Abstract
Day-length and the circadian clock control critical aspects of plant development such as the onset of reproduction by the photoperiodic pathway.1 CONSTANS (CO) regulates the expression of a florigenic mobile signal from leaves to the apical meristem and thus is central to the regulation of photoperiodic flowering.2 This regulatory control is present in all higher plants,3 but the time in evolution when it arose was unknown. We have shown that the genomes of green microalgae encode members of the CONSTANS-like (COL) protein family. One of these genes, the Chlamydomonas reinhardtii CO homolog (CrCO), can complement the co mutation in Arabidopsis.4 CrCO expression is controlled by the clock and photoperiod in Chlamydomonas and at the same time is involved in the correct timing of several circadian output processes such as the accumulation of starch or the coordination of cell growth and division. We have proposed that, since very early in the evolutionary lineage that gave rise to higher plants, CO homologs have been involved in the photoperiod control of important developmental processes, and that the recruitment of COL proteins in other roles may have been crucial for their evolutionary success.
Key words: photoperiod, flowering, constans, signaling, Arabidopsis, Chlamydomomas
Plants have adapted several physiological mechanisms to finely respond to external signals and coordinate the correct timing of important developmental processes.5 This way, different diurnal and seasonal potentially deterring or optimal situations can be predicted and an adequate response prepared in advance. In the case of the control of reproduction, predicting the best time of the year to flower confers a fitness improvement directly related to seed productivity and thus is naturally selected as an important trait in plant evolution.6 Plants and algae posses sophisticated mechanisms to measure time, such as a circadian clock or a photoperiod control to detect day-length and probably temperature.7,8 In photoperiodic flowering, the protein CONSTANS plays a central role because it activates in the leaves the expression of FLOWERING LOCUS T (FT). The small FT protein is able to move through the vascular bundles from the leaves to the apical meristem and trigger the program that transforms it into a reproductive meristem.2 Eventually, these changes induce the production of the flower.
We have recently described4 that in green microalgae the mechanism detecting photoperiod signals involves a CO homolog (CrCO) with a crucial role. CrCO expression is controlled by photoperiod and at the same time regulates several output processes of the clock such as starch accumulation or the onset of cell division.
Algal Genomes Contain CO Homolog Sequences
The recent sequencing of genomes from phylogenetically diverse microalgae allowed the identification of genes that encode homologs of proteins involved in circadian clock and developmental processes in higher plants.9,10 In C. reinhardtii, a chlorophyte microalga who has been extensively studied as a simple model for plant-specific processes like photosynthesis or phototaxia,11 we have identified several proteins that belong to the COL family. One of these proteins, which we have called CrCO, has the domain structure characteristic of CO.12
We also showed that CrCO transcript is regulated by photoperiod, but unlike in Arabidopsis the peak in expression of the gene always occurs during the light period and is actually higher in short days (SD, 8 h light-16 h dark) than in long days (LD, 16 h light-8 h dark). The protein production seems to closely follow the RNA expression so it seems that the control of CrCO in Chlamydomonas is simpler than that of CO in Arabidopsis.13 Observation under the confocal microscopy of GFP fusion tags in onion epidermal cells shows that like CO, CrCO is probably localized in the nuclei, further confirming their physiological analogies.
Expression of CrCO in Plants Under Different Promoters Induces Early Flowering
When we expressed CrCO under the control of a constitutive (35S) promoter and transformed Arabidopsis co mutant plants, we were able to rescue the delay in flowering produced by the lack of CO function. These constructs also induced early flowering in two different wild type ecotypes of Arabidopsis, Ler and col-0. Furthermore, some 35S::CrCO plants phenocopied several growth alterations that had also been described for CO overexpression such as club-like siliques and low polen production.14
Interestingly, when CrCO was expressed under the control of a phloem-specific promoter we were also able to accelerate flowering. This was not the case when CrCO was expressed under the control of a meristem-specific promoter. Thus, as in the case of CO, CrCO expression in the vascular tissues is enough and sufficient to activate flowering. In plants were CrCO was overexpressed we detected both the transcript for the transgene as well as the recombinant protein, except for the meristem-specific construct, were we could detect the transcript at high levels, but not the protein. In all cases, production of CrCO protein was followed by high levels of FT expression both in SD and in LD, which confirmed that CrCO was activating flowering via the canonical CO-FT module previously described for the induction of flowering in Arabidopsis.15 We think that the capacity to activate flowering is due to a lesser functional specificity in the CrCO gene, so that it will be able to activate flowering in a wide range of plant species. To test whether this is the case we are starting to test the effect of CrCO overexpression in other plant model species like tomato and rice.
