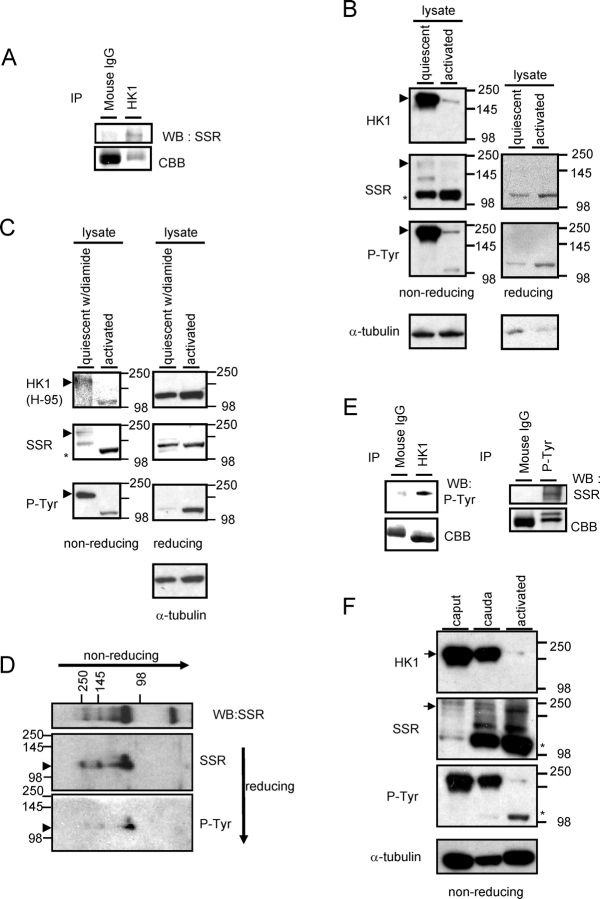
To determine the condition of HK1S protein in quiescent and activated sperm, lysates and pellets were prepared, separated by PAGE under nonreducing conditions, and examined by immunoblotting. A monoclonal antibody (MAB1532) that recognizes a sequence common to HK1 and HK1S was used to detect HK1S on immunoblots of sperm. This was possible because HK1S is the only hexokinase detected in sperm [11]. Immunoprecipitation with HK1 (MAB1532) and immunoblotting with the SSR antiserum confirmed that these antibodies recognize the HK1S protein in sperm (Fig. 2A). The HK1 (MAB1532) antibody recognized a major dimer-size band in quiescent sperm lysates separated under nonreducing conditions (Fig. 2B; HK1, arrowhead). The dimer was substantially more abundant in the lysate than in the pellet (data not shown). In addition, the SSR antibody and another antiserum to HK1 (H-95) recognizing different sequences in the HK1S protein detected minor dimer-size bands in lysates of quiescent sperm separated under nonreducing conditions (Fig. 2, B and C, left panel, SSR, arrowhead; Fig. 2C, left panel, HK1 [H-95]; Supplemental Fig. 1 available at www.biolreprod.org ). Furthermore, HK1 (H-95) recognized monomers under reducing conditions (Supplemental Fig. 1 available at www.biolreprod.org ). The differences in band intensity with the three different antibodies were presumably due to epitope accessibility under nonreducing conditions. Although changes in phosphorylation might also affect antibody binding, only the sequence recognized by HK1 (MAB1532) contains a tyrosine, and the sequences recognized by HK1 (H-95) and SSR antisera do not.
FIG. 2.
HK1S detection by nonreducing and reducing PAGE and immunoblotting. A) Quiescent sperm lysates were immunoprecipitated with antibody to HK1, subjected to PAGE, and immunoblotted with antibody to SSR domain of HK1S. Mouse IgG was used as negative controls. Staining by CBB (lower panels) is indicative of the relative amount of protein in the corresponding region of the gel. B) Left panel is nonreducing PAGE, right panel is reducing PAGE: lysates were prepared from quiescent and activated sperm as described in Materials and Methods. Immunoblots were probed with antibodies to HK1 (MAB1532), the SSR domain of HK1S, P-Tyr, and α-tubulin (loading control). All assays were repeated at least four times with similar results, and representative examples are shown. C) Samples in left panel are lysates separated by PAGE under nonreducing conditions, and samples in right panel are lysates separated by PAGE under reducing conditions. Lysates were prepared from quiescent sperm in PBSTX containing 1 mM diamide and from activated sperm as described in Materials and Methods. Immunoblots were probed with antibodies to HK1 (H-95), the SSR domain of HK1S, P-Tyr, and α-tubulin (loading control). All assays were repeated at least three times with similar results, and representative examples are shown. D) Nonreducing PAGE using quiescent sperm lysate was run in the first dimension, and reducing PAGE was run in the second dimension. Immunoblotting was performed with antibodies to the SSR domain of HK1S and P-Tyr. E) Quiescent sperm lysates were immunoprecipitated with HK1 (MAB1532) antibody (left panel), subjected to PAGE, and immunoblotted with antibody to P-Tyr. Quiescent sperm lysates were immunoprecipitated with antibody to P-Tyr, subjected to PAGE, and immunoblotted with antibody to the SSR domain of HK1S (right panel). Rabbit or mouse IgG was used as a negative control. Staining by CBB (lower panels) is indicative of the relative amount of protein in the corresponding region of the gel. F) Lysates of quiescent sperm from the caput and cauda epididymis and lysates of activated sperm from the cauda epididymis were subjected to nonreducing PAGE and immunoblotting with antibodies to HK1 (MAB1532), the SSR domain of HK1S, P-Tyr, and α-tubulin (loading control). All assays were repeated three times with similar results, and representative examples are shown.
