Abstract
Cell-to-cell communication is crucial for multicellular development, and in plants occurs through specialized channels called plasmodesmata (PD). In our recent manuscript1 we reported the characterization of a PD trafficking mutant, ‘gfp arrested trafficking 1’ (gat1), which carries a mutation in the thioredoxin-m3 (TRX-m3) gene. gat1 mutants showed restricted GFP transport from the phloem to the root meristem that appears to result from structural modifications in the PD channel. We found accumulation of reactive oxygen species (ROS) and callose, as well as a reduction in starch granules in the gat1 root meristem. Application of oxidants to wildtype plants and expression of our GFP reporter in the mutant root meristemless 1 (rml1) mimic the gat1 phenotype. Our results suggest that mutations in GAT1 cause ROS accumulation and induce the biosynthesis of callose, which in turn block PD transport. Therefore, we propose a model whereby GAT1/TRX-m3 is a component of a redox-regulated pathway that maintains PD permeability in Arabidopsis meristems.
Key words: transport, thioredoxin-m3, plasmodesmata, meristem, development, reactive oxygen species
Introduction
Plasmodesmata (PD) are channels that mediate symplasmic communication between plant cells. They facilitate the intercellular movement of viruses, metabolites, proteins and RNAs.2–4 PD also connect companion cells (CC) and sieve elements (SE) mediating the phloem loading and unloading of molecules transported from the source into the sink tissues.5 Although the screening for PD mutants has been pursued by different strategies, few mutants have been isolated suggesting that interference with symplasmic continuity is highly detrimental to plant development.6,7
The transport through PD is in part regulated by the deposition of callose in the channel.8–10 Callose is produced in the cell wall by callose synthases and its synthesis is regulated according to cell type and environmental conditions.11 Callose accumulation is induced in oxidative conditions and as part of the defense response to biotic and abiotic stresses.12–14 Alternatively, ADP-glucose pyrophosphorylase (AGPase), a key enzyme in starch biosynthesis, is activated in reductive conditions.15 This evidence suggests that cell redox homeostasis might play an important role in the regulation of carbon partitioning between growth or stress response processes (cellulose/callose production) and carbon storage (starch production).11,16
Reactive Oxygen Species (ROS) are generated in the plant as a by-product of photosynthesis and photorespiration.17 Oxidative damage by accumulation of ROS is prevented by an efficient antioxidant system.18 At lower concentrations, ROS function in signaling pathways that regulate plant development in response to physiological and environmental cues.19 Although several reports highlight a connection between generation of ROS and the production of callose,12,16 little is known about the mechanism that mediates this process and its potential significance in regulation of PD transport. In our recent manuscript we characterized ‘gfp arrested trafficking 1’ (gat1, pronounced “gate”): a PD trafficking mutant defective in the plastidial thioredoxin-m3 (TRX-m3).1 Thioredoxins regulate the redox activation/inactivation of metabolic and antioxidant proteins.20,21 We propose a role for this thioredoxin in a mechanism that maintains a high level of cell-cell communication in sink tissues by modulating the production of callose in the PD.
Thioredoxin-m3 Regulates PD Transport in Arabidopsis Meristems
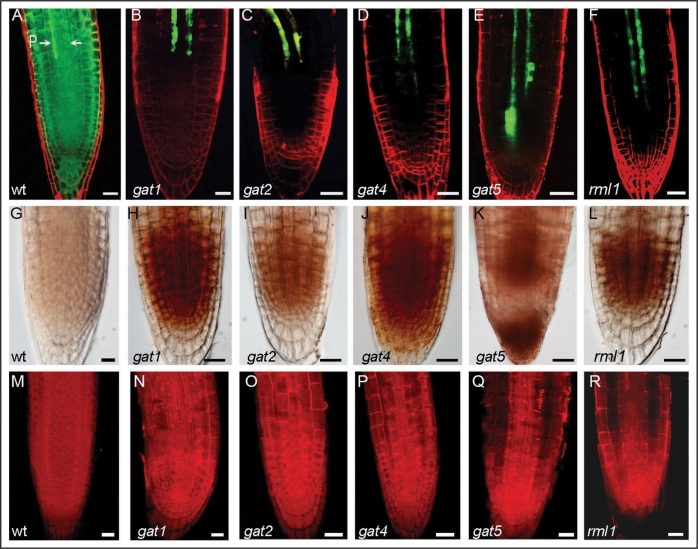
gat1 was isolated in a genetic screen to identify mutants defective in transport of green fluorescent protein (GFP), using a starter line expressing GFP from the phloem CC specific SUCROSE-H+SYMPORTER2 (SUC2) promoter. Wildtype plants show diffusion of the GFP reporter protein out of the phloem into the shoot and root meristems.22 The mutants isolated in the screen showed a reduction in GFP transport out of the phloem (Fig. 1A–E). Most mutants (gat1, 2, 4 and 5) are seedling lethal, indicating that, as expected, phloem transport is essential for seedling development.
