Abstract
Flowering and seed set underpin most of the agriculture production. In the 57 Issue of The Plant Journal, we analysed the gene expression changes in the shoot apical meristem (SAM) during the transition from vegetative to flowering phase in soybean, an important legume crop. We identified a number of genes that are actively transcribed or repressed during the transition to flowering and the annotation of which have allowed us to infer the involvement of at least three hormonal pathways: those that involve abscisic acid (ABA), auxin and jasmonic acid (JA) in the regulation of floral initiation process in soybean. Intriguingly, the induction of known floral homeiotic transcript that includes APETALA1 in the SAM occurred after the induction of these hormonal transcripts adding a likely novel biochemical dimension to the current understanding of floral regulatory pathways. In view of recent studies, a cross-regulatory mechanism involving these hormones is proposed to operate at the SAM to initiate flowering.
Key words: floral transition, soybean, shoot apical meristem, hormone, gene expression, legume
Introduction
Many flowering plants use the change in day length (photoperiod) as signals for flowering. The molecular component underlying the photoperiodic control of flowering remains unknown especially in crop legumes. Molecular studies carried out in the long-day model plant, Arabidopsis thaliana have nevertheless advanced our understanding of floral pathways.1 However, the application of knowledge gained from Arabidopsis to legume crop plants can be challenging as the reproductive phase in Arabidopsis involves the cessation of leaf production to flower initiation while this phase in legumes such as soybean involves both the development of flowering inflorescence and leaves.2 Recent studies involving the use of mutants2 and transcriptome3–5 have started to highlight unique aspects of legume development.
Our current understanding of floral pathways is incomplete. With the exception of giberellic acid, there is a lack of representation of other hormones such as abscisic acid (ABA), auxin or jasmonic acid (JA) in current floral pathways although research to date have implicated the involvement of these hormones in the flowering process. We have used soybean as a model system to uncover molecular events happening at the SAM in response to SD-induced flowering.6 Our result implicates a cross-regulatory mechanism involving ABA, JA and auxin in the floral initiation process in soybean.
Involvement of Multiple Hormonal Pathways in the Floral Initiation Process
We used soybean GeneChip® to investigate the molecular events taking place in the soybean terminal shoot apex that leads to the conversion of the SAM into an inflorescence meristem and the initiation of the floral meristem as a result of short-day (SD) photoperiod.6 To identify events that specifically took place in the SAM, SAMs were dissected under microscope from soybean plants subjected to different length of SD treatment (0, 1, 2, 4 and 6 SD). The resulting microarray data was analyzed using Microarray Significant Profiles (maSigPro)7 to identify transcripts with significantly differential expression profiles in the time-course experiments.
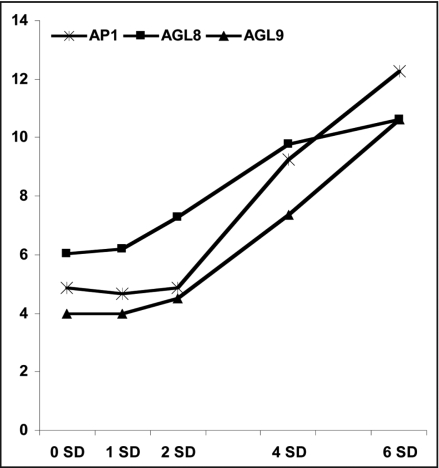
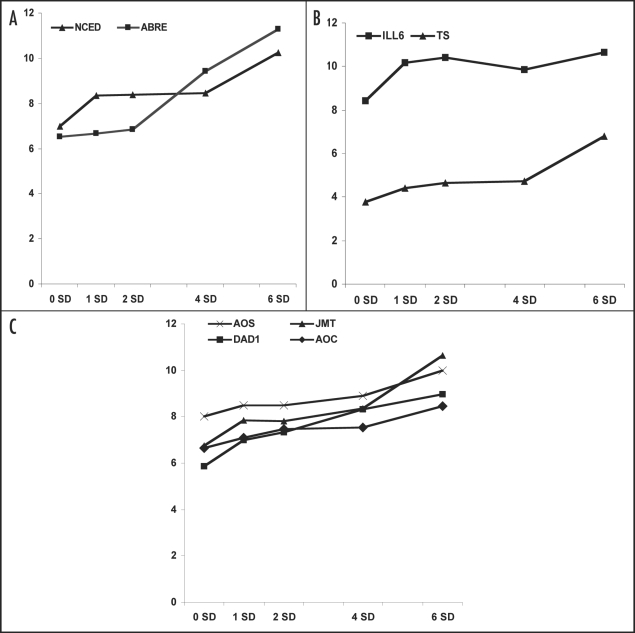
A total of 331 transcripts with significant expression profile changes were identified. Among them are putative soybean orthologs of floral homeotic transcripts that includes APETALA1 (AP1), FRUITFUL (AGL8) and AGL9 MADS BOX gene (Fig. 1). Since we are interested in the molecular events that drive the floral initiation process, we focus on transcripts with significant profile changes on 1 SD i.e., preceding that of floral homeotic sequences. Intriguingly, these are mostly related to ABA, auxin or JA (Fig. 2).