Monomer-size bands were not recognized by the monoclonal antibody to HK1 (MAB1532) under nonreducing conditions (Fig. 2B, HK1), but they were recognized by the SSR antiserum and HK1 (H-95) antiserum in both quiescent and activated sperm lysates under reducing conditions (Fig. 2C, right panel, SSR, asterisk; Fig. 2C, right panel, HK1 [H-95]). Taken together, these results suggest 1) that HK1S is present mainly as a dimer in quiescent sperm and a monomer in activated sperm, 2) that the conversion from dimer to monomer involves reduction of disulfide linkages between HK1S subunits, and 3) that the reduction alters accessibility of the different epitopes recognized by the HK1 (MAB1532) monoclonal antibody, HK1 (H-95) antiserum, and SSR antiserum.
HK1S Phosphotyrosine Levels Are Reduced with Sperm Activation
Sperm lysates were examined by PAGE under nonreducing conditions and immunoblotting with a P-Tyr antibody to determine if changes occur in HK1S tyrosine phosphorylation with sperm activation. While the P-Tyr antibody recognized a prominent band in sperm lysates corresponding in size to the HK1S dimer (Fig. 2B; P-Tyr, arrowhead), only faint dimer- and monomer-size P-Tyr bands were seen following activation for 5 min (Fig. 2B). The bands recognized by P-Tyr antibody were confirmed to be HK1S by two-dimensional PAGE and immunoblotting (Fig. 2D; arrowheads) and by immunoprecipitation (Fig. 2E).
Under nonreducing conditions, the P-Tyr and HK1 antibodies detected dimer-size bands in lysates of quiescent caput sperm that were substantially more prominent than the bands in lysates of activated cauda sperm (Fig. 2F; arrows). In contrast, the monomer-size band recognized by the SSR antibody was considerably more prominent in lysates of quiescent cauda sperm than in lysates of quiescent caput sperm (Fig. 2F; asterisks).
Lysates and pellets of activated sperm and of activated sperm incubated under capacitating conditions for 2 h were subjected to PAGE under reducing conditions followed by immunoblotting with SSR and P-Tyr antibodies. An apparent reduction in immunostaining of the HK1S monomer in the lysate occurred with both antibodies (Supplemental Fig. 2 available at www.biolreprod.org ).
These results indicate that a substantial decrease occurs in the ratio of dimer to monomer and in the tyrosine phosphorylation of HK1S when sperm undergo conversion from quiescent to activated.
Disulfide Bond Reduction Occurs Independently of HK1S Dephosphorylation
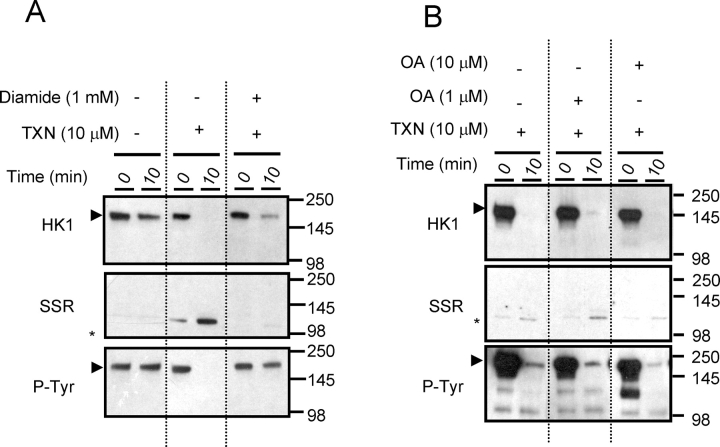
The above studies indicated that when sperm are converted from the quiescent to the activated state, HK1S undergoes both disulfide bond reduction and tyrosine dephosphorylation. To determine the relationship between these processes, we examined the effects of diamide and TXN1 [21] on the dimer-to-monomer conversion in quiescent cauda sperm lysates. The outcome of the assays was determined by PAGE under nonreducing conditions followed by immunoblotting. The cleavage of disulfide bonds in insulin was used as a positive control (data not shown). The conversion of HK1S from dimer (detected with HK1 [MAB1532] and P-Tyr antibodies) to monomer (detected with SSR antibody) occurred by 10 min when TXN1 was present but not when TXN1 was absent or diamide was present (Fig. 3A). In contrast, the TXN1-catalyzed dimer-to-monomer conversion was not prevented in quiescent sperm lysates treated with OA to inhibit serine/threonine phosphatases (Fig. 3B). These results indicate that dephosphorylation of HK1S is not required for dimer-to-monomer conversion and that dephosphorylation occurs independently of disulfide bond reduction.
