Abstract
Light signals perceived by phytochromes (Phys) and cryptochromes (Crys) play key roles in plant growth and development and in photoperiod dependant process such as flowering, tuberization, seasonal growth cessation and dormancy. The integration of the light signals with the endogenous circadian oscillator provides plants with a mechanism to monitor changes in photo-period or day-length. In a recent report, we established that in Vitis vinifera L. cv Thompson Seedless, photoperiod drives the entrance of buds into endodormancy (ED) and modifies the expression of VvPHYA and VvPHYB transcripts in grapevine leaves, suggesting that both VvPHYs could play crucial roles in SD-induced transition of bud into ED. Here, we aimed to establish whether the transition of grapevine buds into ED is a mere consequence of a decision taken in the leaf or whether the bud responds by itself to photoperiod. Results show that in defoliated grapevine canes, bud-ED development is delayed compared with non-defoliated control canes, and that under LD-photoperiod both VvPHYA and VvPHYB transcripts are highly expressed in grapevine buds, whilst under SD-photoperiod both VvPHYs are downregulated and expression can not be detected. Overall, the results suggest that grapevine bud behaves as semi-autonomous organ in sensing the photoperiod signal, and that VvPHYA and VvPHYB gene expression is differently regulated by photoperiod in leaf and bud of grapevines.
Key words: photoperiod, phytochromes, Vitis vinifera
Introduction
Photoperiod, the length of the light period changes according to geographical latitude in a strictly predictable manner during the progression of the season, making it a reliable source of information for the timing of developmental events in living organisms.1,2 To capitalize this situation, plants evolved systems to monitor photoperiod by means of photoreceptors, Phys and Crys, which through the interaction with circadian clock regulates the expression of key genes that control the production of systemic signals.3–6 Time of flowering is one of the most prominent processes regulated by photoperiod, nevertheless a wide variety of other developmental process like stem elongation, bud-dormancy, tuberization, leaf growth and axillary branching are also under photoperiodic regulation.1,7 In grapevines, bud-ED is induced exclusively by decreasing photoperiod8–10 and because of that in the tropic, under constant photoperiod, grapevine buds do not enter into ED and behave as an evergreen tree that growth continuously throughout the whole year.11 These features make SD-induced transition of grapevine buds into ED a good model for studying molecular events involved in photoperiodic dependent processes.
Phytochromes and the Photoperiod Signal
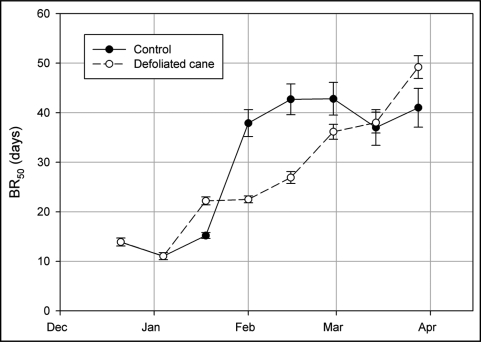
Phys play a crucial role in the perception of photoperiod. For example, poplar responds to SD-photoperiod with a PHYA mediated cessation of growth, terminal bud set and dormancy.12,13 It has been shown that overexpression of oat PHYA in hybrid poplar (P. tremula x P. tremuloide) considerably alters its growing habit, and transgenic poplar does not respond to SD-photoperiod and continues growing without setting buds and entering into ED under that photoperiodic conditions.12 In a recent report, we established that photoperiod in Vitis vinifera cv Thompson Seedless drives the entrance of buds into ED and modifies the expression of VvPHYA and VvPHYB transcripts in grapevine leaves, suggesting that both VvPHYs play a crucial role in SD-induced transition of bud into ED.10 It is generally accepted that photoperiod is perceived in the leaf and that a leaf-produced signal is transmitted towards the apex.2 However, in a recent report it was suggested on the basis of grafting experiments, that the apex itself could respond to photoperiod, and that a combination of signals including those generated in the leaves and others generated within the apex itself are responsible for the photoperiodic response.14 Here, we demonstrate that in defoliated grapevine canes, the entrance of buds into ED is delayed during the season in relation to non-defoliated control canes (Fig. 1). Thus, while in control plants the critical day-length for the transition of buds into ED occurs within the second half of January, in defoliated canes the period of transition extends and shifts towards shorter day-length. This result indicates that the bud by itself responds to photoperiod, and therefore, VvPHYs should express within the bud-tissue.
Figure 1.
Increases in BR50 as function of season progression in buds of control and defoliated canes of Thompson Seedless grapevine grown in Santiago of Chile (33°34′S). BR50 is a parameter that measures the depth or intensity of bud-dormancy in single node cuttings under forcing conditions. Bar represent standard deviation in BR50 values.
