Abstract
Members of casein kinase 1 (CK1) are evolutionarily conserved eukaryotic protein kinases, which play fundamental roles in various cellular, physiological and developmental processes. One of the key mechanisms by which the activity of these multifunctional CK1 members is controlled appears to be their specific spatiotemporal compartmentalization within the cell. Plant genomes encode dozens of CK1 homologs, the function of which are not yet well characterized, however, evolutionary conservation of these genes predicts their fundamental roles in plants. Characterization of Arabidopsis CK1-like 6 (CKL6) that we have recently reported sheds new light on the existence of parallel and unique aspects of the mechanism involved in specific subcellular targeting as well as cellular function of CK1 in plants. In this addendum, I will focus my discussion on the versatility of CKL6 partitioning at different subcellular compartments and propose that this capability likely reflects its multiple functions in modulating an array of cellular targets.
Key words: protein phosphorylation, microtubule dynamics/organization, casein kinase 1, microtubule-binding domain, cell expansion, Arabidopsis
Regulation of CK1 by Spatial Partitioning
CK1 family members are multifunctional protein kinases that target numerous substrates within the cell, including cytoskeletal proteins, membrane receptors and transporters, nuclear factors, vesicle-associated proteins and signal transduction components.1 CK1 members share a similar catalytic activity and substrate specificity as predicted from their highly conserved catalytic domain. Moreover, unlike many other protein kinase families, CK1 does not require an activation mechanism controlled by second messengers. The distinct function of each isoform within the cell is achieved therefore, in part, due to their subcellular targeting to specific compartments or tight association with the target molecules. Domain-swapping experiments performed with yeast CK1 isoforms clearly demonstrate that the interaction between CK1 and its substrates is controlled by the ability of the kinase to associate stably with different subcellular compartments. Budding yeast encodes four CK1 homologues that are essential to yeast vitality, but YCK1/2, and 3 localize to the plasma membrane via palmitoylation, whereas HRR25 the nucleus.2,3 The non-redundant HRR25 and YCKs, however, were shown to functionally complement each other when localization signals were swapped,2 supporting that the subcellular targeting of each isoform is an important determinant for its specific function.
Arabidopsis encodes 14 CK1-like (CKL) members which differentially localize to the cytoplasm, nucleus, ER, or vesicle-like punctate structures.4 Each isoform also appears to differ in overall expression patterns at the cellular and tissue level (https://www.genevestigator.ethz.ch), indicating that CKL members likely perform non-redundant biological functions. Molecular mechanisms underlying subcellular targeting are not well defined for CK1 members in higher animals and plants; however, their association with diverse subcellular compartments1,4 is consistent with the notion that the spatial control of CK1 members is likely a crucial factor for determining specific biological function.
Mechanism of Arabidopsis CKL6 Subcellular Targeting
Arabidopsis CKL members contain a catalytic domain at the N-terminus and a variable domain at the C-terminus4 Several isoforms of yeast and animal CK1 have been shown to require the C-terminal variable domain for substrate specificity, regulation of protein-protein interaction, and/or subcellular localization.5–7 Consistently, targeting of CKL6 to the cortical microtubules is mediated by its C-terminal domain that contains a novel microtubule-binding domain.8 However, the fact that full-length CKL6 associates with not only the cortical microtubules but also distinct punctate structures suggests that the control over subcellular compartmentalization of CKL6 is not that straightforward. Rather, it appears that the association of CKL6 with the microtubules is transient—regulated by the catalytic activity—and the kinase shuttles between microtubules and punctate particles. The following observations corroborate this possibility.
Knocking-out the catalytic activity of CKL6 by introducing a substitution mutation at Lys42 or Asp1329,10 altered the localization pattern of the kinase-inactive (KI) mutant such that it localizes to both punctate structures and microtubules, but with a much stronger affinity to microtubules compared to the wild type CKL6 (Ben-Nissan G and Lee J-Y, unpublished data). Active CKL6 full-length enzyme shows a weaker microtubule labeling than does the KI or the C-terminal domain alone, consistent with the idea that the interaction of CKL6 with microtubules is transient when the enzyme is catalytically active. When a microtubule inhibitor was applied to the plants expressing fluorescently-tagged KI mutant, accumulation of fluorescent signals appeared to shift from depolymerizing microtubules to the punctate structures. By contrast, depolymerized microtubules or tubulins did not accumulate to such structures but dispersed to the cytoplasm. These observations suggest that partitioning of CKL6 between microtubules and the punctate structures is a dynamic and complex event that requires coordination between the microtubule binding activity conferred by the C-terminal domain and the catalytic activity that perhaps counteracts or reverses its binding affinity to the microtubules.