FIG. 3.
In vitro TXN1 assay with lysates of quiescent cauda epididymal sperm. A) Lysates were incubated for 0 or 10 min in the presence or absence of 1 mM diamide and 10 μM TXN1, subjected to nonreducing PAGE, and immunoblotted with antibodies to HK1 (MAB1532), the SSR domain of HK1S, and P-Tyr. B) Lysates were incubated for 0 or 10 min in the presence or absence of 1 or 10 μM of OA and/or 10 μM TXN1, subjected to nonreducing PAGE, and immunostained with antibodies to HK1 (MAB1532), the SSR domain of HK1S, and P-Tyr.
Effects of Disulfide Bond Reduction on Sperm Motility and HK1S Activity
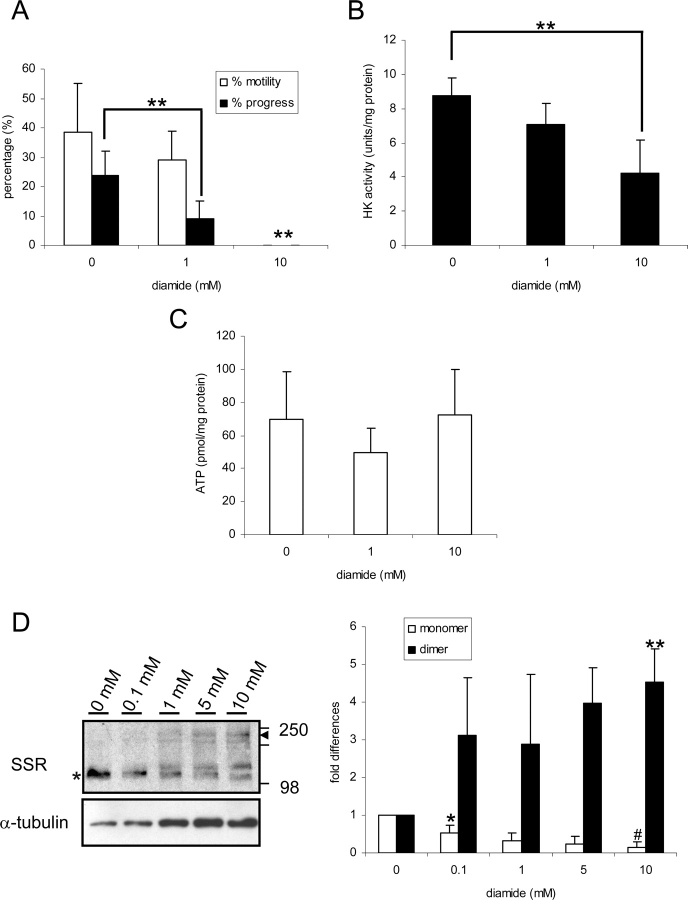
The above studies using sperm lysates showed that during conversion from the quiescent to the activated state, 1) HK1S dimers become monomers, 2) HK1S activity increases, and 3) blocking disulfide bond reduction with diamide blocks the HK1S dimer-to-monomer conversion. The following studies determined if blocking disulfide bond reduction within live sperm with 1 mM or 10 mM diamide alters sperm motility or HK1S activity. Progressive motility was lower in sperm treated with 1 mM diamide than in controls, and no motility was seen with 10 mM diamide treatment (Fig. 4A). The HK1S activity also was lower in sperm treated with 10 mM diamide compared to controls (Fig. 4B). However, the effect of diamide did not appear to be due to cytotoxicity because ATP levels were not changed significantly by 1 mM or 10 mM diamide treatment (Fig. 4C). No changes were seen in motility, HK activity, or ATP levels when OA was added to the medium (Supplemental Fig. 3 available at www.biolreprod.org ). In addition, there appeared to be a decrease in HK1S monomers and an increase in HK1S dimers when sperm were treated with increasing concentrations of diamide (Fig. 4D). These results strongly suggest that reduction of disulfides to thiols is necessary for the initiation of sperm motility and increase in HK1S activity.
FIG. 4.