Figure 1.
Expression dynamics of putative floral homeiotic transcripts during the floral initiation process in the soybean SAM with dramatic induction on 2 SD. Log2-transformed normalized intensities are shown for each time point under study (SD: short-day).
Figure 2.
The expression profile for significantly induced transcripts during the floral transition process in soybean SAM. Activation of selected transcripts predicted to encode: (A) 9 cis-epoxycarotenoid dioxygenase (NCED) and ABA-responsive element binding protein (ABRE); (B) (IAA)-amino acid hydrolase6 (ILL6), tryptophan synthase (TS; Gma.736.1.A1_at); (C) DEFECTIVE IN ANTHER DEHISCENCE1 (DAD1), allene oxide cyclase (AOC; Gma.12166.1.S1_at), allene oxide synthase (AOS; Gma.9894.1.S1_at), s-adenosyl-l-methionine: jasmonic acid carboxyl methyltransferase (JMT; GmaAffx.47649.1.S1_at). Log2-transformed normalized intensities are shown for each time point under study (SD: short-day).
When our dataset was compared with the datasets generated from a recent study in Arabidopsis analyzing the transcriptional effects of ABA,8 one fifth of the transcripts could be identified as Arabidopsis orthologs that are responsive to ABA.6 Subsequent measurement of ABA level in the soybean SAM verified the increase of ABA during the floral initiation process6 and hence implicating the promoting role of ABA in flowering. ABA is perceived as a stress hormone. Since there is a striking occurrence of abiotic stress-related transcripts among the 331 sequences,6 an overlap of the floral signaling pathway in soybean with that of the abiotic stress is therefore very probable. This is in line with a recent report of the existence of a cluster of flowering control proteins in the interaction network associated with abiotic stress.9
Auxin exerts the majority of its effect in plants as indole acetic acid (IAA). Factors that influence the steady-state levels of free IAA in plant cells include the biosynthesis by the tryptophan-dependent pathway and the reversible conjugation with amino acid. The increased expression of a putative IAA-amino acid hydrolase6 (Gma.3543.1.S1_at) and tryptophan synthase (Gma.736.1.A1_at) on 1 SD (Fig. 2), and the subsequent induction of auxin efflux carrier and a number of auxin-responsive proteins imply the increase of auxin level in the SAM during the floral initiation process.6
Meanwhile, transcripts annotated to encode DEFECTIVE IN ANTHER DEHISCENCE1 (DAD1; Gma.12487.1.S1_at), allene oxide cyclase (AOC; Gma.12166.1.S1_at), allene oxide synthase (AOS; Gma.9894.1.S1_at), and s-adenosyl-l-methionine: jasmonic acid carboxyl methyltransferase (JMT; GmaAffx.47649.1.S1_at) are also among the transcripts induced at 1 SD (Fig. 2). Similar genes are known to be involved in the biosynthesis of JA and its methylated form, methyl jasmonate (MeJA). For example, in Arabidopsis DAD1 has been reported to encode a novel phospholipase protein catalysing the initial step of jasmonic acid biosynthesis10 while JMT has been reported to catalyse the methylation of JA to form MeJA.11 All these provide strong evidence of an increased level of JA in the SAM during the floral initiation process.