Several Output Processes from the Circadian Clock are Disrupted in Chlamydomonas by Modification of CrCO Expression
The function of CO described to date is to induce expression of FT in the vascular tissue of photosynthetic tissues and thus generate a signal that is transmitted to the meristem to activate flowering.16 In microalgae, there is no clear FT homolog, neither a distance signal is needed to activate any developmental process. So, the question remained about the role of CrCO in Chlamydomonas. To answer this, CrCO overexpressor (CrCOox) and CrCO suppressor (CrCOas) microalgal lines were produced and their effects on starch accumulation and cell division studied. Both CrCO suppression and overexpression had a drastic effect in the clock outputs analyzed thus showing that CrCO is important in the control of both processes. CrCO overexpression caused the asynchrony between cell growth and division. Synchronous growth is a common feature of algal cultures.17 CrCOox lines also showed lower accumulation of starch and different capacity to accumulate it during the day both in SD and LD. We further demonstrated that CrCOox lines presented aberrant cell morphotypes that were probably caused by desynchronized growth and division. Furthermore, CrCOas lines had a deterred growth capacity, many of them never surviving the first rounds of duplication in restrictive (high light) conditions. It seemed then that, at least in Chlamydomonas, CrCO function was crucial for survival.
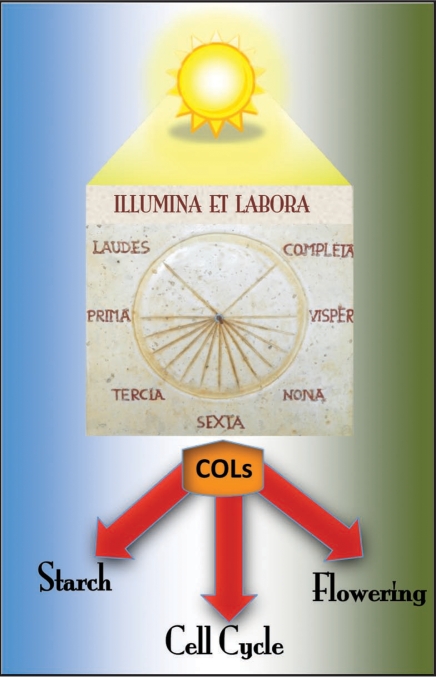
The fact that in Chlamydomonas CrCO has a role in starch accumulation and cell division opens the possibility that this role might be maintained in higher plants CO (Fig. 1). It has been previously reported that starch accumulation is under circadian clock control,18,19 the gi mutant showed elevated levels of starch20 and the cell cycle might be controlled by the clock.21 These observations suggest that the transition to flowering might be coordinated with cell division and carbon metabolism through outputs of the circadian clock, and CO arises as a putative integrator of all these processes (Fig. 1).
Figure 1.
ILLUMINA ET LABORA. COL proteins receive signals from day-length and the circadian clock and are activated by light. During evolution they have developed roles from the regulation of starch synthesis and cell cycle (algae, blue side) to the control of flowering time (plants, green side).
Conclusions
The functional complementation of CO by CrCO is remarkable for several reasons. Firstly, because it implies that the mechanisms that control daylength and clock dependent processes in higher plant are already present in microalgae. Secondly, because our results show that this process is crucial for algal growth and viability, and although CO mutation is not lethal in Arabidopsis, presumably due to function redundancy in the different CO homologs, all in all, the family of COL proteins may have important functions still concealed in higher plants. In the third case, it is remarkable that after the great evolutionary distance between CO and CrCO proteins and sharing only 27% identity in amino acid sequence, the function is still highly conserved. That means that the structure of CO and CrCO proteins shares a high degree of homology, so that CrCO can substitute CO in the quaternary complex that is able to influence gene expression. Although the molecular mechanism controlling CrCO function in microalgae is still largely unknown, parallel proteomic studies in both CO and CrCO may give new lights into the function-structure of this group of proteins and help understand how photosynthetic organisms measure time in a daily and seasonal scale.