Effects of diamide on motility, HK activity, and ATP levels in sperm from the cauda epididymis. A) Sperm were incubated in the presence of 0, 1, or 10 mM diamide as described in Materials and Methods. The percentages of sperm that were motile and of sperm showing progressive motility are shown (** = significantly different, P < 0.05). B) HK activity in the presence of 0, 1, or 10 mM diamide (** = significantly different, P < 0.05). C) ATP levels in the presence of 0, 1, or 10 mM diamide. D) Effects of diamide concentration on HK1S bands recognized by SSR antibody. Sperm were incubated in the presence of 0, 0.1, 1, 5, or 10 mM diamide; lysates were subjected to nonreducing PAGE, and immunoblotting was done with antibody to the SSR domain of HK1S and with antibody to α-tubulin as loading control. Expression levels are shown as fold differences relative to the level on 0 mM diamide, with that level set at 1. Data are expressed as a mean ± SD of three different experiments (* = significantly different, P < 0.05; ** = significantly different, P < 0.01; # = significantly different, P < 0.001).
Sperm Thiol Proteins Are Oxidized in the Epididymis
To determine if these findings were a reflection of the general redox status of thiol proteins in the epididymis, sperm were labeled with mBBr to detect free thiols [16, 23]. Cauda sperm incubated for 30 min in PBS were moderately labeled with mBBr in the head, middle, and principal pieces (Fig. 5A), but addition of the disulfide bond-reducing reagent DTT resulted in strong mBBr labeling over the head, middle piece, and principal piece regions (Fig. 5B). The sperm head expanded noticeably with DTT treatment (Fig. 5B). In contrast, cauda sperm incubated in PBS containing NEM to protect against disulfide bond reduction labeled weakly with mBBr only in the middle piece (Fig. 5, C and E). At the concentrations used in these studies, the effect of NEM was abrogated by addition of DTT (Fig. 5D). These observations are consistent with studies in other species indicating that sperm thiols are oxidized and form disulfide bonds in the epididymis [16, 23]. The labeling by mBBr of the principal piece region in the sperm flagellum corresponds to the location of HK1S in mouse sperm, and the increased mBBr binding to this region after DTT treatment is consistent with the effect of diamide on sperm HK1S activity, HK1S dimer-to-monomer conversion, and sperm motility.
FIG. 5.
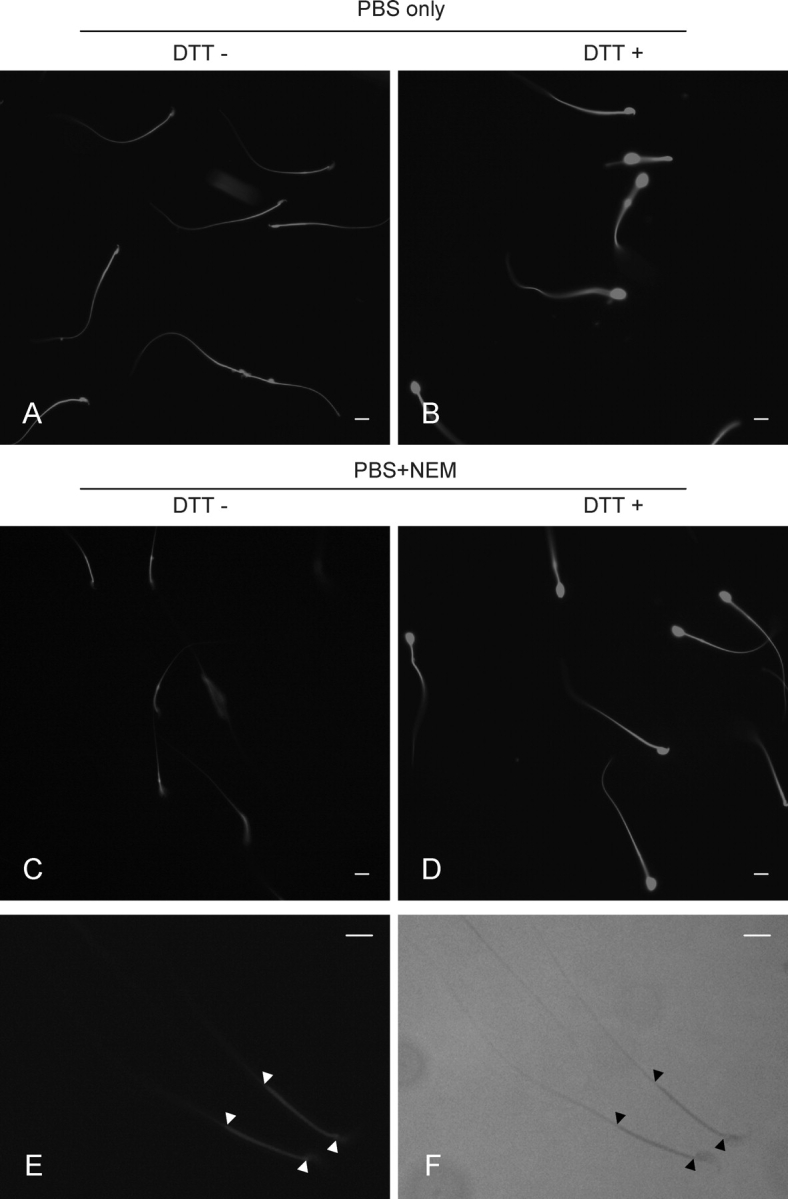
Oxidization of thiol proteins in cauda sperm assayed by mBBr labeling. A) Sperm incubated with PBS alone. B) Sperm incubated in PBS containing DTT. C) Sperm incubated in PBS containing NEM. D) Sperm incubated in PBS, NEM, and DTT. E) Higher magnification of sperm incubated in PBS containing NEM. F) Phase image of same sperm in E. Bars = 8 μm. The exposure time was the same for each panel.