Auxin has been found to be essential for inducing organogenesis in the Arabidopsis inflorescence meristem and is believed to be present in floral primordia from an early stage.12–14 DAD1 protein is found to be essential for synchronizing pollen maturation, anther dehiscence and flower opening in Arabidopsis10 and the accumulation of a high level of JA in the soybean flowers has also been reported.15 Although these findings are associated with the later stage of floral organ development, our study has indicated that JA or auxin may play a novel role in regulating the floral evocation process. Interestingly, in addition to the increase of JA in the soybean flower,15 an independent study has also uncovered the increased level of JA in soybean leaves during the flowering process.16 Thus, there exists the possibility that JA may originate from distant plants parts such as leaves and thus participate in long distance signaling forming part of the floral inductive stimulus transmitted from the leaves. Once it is in the SAM, it may trigger further increase of JA level by activating the expression of transcripts related to JA biosynthesis as observed in this study. Future experiments characterizing the phloem sap from soybean plants undergoing the floral transition process shall assess the possibility of hormones reported in this study as part of the floral inductive stimulus transported from leaves.
Recent studies have suggested that cross-regulatory mechanisms are often involved in hormone signaling which increase the specificity of responses in different cellular contexts.17 For instance, there has been evidence suggesting an interplay between ABA and auxin during lateral root initiation with the existence of an ABA signaling cascade controlling meristem activity after lateral root emergence.18 It is more than likely that similar cross-regulatory mechanism involving these hormones operates at the SAM to regulate flowering. Further double-mutant genetic analyses and detailed spatial localization studies shall help to resolve these possibilities.
Potential Floral Repressors
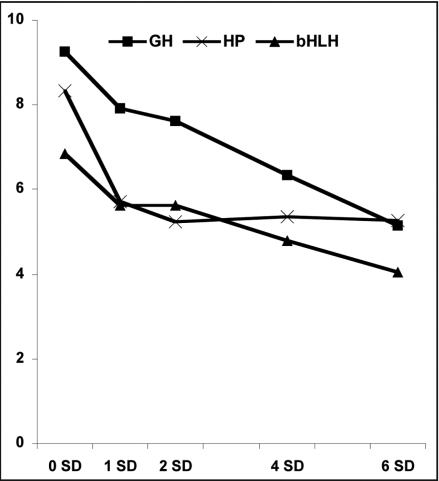
Floral initiation process is also characterized by the downregulation of genes and these could represent potential floral repressors with their transcription inactivated for the floral evocation process to occur in the incipient floral primordia. Transcripts downregulated at 1SD include transcripts predicted to encode glycosyl hydrolase family 1 protein (GH; Gma.16515.2.S1_a_at), hypoxia-responsive family protein (HP; Gma.3013.1.S1_s_at) and basic helix-loop-helix (bHLH; GmaAffx.79175.1.S1_at) family protein (Fig. 3). It is intriguing that the Arabidopsis counterpart of the identified GH is reported to encode myrosinase and there has been speculative model associating myrosinase with ABA signaling19 while hypoxia has been reported to interfere with ABA metabolism.20 Whether the downregulation of these transcripts is related to ABA during the floral transition process in soybean SAM awaits further study. Meanwhile, the bHLH proteins are a superfamily of transcription factors that can bind to DNA target sites and hence play important regulatory roles in diverse biological process. The transcript encoding bHLH identified in this study likely represents a novel floral repressor.
Figure 3.
The expression profile of transcripts significantly repressed during the floral transition process in soybean SAM. Repression of selected transcripts predicted to encode glycosyl hydrolase family 1 protein (GH; Gma.16515.2.S1_a_at), hypoxia-responsive family protein (HP; Gma.3013.1.S1_s_at) and basic helix-loop-helix (bHLH; GmaAffx.79175.1.S1_at) family protein. Log2-transformed normalized intensities are shown for each time point under study (SD: short-day). Log2-transformed normalized intensities are shown for each time point under study (SD: short-day).