Figure 1.
GFP transport, staining of ROS and callose in wildtype, gat and rml1 mutants. (A–F) Confocal images of root apices expressing cytoplasmic GFP driven by the SUC2 promoter from wildtype (wt) and mutants (gat1, gat2, gat4, gat5 and rml1) seedlings. Note that GFP diffuses out of the phloem (P) into the root meristem in wildtype seedlings but not in the mutants. (G–L) show diamino benzidine staining of ROS in the roots of wildtype and the mutant seedlings. (M–R) show aniline blue staining of callose in the roots of wildtype and the mutant seedlings. Note that the mutants accumulate higher levels of ROS and callose. Scale bar = 20 µm.
Further characterization of the gat1 developmental phenotype revealed defects in meristem maintenance. We also found that PD in the gat1 root meristem were sometimes branched or occluded, suggesting a reduced transport capability. Therefore, we proposed that GAT1 regulates PD permeability and this regulation is essential for meristem development. Positional cloning followed by complementation analysis indicated that GAT1 encodes the predicted thioredoxin TRX-m3 (At2g15570). Although devoid of any mutation in the coding and promoter regions, methylation profiling of the original gat1 allele indicated that the mutant allele was hypermethylated. Additional gat1 alleles also showed restricted transport and developmental defects, confirming that mutations in TRX-m3 led to the observed trafficking phenotype.
Interestingly, ectopic expression of GAT1 in mature leaves enhanced GFP diffusion, indicating that GAT1 is not only necessary, but also sufficient, to regulate intercellular transport. These transgenic plants also exhibited late flowering and delayed senescence phenotypes, indicating that altered PD transport might affect these developmental transitions. Together, these results suggest that GAT1 plays a role in the regulation of intercellular PD transport that is critical for the maintenance of stem cell populations and developing tissues.
Exposing Plants to Oxidative Conditions Mimics the gat1 Phenotype
Thioredoxins are proposed to function in the post-translational regulation of target proteins that participate in different metabolic processes or act as antioxidants.20,21 The finding that gat1 mutants accumulate ROS in the root tip provides an interesting link between regulation of intercellular transport and cell redox homeostasis (Fig. 1H). Moreover, the exogenous application of oxidants to wildtype plants expressing pSUC2-GFP, and the expression of this reporter in root meristemless 1 mutants (rml1), which are defective in the synthesis of the antioxidant glutathione23 (Fig. 1F), phenocopied the gat1 trafficking defects. These results suggest that the transport and developmental defects observed in gat1 are linked to an abnormal accumulation of ROS. However, antioxidant treatments (reduced glutathione, dithiothreitol or sodium ascorbate) were unable to rescue the gat1 GFP trafficking phenotype (unpublished results), suggesting that the mechanism that triggers PD modification in the gat1 mutants occurs early in development and may be irreversible at the time of treatment (during germination).
Since callose has been associated with reduced PD permeability in mature tissues, and the synthesis of this polymer is dependent on oxidative conditions, we assayed callose accumulation in gat1, rml1 and oxidant-treated seedlings and found that in each case its accumulation was enhanced in the root meristem (Fig. 1M, N and R). Additional trafficking mutants isolated in our screen (gat2, gat4 and gat5) also showed increased ROS production and callose deposition in the root tip (Fig. 1I–K, O–Q). Therefore, these mutants, together with gat1 and rml1, might be part of the same or a similar pathway controlling symplasmic unloading in the root meristem via oxidative regulation of callose metabolism.