DISCUSSION
Sperm in the cauda epididymis are immotile and quickly become motile when diluted in physiological media, but little is known about the changes that occur with activation of sperm motility. We predicted that one change would be an increase in ATP production by glycolysis. Although oxidative phosphorylation is the main source and glycolysis is a minor source of ATP in most cell types, fertilization was not prevented by pharmacological inhibition of oxidative phosphorylation [22, 24] or disruption of the testis-specific cytochrome C (Cyct) gene [25]. However, in vitro fertilization failed if the medium lacked glucose [24, 26–29], suggesting that glycolysis produces most of the ATP needed for sperm function. This was shown convincingly by using gene targeting to disrupt the sperm-specific Gapdhs gene. Sperm from mice lacking the GAPDHS glycolytic enzyme had very low ATP levels and severely compromised motility, causing the male mice to be infertile [9].
The rate-limiting enzyme for glycolysis in brain and red blood cells is HK1 [30, 31], suggesting that sperm activation might involve an increase in HK1S activity that enhances the rate of glycolysis and ATP production. Our study showed that HK1S activity and ATP levels are significantly higher in activated than in quiescent sperm, findings consistent with this idea. A clue to the regulation of this change was the observation that HK1S is present mainly as a dimer in quiescent sperm and a monomer in activated sperm. At the low concentrations used for kinetic studies, HK1 is a monomer, but dimerization can occur at higher concentrations in the presence of glucose and glucose 6-phosphate (G6P) [32] and during crystallization [33–35]. Interaction between the N- and C-terminal domains of HK1S is believed necessary for the monomer to function [36], but this interaction is constrained in the dimer form [32], suggesting that dimerization of HK1S subunits causes HK enzymatic activity to be lower in quiescent than in activated sperm.
A key observation for understanding the dimer-to-monomer conversion occurred when sperm lysates were analyzed by PAGE and dimers were found only under nonreducing conditions. In addition, treatment of quiescent sperm lysates with diamide to block disulfide-bond reduction inhibited the dimer-to-monomer conversion catalyzed by TXN1. Although these observations indicated that HK1S dimers probably are stabilized by disulfide bonds, x-ray crystallographic studies found that HK1 dimers were maintained by hydrogen bonds and not intramolecular disulfide bonds [37]. However, the conformation of HK1 dimers in crystals presumably is different than their conformation in solution and not necessarily that of HK1S in the sperm flagellum. Furthermore, a cysteine residue in the SSR domain of HK1S (and not present in the PBD domain of HK1) is available for intramolecular disulfide bond formation between HK1S subunits.
Although the conversion of HK1S dimers to monomers in vitro occurred by disulfide bond reduction, it remained to be determined if the same occurs during activation of live sperm. We found that treating sperm with diamide to inhibit disulfide bond cleavage resulted in the reduction of both HK1S activity and sperm motility. The ATP levels remained unchanged for several minutes in the presence of diamide, suggesting that its effect was not due to cytotoxicity. However, the structure and activity of another glycolytic enzyme, muscle phosphofructokinase (PFKM), was reported to be sensitive to the oxidation state of protein sulfhydryl groups [38]. Thus, at least some of the effect of diamide on HK1S activity might be indirect. If diamide inhibits PFKM or other glycolytic enzymes, this might result in the accumulation of G6P and lead to product inhibition of HK1S activity. Further studies will be needed to examine the possible role of sulfhydryl reduction in other glycolytic enzymes during sperm activation.
Cysteine residues of proteins in the principal piece were minimally detected by mBBr in quiescent sperm but were readily detected in activated sperm. The majority of HK1S is present in the principal piece region of the flagellum, and presumably some of the thiol groups labeled by mBBr were on HK1S monomers. It is not unusual for redox-sensitive proteins to form transient disulfide bonds under oxidizing conditions, and the presence of HK1S as a dimer in the epididymis is consistent with the previously reported redox status of sperm proteins in the epididymis [16]. However, our studies strongly suggest that HK1S disulfide bond reduction contributes significantly to the increase in ATP levels and the initiation of motility that occur when mouse sperm undergo conversion from the quiescent to activated state.
