Abstract
The induction and regulation of wound-healing (WH) processes in potato tubers and other vegetables are of great nutritional and economic importance. The rapid accumulation of waxes to restrict water vapor loss and formation of suberin barriers to block infection are crucial components of WH. Recently we determined the regulatory involvement of abscisic acid (ABA) and ethylene in WH. In this addendum we integrate and interpret features from this recent research with additional information on ABA and data on the association of jasmonic acid (JA) in tuber WH. Results show that wounding dramatically increased tuber ethylene production and ABA and JA content. Blockage of wound-induced ABA biosynthesis and ethylene action/biosynthesis showed that ABA is a potent regulator in reduction of water vapor loss and hastening of suberization while ethylene had no discernable effect. The collective results also imply that ethylene has no effect on ABA regulation of WH. JA content in dormant and non-dormant minitubers is very low (≤l ng gFW−1) but rapidly increases upon wounding then decreases, all before wound-induced ABA or ethylene accumulation reach their maxima. Results gathered to date do not support a role for ethylene in potato tuber WH but do implicate ABA in this process. Although JA content increases rapidly after wounding, its role in tuber WH remains speculative.
Key words: wound-healing, suberization
Wound-healing involves a broad range of biological processes induced and regulated by poorly defined cognate signals. The processes of suberization and associated wax accumulation are commonly considered to be synonymous with WH.1 These processes appear to be ubiquitous mechanisms of WH in plants,2 moreover they are crucial in durable wound protection.1 The importance of the signals regulating WH is underscored by the critical nature of the constituent processes.
The biological processes involved in the rapid reduction in water vapor loss directly after wounding are important in preventing desiccation during healing, presumably through accumulation of waxes at the wound site.3–5 Also, accumulation of waxes and other aliphatic compounds has been shown to be involved in reduction of water vapor loss during maturation of fully formed native periderm.6,7 Desiccation and resulting death of cells at and near the wound surface would terminate the cellular responses necessary for induction of WH processes that protect underlying parenchyma tissues. The accumulation of suberin poly(phenolic(s)) (SPP) and suberin poly(aliphatic(s)) (SPA) during WH is important because these biopolymers provide robust barriers to bacterial and fungal infection respectively.8–10 The signaling mechanisms regulating the formation and deposition of these biopolymers are economically important areas of research. Until recently, regulation of these processes had largely been suggested through correlative studies. However, our recent work employed liquid chromatography mass spectrometry (LC-MS) detection coupled with inhibition of regulator action or biosynthesis to determine if the process is impaired. Subsequent reversal by exogenous regulator was then used to definitively determine if the process is restored by the signal in question.
Wounding induces the biosynthesis of several hormones, most notably ABA, ethylene and JA in many tissues.11 The involvement of two important regulatory compounds, ethylene12 and ABA,13 in potato tuber WH was determined in our laboratories by quantifying their content and using depletion and blocking tactics during the period of wound response. A third regulatory compound, JA, continues to be investigated in our laboratory. Herein, we provide addenda information on these regulatory findings and combine the results to further describe the interactive involvement of these three signaling compounds in WH.