Expression of VvPHYA and VvPHYB Transcripts in Grapevine Buds
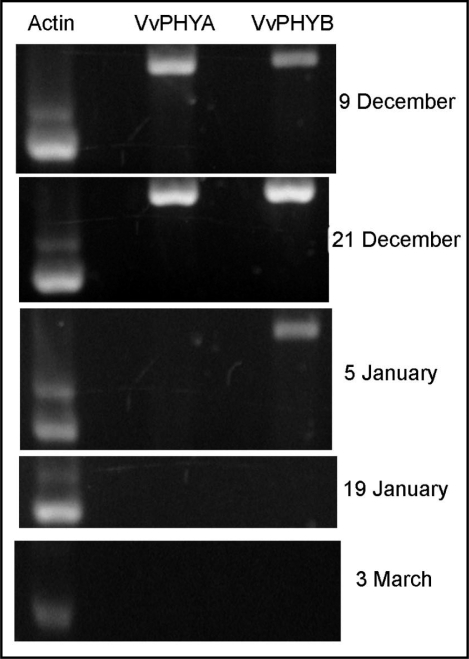
The expression of VvPHYA and VvPHYB transcripts was analyzed by semi quantitative RT-PCR in grapevine buds before, during and after the critical day-length for the transition of buds into ED, as defined previously.10 The result shows that both VvPHYs are highly expressed under LD-photoperiod, however, under SD-photoperiod its expression is down-regulated and it is not detected (Fig. 2). These results contrast with those obtained in grapevine leaves,10 in which both VvPHYs are highly expressed under SD-conditions. Moreover, in grapevine leaves the expression of both VvPHYs oscillates with a daily rhythm under LD conditions, while under SD conditions the oscillation disrupts and both transcripts express uniformly at high levels. Although we have not analyzed yet the daily expression profile of VvPHYA and VvPHYB under different photoperiod in grapevine buds, preliminary results indicates that photoperiod regulates VvPHYA and VvPHYB expression differentially in grapevine buds and in grapevine leaves. Since as it is shown here, the expression of both VvPHYs is not detected under SD-photoperiod in grapevine buds, while in grapevine leaves under similar photoperiod conditions, both VvPHYs are highly expressed.10 The downregulation of VvPHYA and VvPHYB expression under SD-photoperiod suggests that buds become isolated from light signals once they enter into ED, and therefore, the development of the whole ED program occurs independently of light signals. Therefore, it is reasonable to envisage that downregulation of VvPHYA and VvPHYB expression by SD-photoperiod could be a signal for the entrance of buds into ED, since in Arabidopsis it has been demonstrated that most light responsiveness genes are mediate by PHYA or PHYB.15 Hence in the absence of PHYs expression bud transcriptome could be highly modified and metabolism depressed, favoring thus a recess stage characteristic of bud-ED.
Figure 2.
Expression analysis of VvPHYA and VvPHYB transcripts in grapevine buds before, during, and after the critical day-length for transition of buds into ED in Santiago of Chile (33°34′S). Analysis was performed by semi-quantitative RT-PCR using VvACTIN gene as control.
Acknowledgements
Financial support of Fondecyt project 1080013 is gratefully acknowledged.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8907
References
- 1.Kobayashi Y, Weigel D. Move on up, it's time for change, mobile signals controlling photoperiod-dependent flowering. Genes Dev. 2008;21:237–284. doi: 10.1101/gad.1589007. [DOI] [PubMed] [Google Scholar]
- 2.Turck F, Fornara F, Coupland G. Regulation and identity of florigen: Flowering locus T moves center stage. Annu Rev Plant Physiol Plant Mol Biol. 2008;59:573–594. doi: 10.1146/annurev.arplant.59.032607.092755. [DOI] [PubMed] [Google Scholar]
- 3.Yanovsky MJ, Kay S. Molecular basis of seasonal time measurement in Arabidopsis. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]
- 4.Suárez-López P, Wheatley K, Robson F, Onuchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1119. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- 5.Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptors regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- 6.An H, Roussot C, Suarez-López P, Corbesier L, Vincent C. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development. 2004;131:3615–3626. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Falcon M, Bou J, Prat S. Seasonal control of tuberization in potato: Conserved elements with the flowering response. Annu Rev Plant Biol. 2006;5:15–80. doi: 10.1146/annurev.arplant.57.032905.105224. [DOI] [PubMed] [Google Scholar]
- 8.Fennell A, Hoover E. Photoperiod influences growth, bud-dormancy and cold acclimation in V. labruscana and V. riparia. J Am Soc Hortic Sci. 1991;116:270–273. [Google Scholar]
- 9.Salzman RA, Bressan RA, Hasegawa PM, Ashworth EN, Bordelon BP. Programmed accumulation of LEA-like proteins during dessication and cold acclimation of overwinter grape buds. Plant Cell Environm. 1996;19:713–720. [Google Scholar]
- 10.Kühn N, Ormeño-Nuñez J, Jaque-Zamora G, Pérez FJ. Photoperiod modifies the diurnal expression profile of VvPHYA and VvPHYB transcripts in field grown grapevine leaves. J Plant Physiol. 2009 doi: 10.1016/j.jplph.2009.01.005. In Press (doi: 1016/j.jplph.220.01.005) [DOI] [PubMed] [Google Scholar]
- 11.Possingham JV. Proc Intl Symp Table Grapes. Anaheim Calif. USA: Pub Am Soc Enol—Vitic Davis Calif USA; 1994. Production of table grapevines in India. [Google Scholar]
- 12.Olsen JE, Juntilla O, Nilsen J, Eriksson ME, Martinussen I, Olsson O, et al. Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J. 1997;12:1339–1350. [Google Scholar]
- 13.Welling A, Moritz T, Palva ET, Junttila O. Independent activation of cold acclimation by low temperatures and short photoperiod in hybrid aspen. Plant Physiol. 2002;129:1633–1641. doi: 10.1104/pp.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruonala R, Rinne PLH, Kangasjärvi J, van der Schoot C. CENL1 Expression in the Rib meristem affects stem elongation and the transition to dormancy in Populus. Plant Cell. 2008;20:59–74. doi: 10.1105/tpc.107.056721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quail PH. Phytochrome-regulated gene expression. J Integr Plant Biol. 2007;49:11–20. [Google Scholar]