Conclusions
Our study has implicated the involvement of multiple hormones during the floral initiation process in soybean. Future challenge will thus be to identify how these integrate into the current floral regulatory pathways and whether any of the potential floral repressor identified represents key players in such pathway.
Acknowledgements
We thank the Australian Research Council for financially supporting this project as a part of the ARC Centre of Excellence grant.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8978
References
- 1.Corbesier L, Coupland G. The quest for florigen: a review of recent progress. J Exp Bot. 2006;57:3395–3403. doi: 10.1093/jxb/erl095. [DOI] [PubMed] [Google Scholar]
- 2.Domoney C, Duc G, Ellis THN, Ferrandiz C, Firnhaber C, Gallardo K, et al. Genetic and genomic analysis of legume flowers and seeds. Curr Opin Plant Biol. 2006;9:133–141. doi: 10.1016/j.pbi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Wong CE, Bhalla PL, Ottenhof H, Singh MB. Transcriptional profiling of the pea shoot apical meristem reveals processes underlying its function and maintenance. BMC Plant Biol. 2008;8:73. doi: 10.1186/1471-2229-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haerizadeh F, Wong CE, Singh MB, Bhalla PL. Genome-wide analysis of gene expression in soybean shoot apical meristem. Plant Molec Biol. 2009;69:711–727. doi: 10.1007/s11103-008-9450-1. [DOI] [PubMed] [Google Scholar]
- 5.Haerizadeh F, Wong CE, Bhalla PL, Gresshoff PM, Singh MB. Genomic expression profiling of mature soybean (Glycine max) pollen. BMC Plant Biol. 2009:9. doi: 10.1186/1471-2229-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong CE, Singh MB, Bhalla PL. Molecular processes underlying the floral transition in the soybean shoot apical meristem. Plant J. 2009;57:832–845. doi: 10.1111/j.1365-313X.2008.03730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conesa A, Nueda MJ, Ferrer A, Talon M. maSigPro: a method to identify significantly differential expression profiles in time-course microarray experiments. Bioinformatics. 2006;22:1096–1102. doi: 10.1093/bioinformatics/btl056. [DOI] [PubMed] [Google Scholar]
- 8.Nemhauser JL, Hong FX, Chory J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell. 2006;126:467–475. doi: 10.1016/j.cell.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 9.Tardif G, Kane NA, Adam H, Labrie L, Major G, Gulick P, et al. Interaction network of proteins associated with abiotic stress response and development in wheat. Plant Molec Biol. 2007;63:703–718. doi: 10.1007/s11103-006-9119-6. [DOI] [PubMed] [Google Scholar]
- 10.Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence and flower opening in Arabidopsis. Plant Cell. 2001;13:2191–2209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo HS, Song JT, Cheong J-J, Lee Y-H, Lee Y-W, Hwang I, et al. Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proc Natl Acad Sci USA. 2001;98:4788–4793. doi: 10.1073/pnas.081557298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12:507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 14.Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- 15.Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 1995;92:4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koda Y, Yoshida K, Kikuta Y. Evidence for the involvement of jasmonic acid in the control of the stem-growth habit of soybean plants. Physiol Plant. 1991;83:22–26. [Google Scholar]
- 17.Kuppusamy K, Walcher C, Nemhauser J. Cross-regulatory mechanisms in hormone signaling. Plant Molec Biol. 2009;69:375–381. doi: 10.1007/s11103-008-9389-2. [DOI] [PubMed] [Google Scholar]
- 18.De Smet I, Zhang HM, Inze D, Beeckman T. A novel role for abscisic acid emerges from underground. Trends Plant Sci. 2006;11:434–439. doi: 10.1016/j.tplants.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Z, Zhang W, Stanley BA, Assmann SM. Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell. 2008;20:3210–3226. doi: 10.1105/tpc.108.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benech-Arnold RL, Gualano N, Leymarie J, Come D, Corbineau F. Hypoxia interferes with ABA metabolism and increases ABA sensitivity in embryos of dormant barley grains. J Exp Bot. 2006;57:1423–1430. doi: 10.1093/jxb/erj122. [DOI] [PubMed] [Google Scholar]