Regulation of Cortical Microtubule and Cell Expansion by CKL6
Various protein kinases are involved in regulating microtubule dynamics by phosphorylating microtubule associated proteins (MAPs).11,12 Regulation of microtubules by direct phosphorylation of microtubule building blocks tubulins is not as widely observed. However, two recent reports8,13 denote that direct modulation of tubulins by phosphorylation may add to the multi-layered regulation of microtubule dynamics in both mammalian and plant systems. Cyclin-dependent kinase Cdk1 was shown to phosphorylate Ser172 of tubulin β and alters microtubule dynamics during the mitotic phase.13 We have shown that CKL6 phosphorylates Ser413/420 of tubulin β and overexpression of CKL6 can alter the cortical microtubule organization during interphase.8 The fact that these phosphorylation sites are evolutionarily conserved implies that both post-translational modifications may constitute a fundamental mechanism to fine-tune the microtubule function.
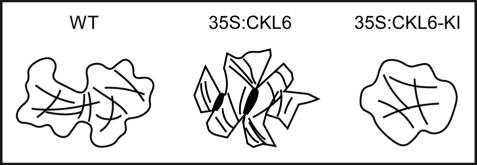
CKL6 is ubiquitously expressed in various tissues at a relatively low level not detectable by conventional Northern analysis (Ben-Nissan G and Lee J-Y, unpublished data). Genevestigator expression data (https://www.genevestigator.ethz.ch) show that CKL6 expression is relatively higher in two cell types, pollen and root hair cells, than in other tissues. One biological theme common to these two cell types includes the polarized cell growth essential for pollen germination and tube growth and root hair cell elongation. It is tempting to speculate that CKL6 may play a specific role in these cell types through modulation of microtubules given that ectopic expression of active and inactive CKL6 perturbs cortical microtubule organization and polarized cell expansion (Fig. 1). Another possibility is a potential involvement of CKL6 in vesicle trafficking which is also a crucial process for those cell types. Exploiting these cellular systems may prove to be advantageous in gaining new insight into the mechanism that CKL6 is involved to modulate cell growth and shape formation.
Figure 1.
Altered microtubule and cell morphology phenotypes induced by overexpression of active and inactive CKL-6. Cartoons illustrating the cell shape and cortical microtubule phenotypes observed in the wild type (WT), 35S:CKL6, and 35S:CKL6-KI leaf epidermal pavement cells are presented based on the published data.8
Role in Vesicle Trafficking?
The role of CK1 in membrane trafficking was suggested by the observation that animal CK1δ isoform localizes to punctate structures that were found in the trans-Golgi or the cytoplasm including small synaptic vesicles containing the components phosphorylated by CK1.14,15 Moreover, yeast CK1/2, and 3 associate with plasma membrane through isoprenylation sites at their C-termini.2 In Arabidopsis, at least five CKL members including CKL6 localizes to punctate particles, identities of which are not yet known.4 Do these CKLs have roles in membrane trafficking?
I propose that the following circumstantial evidence correlates with a role at least for CKL6 in membrane trafficking. CKL6 was found to co-fractionate with microsomal membranes and partially overlap with late endosomes enriched with prevacuolar markers when separated on a sucrose gradient (Yang Y and Lee J-Y, unpublished data). In the absence of predictable membrane-spanning domains or lipid modification sites, a recognition/interaction with membrane components would be essential for CKL6 to associate with membrane compartments. It remains to be elucidated whether the punctate particles labeled by fluorescently-tagged CKL6 are vesicular compartments, but it is noteworthy that these particles are motile along the actin filaments (Ben-Nissan G and Lee J-Y, unpublished data). Coexpression analysis available for CKL6 (http://atted.jp/) revealed a strong expression correlation with phosphatidyl inositol signaling pathway, which constitutes an important membrane trafficking pathway. Involvement of CK1 in this signaling pathway during asymmetric cell division has been demonstrated in C. elegance.16 Although likely, it remains to be seen whether this process is also conserved in plants. Future investigation into isolation of membrane trafficking components as targets of CKL6 (or other members) will be one of the imperative tasks in order to firmly establish a role for CKL in membrane trafficking.