Sperm are generators of reactive oxygen species (ROS) [39–41], and redox-sensitive proteins form transient disulfide bonds under oxidizing conditions. This suggests that a redox reaction is responsible for the conversion of HK1S from a dimer to a monomer during sperm activation [42], which is consistent with other studies indicating that redox reactions are involved in the induction of progressive sperm motility [43].
Cells have two major redox-regulated systems, glutathiones (GSH) and thioredoxins (TRX) [44–46]. Sperm are known to have an active glutathione reductase and glutathione (GSH) system for protecting proteins from oxidative damage [47]. They also have a thioredoxin system for regulation of redox, and spermatogenic cell-specific thioredoxins TXNDC2, TXNDC3, and TXNDC8 (formerly called SPTRX-1, SPTRX-2, and SPTRX-3) [46, 48, 49] have been identified recently. One or both of these systems might be responsible for catalyzing the HK1S dimer-to-monomer conversion during sperm activation, and current studies are evaluating this possibility.
The present studies found that HK1S undergoes dephosphorylation during sperm activation, which seems contrary to precious reports that HK1S is tyrosine phosphorylated in sperm [11, 50]. However, these results typically were determined after isolation and wash steps taking 15–20 min. This suggests that the tyrosine-phosphorylated HK1S observed in previous studies resulted from rephosphorylation of monomers dephosphorylated during sperm activation.
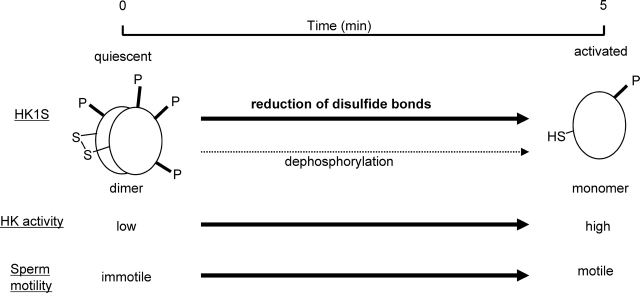
These studies indicate that a series of molecular and functional changes occur when sperm undergo conversion from quiescent to activated (Fig. 6). These include 1) reduction of disulfide bonds associated with HK1S dimers, 2) conversion of HK1S dimers to monomers, 3) HK1S dephosphorylation of HK1S, 4) an increase in HK activity, and 5) sperm becoming motile. Treatment with diamide impedes 1) reduction of disulfide bonds, 2) dimer-to-monomer conversion, and 3) increases in sperm motility. Dephosphorylation of HK1S occurs independently of disulfide bond reduction and is not required for dimer-to-monomer conversion. However, the molecular mechanisms responsible for the disulfide bond reduction and dephosphorylation that occur during sperm activation remain to be defined.
FIG. 6.
Correlation during sperm activation of changes in conformation of HK1S, HK1S activity, redox status, and sperm motility. The HK1S in quiescent sperm is present mainly as a dimer. During 5 min of incubation in M2 medium, there is a reduction in disulfide bonds and a conversion of HK1S dimmers to monomers. The dephosphorylation of HK1S during this period appears to occur independently of disulfide bond reduction. The reduction of disulfides (HK1S dimers) to thiols (HK1S monomers) is accompanied by activation of sperm motility and increases in HK activity.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the help provided by Gina Goulding with animal procedures, by Clyde Rogers for animal care, Dr. Furong Yu for technical suggestions, Drs. Carmen Williams and Yukitomo Arao for valuable suggestions regarding the manuscript, and the other members of the Gamete Biology Group for many helpful discussions.
Footnotes
Supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences and in part by a contract of the Spanish Ministry of Education and Science under the Ramón y Cajal Programme (A.M.-V.).