Acknowledgements
This work is supported by project BIO2007-61837 and “Ramón y Cajal” contract to F.V. and project BIO2008-02292 to J.M.R. from the Spanish Ministry of Science; and from the Excellence Project P08-AGR-03582 and group helps BIO-261 and BIO-281 from the Andalusian Government.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8975
References
- 1.Imaizumi T, Kay SA. Photoperiodic control of flowering: not only by coincidence. Trends Plant Sci. 2006;11:567. doi: 10.1016/j.tplants.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi Y, Weigel D. Move on up, it's time for change-mobile signals controlling photoperiod-dependent flowering. Genes Dev. 2007;21:2371–2384. doi: 10.1101/gad.1589007. [DOI] [PubMed] [Google Scholar]
- 3.Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science. 2006;312:1040–1043. doi: 10.1126/science.1126038. [DOI] [PubMed] [Google Scholar]
- 4.Serrano G, Herrera-Palau R, Romero JM, Serrano A, Coupland G, Valverde F. Chlamydomonas CONSTANS and the evolution of plant photoperiodic signaling. Curr Biol. 2009;19:359–368. doi: 10.1016/j.cub.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 5.Bäurle I, Dean C. The timing of developmental transitions in plants. Cell. 2006;125:655–664. doi: 10.1016/j.cell.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi Y, Teshima KM, Yokoi S, Innan H, Shimamoto K. Variations in Hd1 proteins, Hd3a promoters and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc Natl Acad Sci USA. 2009;106:4555–4560. doi: 10.1073/pnas.0812092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould PD, Locke JCW, Larue C, Southern MM, Davis SJ, Hanano S, et al. The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell. 2006;18:1177–1187. doi: 10.1105/tpc.105.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittag M, Kiaulehn S, Johnson H. The Circadian clock in Chlamydomonas reinhardtii. What is it for? What is it similar to? Plant Physiol. 2005;137:399–409. doi: 10.1104/pp.104.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merchant SS, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–251. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo T, Okamoto K, Onai K, Niwa Y, Shimogawara K, Ishiura M. A systematic forward genetic analysis identified components of the Chlamydomonas circadian system. Genes Dev. 2008;22:918–930. doi: 10.1101/gad.1650408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris EH. The Chlamydomonas Source Book. San Diego: Academic Press; 1989. [Google Scholar]
- 12.Robson F, Costa MMR, Hepworth S, Vizir I, Piñeiro M, Reeves PH, et al. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001;28:619–631. doi: 10.1046/j.1365-313x.2001.01163.x. [DOI] [PubMed] [Google Scholar]
- 13.Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein and the mechanism of photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- 14.Onouchi H, Igeño MI, Perilleux C, Graves K, Coupland G. Mutagenesis of plants over-expressing CONSTANS demonstrates novel interactions among Arabidopsis flowering-time genes. Plant Cell. 2000;12:885–900. doi: 10.1105/tpc.12.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- 16.Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, et al. FT protein movement contributes to long-distance signalling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 17.Goto K, Johnson CH. Is the cell division cycle gated by a circadian clock? The case of Chlamydomonas reinhardtii. J Cell Biol. 1995;129:1061–1069. doi: 10.1083/jcb.129.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mérida A, Rodríguez-Galán JM, Vincent C, Romero JM. Expression of the granule-bound starch synthase I (waxy) gene from Antirrhinum majus is developmentally and circadian-clock regulated. Plant Physiol. 1999;120:401–409. doi: 10.1104/pp.120.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tenorio G, Orea A, Romero JM, Mérida A. Oscillation of mRNA level and activity of granule-bound starch synthase I in Arabidopsis leaves during the day/night cycle. Plant Mol Biol. 2003;51:949–958. doi: 10.1023/a:1023053420632. [DOI] [PubMed] [Google Scholar]
- 20.Eimert K, Wang SM, Lue WI, Chen J. Monogenic recessive mutations causing both late floral initiation and excess starch accumulation in Arabidopsis. Plant Cell. 1995;7:1703–1712. doi: 10.1105/tpc.7.10.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouget F-Y, Moulager M, Corellou F. Circadian regulation of cell division. In: Verma DPS, Hong Z, editors. Cell Division Control in Plants. Plant Cell Monogr. Vol. 9. Springer; 2008. pp. 3–11. ISBN: 3540734864. [Google Scholar]