Tuber ABA Content and Regulation
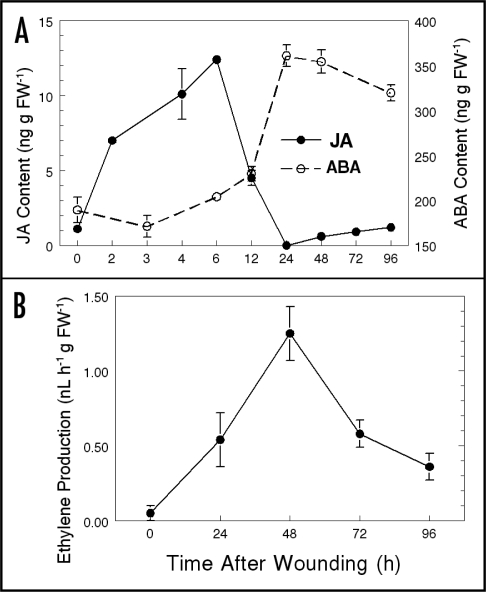
LC-MS determinations showed that tuber basal ABA content varied depending on how long the potatoes were stored after harvest. Surprisingly, total tuber ABA content was lowest at harvest, a period when wound response and dormancy appear to be the most robust. After harvest, basal ABA content then increased at three and six months of storage and decreased at nine months of storage. In these experiments, discs (16 mm × 3 mm) were excised from tubers to provide tissues with a reproducible global wound response that was greater than that of tubers cut in half which had only a single surface yielding a wound response. ABA content in tuber discs decreased dramatically to near non-detectable amounts one or two days after wounding then increased unless precursor biosynthesis was blocked by fluridone.13 Here, we show additional data from determinations of ABA content in less traumatized WH half tubers (single wound surface) obtained at six months of storage, after the break of dormancy (Fig. 1A). Although these less traumatized tissues did not undergo the dramatic decrease in ABA content found in globally wounded tuber disks, they did produce a similar large wound-induced increase in total ABA content. Wounding clearly induces increases in ABA content several-fold, but the increase occurs one to three days after cutting for less and more traumatized tissues respectively. In addition, both the basal ABA content and wound-induce increase in ABA content during wound-healing was lower in freshly harvested tubers than that in tubers stored three, six and nine months. These data indicate that the mechanisms associated with postharvest wound-induced ABA degradation and biosynthesis changed significantly during storage, perhaps reflecting dormancy-related changes in the expression of genes involved in ABA metabolism.14
Figure 1.
The effect of wounding on: (A) ABA and JA accumulation, and (B) ethylene production in non-dormant potato tuber tissue. (A) Note that tuber basal JA content is very low, but rapidly increases within hours after wounding then decreased to nil or low content by 24 h followed by a small increase. Basal ABA content (∼190 ng g FW−1) was high enough to be easily measured and increased greatly by 24 h after wounding. Global wounding, data not shown,13 results in a strong decrease in ABA content followed by an increase. (B) Ethylene production is low in non-wounded tuber tissue but increases by 24 h after wounding, reaches a maximum around 48 h after wounding and then decreases. All three of these signaling compounds are wound-inducible.
ABA Regulation of Phenylalanine Ammonia Lyase (PAL) and Accumulation of SPP and SPA
Little or no PAL activity can be detected in non-wounded tuber tissue. Wounding of potato tuber provides a classic example of induction of de novo synthesis of PAL from little/no activity to a robust activity by 24 to 48 h after wounding15 depending on the age of the tuber.16 Wound induction of PAL activity was moderately reduced (1/3 to 2/3) by blockage of ABA biosynthesis, but to a lesser extent than other processes occurring further downstream in the procession of wound responses. Addition of exogenous ABA restored the partial loss of PAL induction in ABA blocked tissues. Although addition of exogenous ABA to non-blocked tissue immediately after tuber wounding increased PAL, significant increases in wound-induced ABA content occur well after initial PAL induction. Perhaps endogenous ABA, or another signal/regulator, present upon wounding plays a crucial role in PAL induction, while wound-induced ABA plays a smaller role. This signaling is important because PAL is the first committed step in the phenylpropanoid pathway of SPP biosynthesis and many other wound related processes, which in turn are regulated by ABA.
Wounding appears to induce cell wall modifications at the closing layer as a prelude to major SPP accumulations (a robust barrier to bacterial infection) because blockage of ABA biosynthesis resulted in erratic and reduced fluorescence at the wound surface, indicating absence or reduction of SPP accumulations. Importantly, these SPP-barren cell walls were visibly altered with a darkened color and/or muted background fluorescence. Addition of ABA restored the accumulation of brilliant blue autofluorescent SPP on the closing layer cell walls.
The second suberin biopolymer to accumulate, the SPA component (a robust barrier to fungal infection), was also significantly reduced by blockage of ABA biosynthesis. SPA accumulation and other suberization processes adversely affected by blockage of ABA biosynthesis were restored by exogenous ABA. This restoration included control of water vapor loss, a vital process associated with wax accumulation in suberizing cells. Blockage of ABA biosynthesis resulted in an increase in water vapor loss of up to 123% over that of the control during WH.