REFERENCES
- Yanagimachi R.Mammalian fertilization. Knobil E, Neill JD.The Physiology of Reproduction, 2nd ed New York:Raven Press;1994: 189–317. [Google Scholar]
- Inaba K.Molecular architecture of the sperm flagella: molecules for motility and signaling. Zool Sci 2003; 20: 1043–1056. [DOI] [PubMed] [Google Scholar]
- Urner F, Sakkas D.Protein phosphorylation in mammalian spermatozoa. Reproduction 2003; 125: 17–26. [DOI] [PubMed] [Google Scholar]
- McCarrey JR, Thomas K.Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature 1987; 326: 501–505. [DOI] [PubMed] [Google Scholar]
- Sakai I, Sharief FS, Li SSL.Molecular cloning and nucleotide sequence of the cDNA for sperm-specific lactate dehydrogenase-C from mouse. Biochem J 1987; 242: 619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JE, Schatte EC, O'Brien DA, Eddy EM.Expression of a glyceraldehyde 3-phosphatase dehydrogenase gene specific to mouse spermatogenic cells. Biol Reprod 1992; 46: 869–878. [DOI] [PubMed] [Google Scholar]
- Mori C, Welch JE, Fulcher KD, O'Brien DA, Eddy EM.Unique hexokinase messenger ribonucleic acids lacking the porin-binding domain are developmentally expressed in mouse spermatogenic cells. Biol Reprod 1993; 49: 191–203. [DOI] [PubMed] [Google Scholar]
- Storey BT, Kayne FJ.Energy metabolism of spermatozoa. V. The Embden-Myerhof pathway of glycolysis: activities of pathway enzymes in hypotonically treated rabbit epididymal spermatozoa. Fertil Steril 1975; 26: 1257–1265. [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA.Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A 2004; 101: 16501–16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori C, Nakamura N, Welch JE, Gotoh H, Goulding EH, Fujioka M, Eddy EM.Mouse spermatogenic cell-specific type 1 hexokinase mHk1-s transcripts are expressed by alternative splicing from the mHk1 gene and the HK1-S protein is localized mainly in the sperm tail. Mol Reprod Dev 1998; 49: 374–385. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Shibata H, O'Brien DA, Mori C, Eddy EM.Spermatogenic cell-specific type 1 hexokinase is the predominant hexokinase in sperm. Mol Reprod Dev 2008; 75: 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab P, Visconti P, Leclerc P, Kopf GS.p95, the major phosphotyrosine-containing protein in mouse spermatozoa, is a hexokinase with unique properties. J Biol Chem 1994; 269: 3810–3817. [PubMed] [Google Scholar]
- Visconti PE, Olds-Clarke P, Moss SB, Kalab P, Travis AJ, de las Heras M, Kopf GS.Properties and localization of a tyrosine phosphorylated form of hexokinase in mouse sperm. Mol Reprod Dev 1996; 43: 82–93. [DOI] [PubMed] [Google Scholar]
- Wilson JE.Hexokinase. Rev Physiol Biochem Pharmacol 1995; 126: 65–198. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB.Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem 2001; 276: 7630–7636. [DOI] [PubMed] [Google Scholar]
- Shalgi R, Seligman J, Kosower NS.Dynamics of the thiol status of rat spermatozoa during maturation: analysis with the fluorescent labeling agent monobromobimane. Biol Reprod 1989; 40: 1037–1045. [DOI] [PubMed] [Google Scholar]
- Seligman J, Newton GL, Fahey RC, Shalgi R, Kosower NS.Nonprotein thiols and disulfides in rat epididymal spermatozoa and epididymal fluid: role of gamma-glutamyl-transpeptidase in sperm maturation. J Androl 2005; 26: 629–637. [DOI] [PubMed] [Google Scholar]
- Joshi MD, Jagannathan V.Hexokinase I. Brain. Methods Enzymol 1966; 9: 371–376. [Google Scholar]
- Tsao T-S, Burcelin R, Charron MJ.Regulation of hexokinase II gene expression by glucose flux in skeletal muscle. J Biol Chem 1996; 271: 14959–14963. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Whittingham DG, Overstreet JW, Yanagimachi R.Tolerance of the mouse sperm nuclei to freeze-drying depends on their disulfide status. Biol Reprod 2003; 69: 1859–1862. [DOI] [PubMed] [Google Scholar]
- Wollman EE, Auriol L, Rimsky L, Shaw A, Jacquot J-P, Wingfield P, Graber P, Dessarps F, Robin P, Galibert F, Bertoglio J, Fradeliz D.Cloning and expression of cDNA for human thioredoxin. J Biol Chem 1988; 263: 15506–15512. [PubMed] [Google Scholar]
- Mukai C, Okuno M.Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod 2004; 71: 540–547. [DOI] [PubMed] [Google Scholar]
- Seligman J, Kosower NS, Weissberg R, Shalgi R.Thiol-disulfide states of human sperm proteins. J Reprod Fertil 1994; 101: 435–443. [DOI] [PubMed] [Google Scholar]
- Fraser LR, Quinn PJ.A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse. J Reprod Fertil 1981; 61: 25–35. [DOI] [PubMed] [Google Scholar]
- Narisawa S, Hecht NB, Goldberg E, Boatright KM, Reed JC, Millán JL.Testis-specific cytochrome c-null mice produce functional sperm but undergo early testicular atrophy. Mol Cell Biol 2002; 22: 5554–5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe PC.Glucose requirement for mouse sperm capacitation in vitro. Biol Reprod 1976; 15: 39–45. [DOI] [PubMed] [Google Scholar]