Conclusions
Characterization of CKL6 in terms of its subcellular targeting to cortical microtubules, the molecular mechanism underlying interaction with microtubules, and in vitro tubulin phosphorylation has identified this member of CK1 as a novel MAP. Cytoskeleton association of animal CK1 members has been known, but the molecular mechanism or determinant by which this association is accomplished remained elusive. On one hand, our study provides new insight into the role of this evolutionarily conserved protein kinase in the regulation of cortical microtubule organization crucial for the polarized cell growth and shape formation. Targeting of CKL6 to punctate particles in addition to microtubules, on the other hand, suggests that CKL6 likely perform multiple functions in multiple locations. Elucidating the molecular mechanisms underlying the activation and localization, identifying substrates, and assigning the specific function of each plant CK1 may provide new opportunities to discover many basic principles of eukaryotic cellular processes as well as unique mechanisms evolved in plant cells.
Acknowledgements
This work was supported by the National Science Foundation (grant no. MCB 0445626 to J.-Y.L.) and the National Institutes of Health (grant no. NCRR COBRE P20 RR-15588 to J.-Y.L.). I thank Jeanette Miller for proofreading this manuscript.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8991
References
- 1.Knippschild U, et al. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Vancura A, et al. A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J Biol Chem. 1994;269:19271–19278. [PubMed] [Google Scholar]
- 3.Wang X, et al. Prenylated isoforms of yeast casein kinase I, including the novel Yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase. Mol Cell Biol. 1996;16:5375–5385. doi: 10.1128/mcb.16.10.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JY, et al. Plasmodesmal-associated protein kinase in tobacco and Arabidopsis recognizes a subset of non-cell-autonomous proteins. Plant Cell. 2005;17:2817–2831. doi: 10.1105/tpc.105.034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babu P, et al. Plasma membrane localization of the Yck2p yeast casein kinase 1 isoform requires the C-terminal extension and secretory pathway function. J Cell Sci. 2002;115:4957–4968. doi: 10.1242/jcs.00203. [DOI] [PubMed] [Google Scholar]
- 6.Swiatek W, et al. Regulation of casein kinase I epsilon activity by Wnt signaling. J Biol Chem. 2004;279:13011–13017. doi: 10.1074/jbc.M304682200. [DOI] [PubMed] [Google Scholar]
- 7.Gietzen KF, Virshup DM. Identification of inhibitory autophosphorylation sites in casein kinase I epsilon. J Biol Chem. 1999;274:32063–32070. doi: 10.1074/jbc.274.45.32063. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Nissan G, et al. Arabidopsis casein kinase 1-like 6 contains a microtubule-binding domain and affects the organization of cortical microtubules. Plant Physiol. 2008;148:1897–1907. doi: 10.1104/pp.108.129346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J, et al. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell. 1998;93:851–861. doi: 10.1016/s0092-8674(00)81445-2. [DOI] [PubMed] [Google Scholar]
- 10.Peters JM, et al. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 11.Vasquez RJ, Gard DL, Cassimeris L. Phosphorylation by CDK1 regulates XMAP215 function in vitro. Cell Motil Cytoskeleton. 1999;43:310–321. doi: 10.1002/(SICI)1097-0169(1999)43:4<310::AID-CM4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Sasabe M, et al. Phosphorylation of NtMAP65-1 by a MAP kinase downregulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev. 2006;20:1004–1014. doi: 10.1101/gad.1408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fourest-Lieuvin A, et al. Microtubule regulation in mitosis: tubulin phosphorylation by the cyclin-dependent kinase Cdk1. Mol Biol Cell. 2006;17:1041–1050. doi: 10.1091/mbc.E05-07-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross SD, et al. A phosphatidylinositol 4,5-bisphosphate-sensitive casein kinase I alpha associates with synaptic vesicles and phosphorylates a subset of vesicle proteins. J Cell Biol. 1995;130:711–724. doi: 10.1083/jcb.130.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrend L, et al. Interaction of casein kinase 1delta (CK1delta) with post-Golgi structures, microtubules and the spindle apparatus. Eur J Cell Biol. 2000;79:240–251. doi: 10.1078/s0171-9335(04)70027-8. [DOI] [PubMed] [Google Scholar]
- 16.Panbianco C, et al. A casein kinase 1 and PAR proteins regulate asymmetry of a PIP(2) synthesis enzyme for asymmetric spindle positioning. Dev Cell. 2008;15:198–208. doi: 10.1016/j.devcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]