Ethylene
After harvest and prior to sprouting, potato tubers produce a low (ca. 1 pL g FW−1 h−1) and constant amount of ethylene.17 Wounding induces a significant increase in ethylene evolution.12 However, ethylene production did not reach maximum until two days after wounding (Fig. 1B) and blockage of ethylene biosynthesis and action had no discernable effect on SPP or SPA accumulation in WH tubers.12 Further, exogenous ethylene had no noticeable effect on accumulation of SPP or SPA. These results indicate that, although ethylene is not essential for wound induced suberization, it may participate in other wound responses such as glycoalkaloid accumulation.18 Collectively, these results also imply that ethylene is not required for the wound induction of ABA biosynthesis, nor does ethylene play a role in the ABA-mediated regulation of the above WH processes. The signaling roles of wound-induced ethylene in healing tuber tissues, if any, have not been determined.
JA Content and Wound Induction
Oxylipins, particularly JA and methyl jasmonate (MJ), have been shown to play a variety of important signaling roles in wounded leaf tissue.11,19 The volatile nature of MJ facilitates transmission of the wound signal throughout foliar structures and to adjacent plants. However, little is known about the involvement of JA in tuber wound responses and healing. The dense nature and high water content of tuber tissue could effect such signaling transmission. Koda and Kikuta20 used HPLC to show that tuber JA content increased upon wounding, but only in dormant tubers (cv. Irish cobbler) stored 4 months or less. Using LC-MS detection for JA, we recently determined that after twelve months of storage, i.e., well after breaking dormancy, the basal JA content in resting minitubers (certified seed, cv. Russet Burbank) was barely detectable (≤1 ng g FW−1). However, within two to six h after wounding, JA content rapidly increased to ∼12 ng gfw−1. JA content then decreased dramatically to low or non-detectable amounts by 12 to 24 h after wounding followed by slight increases to ∼1 ng gfw−1 at 48 to 96 h after wounding (Fig. 1A). Similar results were obtained with dormant tubers. This wound-induced increase in JA is more rapid than that for ABA or ethylene, with the increase and decrease in JA occurring before that of ABA and ethylene. Also, these results indicate that increased JA production is associated with the tuber wound-response in dormant and non-dormant tubers and that basal JA content is at or near non-detectable concentrations. Additional JA involvement in WH is being investigated in our laboratories.
Conclusions
The integration of regulatory mechanisms from various signaling compounds continues to be vigorously investigated in leaf tissue. However, little is known or has been investigated regarding signaling crosstalk among regulatory compounds in the economically important area of tuber WH. Our collective research shows that wounding induces changes in tuber ABA, ethylene and JA content and that ABA is an important signal in the regulation of WH processes. However, specific roles of wound-induced ethylene and JA in tuber healing are not clear, although ABA appears to regulate the above processes independent of these two signaling compounds.