- Hoshi K, Tsukikawa S, Sato A.Importance of Ca2+, K+ and glucose in the medium for sperm penetration through the human zona pellucida. Tohoku J Exp Med 1991; 165: 99–104. [DOI] [PubMed] [Google Scholar]
- Urner F, Sakkas D.Glucose participates in sperm-oocyte fusion in the mouse. Biol Reprod 1996; 55: 917–922. [DOI] [PubMed] [Google Scholar]
- Williams AC, Ford WC.The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl 2001; 22: 680–695. [PubMed] [Google Scholar]
- Rapoport S.Regulation of metabolism in red cells. Bibl Haematol 1968; 29: 133–145. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV.The relationships between substrates and enzymes of glycolysis in brain. J Biol Chem 1964; 239: 31–42. [PubMed] [Google Scholar]
- Sebastian S, Kenkare UW.Stimulation of brain hexokinase gene expression by recombinant brain insulin-like growth factor in C6 glial cells. Exp Cell Res 1999; 246: 243–247. [DOI] [PubMed] [Google Scholar]
- Aleshin AE, Zeng C, Bourenkov GP, Bartunik HD, Fromm HJ, Honzatko RB.The mechanism of regulation of hexokinase: new insights from the crystal structure of recombinant human brain hexokinase complexed with glucose and glucose and glucose-6-phosphate. Structure 1998; 15: 39–50. [DOI] [PubMed] [Google Scholar]
- Aleshin AE, Fromm HJ, Honzatko RB.Multiple crystal forms of hexokinase I: new insights regarding conformational dynamics, subunit interactions, and membrane association. FEBS Lett 1998; 434: 42–46. [DOI] [PubMed] [Google Scholar]
- Aleshin AE, Zeng C, Bartunik HD, Fromm HJ, Honzatko RB.Regulation of hexokinase I: crystal structure of recombinant human brain hexokinase complexed with glucose and phosphate. J Mol Biol 1998; 282: 345–347. [DOI] [PubMed] [Google Scholar]
- Tsai HJ, Wilson JE.Functional organization of mammalian hexokinases: characterization of chimeric hexokinases constructed from the N- and C-terminal domains of the rat type I and type II isozymes. Arch Biochem Biophys 1995; 316: 206–214. [DOI] [PubMed] [Google Scholar]
- Mulichak AM, Wilson JE, Padmanabhan K, Garavito RM.The structure of mammalian hexokinase-1. Nat Struct Biol 1998; 5: 555–560. [DOI] [PubMed] [Google Scholar]
- Luther MA, Gilbert HF, Lee JC.Self-association of rabbit muscle phosphofructokinase: role of subunit interaction in regulation of enzymatic activity. Biochemistry 1983; 22: 5494–5500. [DOI] [PubMed] [Google Scholar]
- Tosic J, Walton A.Metabolism of spermatozoa. The formation and elimination of hydrogen peroxide by spermatozoa and effects on motility and survival. Biochem J 1950; 47: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland MK, Storey BT.Oxygen metabolism of mammalian spermatozoa. Generation of hydrogen peroxide by rabbit epididymal spermatozoa. Biochem J 1981; 198: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Clarkson JS.Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 1987; 81: 459–469. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, O'Flaherty C.Sperm activation: role of reactive species and kinases. Biochim Biophys Acta 2008; 1784: 106–115. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Smyth TB, Vindivich D, Harter C, Robinson J, Chang TSK.Induction and enhancement of progress motility in hamster caput epidydimal spermatozoa. Biol Reprod 1986; 35: 1065–1074. [DOI] [PubMed] [Google Scholar]
- Rietsch A, Beckwith J.The genetics of disulfide bond metabolism. Annu Rev Genet 1998; 32: 163–184. [DOI] [PubMed] [Google Scholar]
- Arner ES, Holmgren A.Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 2000; 267: 6102–6109. [DOI] [PubMed] [Google Scholar]
- Miranda-Vizuete A, Ljung J, Damdimopoulos AE, Gustafsson JA, Oko R, Pelto-Huikko M, Spyrou G.Characterization of Sptrx, a novel member of the thioredoxun family specifically expressed in human spermatozoa. J Biol Chem 2001; 276: 31567–31574. [DOI] [PubMed] [Google Scholar]
- Alvarez JG, Storey BT.Lipid peroxidation and the reactions of superoxide and hydrogen peroxide in mouse spermatozoa. Biol Reprod 1984; 30: 833–841. [DOI] [PubMed] [Google Scholar]
- Miranda-Vizuete A, Tsang K, Yu Y, Jiménez A, Pelto-Huikko M, Flickinger CJ, Sutovsky P, Oko R.Cloning and developmental analysis of murid spermatid-specific thioredoxin-2 (SPTRX-2), a novel sperm fibrous sheath protein and autoantigen. J Biol Chem 2003; 278: 44874–44885. [DOI] [PubMed] [Google Scholar]
- Jimenez A, Zu W, Rawe VY, Pelto-Huikko M, Flickinger CJ, Sutovsky P, Gustafsson JA, Oko R, Miranda-Vizuete A.Spermatocyte/spermatid-specific thioredoxin-3, a novel Golgi apparatus-associated thioredoxin, is a specific marker of aberrant spermatogenesis. J Biol Chem 2004; 279: 34971–34982. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS.Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995; 121: 1129–1137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.