Abbreviations
- ABA
abscisic acid
- JA
jasmonic acid
- LC-MS
liquid chromatography mass spectrometry
- MJ
methyl jasmonate
- PAL
phenylalanine ammonia lyase
- SPA
suberin poly(aliphatic(s))
- SPP
suberin poly(phenolic(s))
- WH
wound-healing
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8922
References
- 1.Lulai EC. Skin-set, wound-healing and related defects. In: Vreugdenhil Dick., editor. Potato Biology and Biotechnology: Advances and Perspectives. Amsterdam, The Netherlands: Elsevier Limited; 2007. pp. 471–496. [Google Scholar]
- 2.Dean BB, Kolattukudy PE. Synthesis of suberin during wound-healing in jade leaves, tomato fruit and bean pods. Plant Physiol. 1976;58:411–416. doi: 10.1104/pp.58.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lulai EC, Orr PH. Porometric measurements indicate wound severity and tuber maturity affect the early stages of wound-healing. Am Potato J. 1995;72:225–241. [Google Scholar]
- 4.Schreiber L, Franke R, Harmann K. Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpirations. Planta. 2005;220:520–530. doi: 10.1007/s00425-004-1364-9. [DOI] [PubMed] [Google Scholar]
- 5.Soliday CL, Kolattukudy PE, Davis RW. Chemical and ultrastructural evidence that waxes associated with the suberin polymer constitute the major diffusion barrier to water vapor in potato tuber (Solanum tuberosum L.) Planta. 1979;146:607–614. doi: 10.1007/BF00388840. [DOI] [PubMed] [Google Scholar]
- 6.Serra O, Soler M, Hohn C, Sauveplane V, Pinot F, Franke R, et al. CYP86A33-Targeted gene silencing in potato tuber alters suberin composition, distorts suberin lamellae and impairs the periderm's water barrier function. Plant Physiol. 2009;149:1050–1060. doi: 10.1104/pp.108.127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogt E, Schonherr J, Schmidt HW. Water permeability of periderm membranes isolated enzymatically from potato tubers (Solanum tuberosum L.) Planta. 1983;158:294–301. doi: 10.1007/BF00397330. [DOI] [PubMed] [Google Scholar]
- 8.Lulai EC. Non-wound induced suberization of tuber parenchyma cells: a physiological response to the wilt disease pathogen Verticillium dahliae. Am J Potato Res. 2005;82:433–440. [Google Scholar]
- 9.Lulai EC, Corsini DL. Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiol Mol Plant Pathol. 1998;53:209–222. [Google Scholar]
- 10.Lulai EC, Weiland JJ, Suttle JC, Sabba RP, Bussan AJ. Pink eye is an unusual periderm disorder characterized by aberrant suberization: a cytological analysis. Am J Potato Res. 2006;83:409–421. [Google Scholar]
- 11.Leon J, Rojo E, Sánchez-Serrano JJ. Wound signaling in plants. J Exp Bot. 2001;52:1–9. doi: 10.1093/jexbot/52.354.1. [DOI] [PubMed] [Google Scholar]
- 12.Lulai EC, Suttle JC. The involvement of ethylene in wound-induced suberization of potato tuber (Solanum tuberosum L.): a critical assessment. Postharvest Biol Technol. 2004;34:105–112. [Google Scholar]
- 13.Lulai EC, Suttle JC, Pederson SM. Regulatory involvement of abscisic acid in potato tuber wound-healing. J Exp Bot. 2008;59:1175–1186. doi: 10.1093/jxb/ern019. [DOI] [PubMed] [Google Scholar]
- 14.Destefano-Beltrán L, Knauber D, Huckle L, Suttle JC. Effects of postharvest storage and dormancy status on ABA content, metabolism and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Mol Biol. 2006;61:687–697. doi: 10.1007/s11103-006-0042-7. [DOI] [PubMed] [Google Scholar]
- 15.Zucker M. Sequential induction of phenylalanine ammonia-lyase and a lyase-inactivating system in potato tuber disks. Plant Physiol. 1968;43:365–374. doi: 10.1104/pp.43.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar GN, Knowles NR. Wound-induced superoxide production and PAL activity decline with potato tuber age and wound healing ability. Physiol Plant. 2003;117:108–117. [Google Scholar]
- 17.Suttle JC. Ethylene is not involved in hormone- and bromoethane-induced dormancy break in Russet Burbank minitubers. Amer J Potato Res. 2009 In Press. [Google Scholar]
- 18.Bergenstråhle A, Tilberg E, Jonsson L. Effects of ethephon and norbornadiene on sterol and glycoalkaloid biosynthesis in potato tuber discs. Physiol Plant. 1993;89:301–308. [Google Scholar]
- 19.Schaller A, Stintzi A. Jasmonate biosynthesis and signaling for induced plant defense against herbivory. In: Schaller A, editor. Induced Plant Resistance to Herbivory. Stuttgart: Springer; 2008. pp. 349–366. [Google Scholar]
- 20.Koda Y, Kikuta Y. Wound-induced accumulation of jasmonic acid in tissues of potato tubers. Plant Cell Physiol. 1994;35:751–756. [Google Scholar]