Hypothesis: GAT1 Plays a Physiological Role in the Redox-Dependent Metabolic balance of Sink Tissues
In contrast to the other Arabidopsis m-type thioredoxins, TRX-m3 is most highly expressed in non-green plastids located in meristems and in the vasculature. In addition, TRX-m3 does not exhibit in vitro affinity or activity with standard substrates (NADP-MDH, FBPase or 2-Cys Prx) and cannot complement the yeast thioredoxin-null mutant phenotype.24 These evidences suggest that TRX-m3 may have a specialized function in regulating the activity of distinct targets.
The reduced number of starch granules found in gat1 columella cells and the partial rescue by sucrose addition support a role for GAT1/TRX-m3 in the regulation of starch metabolism and/or in the phloem transport of sucrose into the Arabidopsis root. The transport (and storage) of photo-assimilates provides the energy that supports the high rate of cell division and growth in meristematic tissues.25 We propose that the defects in meristem maintenance that lead to the arrest of growth, and ultimately lethality in gat1, are a consequence of an impaired regulation in carbon allocation and partitioning. This physiological function of GAT1 might be executed by reductive activation/inactivation of plastidial enzymes that participate in starch metabolism. This redox modulation of carbon metabolism might affect sugar signaling pathways since it has been shown that sugar and thiol-disulfide signals may share common metabolic targets.26 However, further proteomic studies to identify specific targets of TRX-m3 in meristems will be needed to elucidate this pathway.
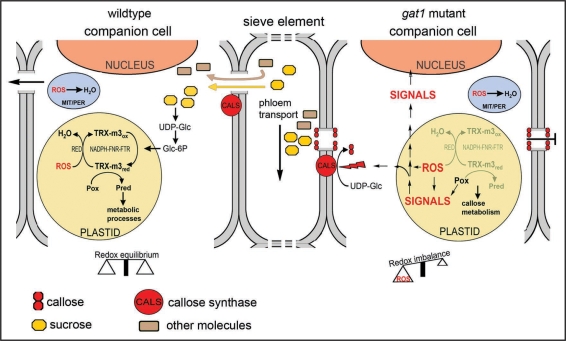
Based on our results, we propose a model that links the loss of GAT1/TRX-m3 with altered carbohydrate metabolism and permeability of PD channels (Fig. 2). In physiological conditions, it has been shown that sucrose transported by the phloem to the sink tissues activates the reduction of TRX-m by a NADPH-FNR-FTR pathway.20,21,27 Reduced TRX participates in the reductive regulation of metabolic enzymes and in ROS detoxification mediated by reductants (antioxidants).20,21,27 We propose that loss of GAT1/TRX-m3 blocks ROS scavenging and inactivates essential proteins involved in metabolic processes required for normal development (such as carbon storage). The resulting oxidative environment and changes in metabolic activity induce the synthesis of callose by callose synthases (CALS) as described in other instances.11,12,14,16 Alternatively, TRX-m3 may regulate unidentified target proteins, which are involved in an early step of callose metabolism, or produce signaling molecules that (directly or by regulation of gene expression) activate this process. Deposition of callose plugs the PD channels and blocks symplasmic unloading as indicated by restricted GFP diffusion (Fig. 2).
Figure 2.
Model for the role of GAT1/TRX-m3 in the regulation of PD transport in Arabidopsis meristems (based on Schurmann and Buchanan, 2008). Metabolites are transported through the phloem from source to sink tissues and are unloaded symplasmically into companion cells from the sieve elements. Sucrose, the main form in which photoassimilates are transported, is cleaved to UDP-glucose (UDP-Glc), which is thereafter metabolized to glucose 6-phosphate (Glc-6P). TRX reduction is induced by Glc-6P via NADPH-FNR-FTR pathway. Reduced TRX regulates the activity of target proteins (Pox: oxidized protein, Pred: reduced protein) involved in metabolic processes or in ROS detoxification (RED: reductants). The reduction of ROS (generated during metabolism) also takes place in other organelles such as mitochondria and peroxisomes (MIT/PER), which, together with the plastids, maintain cell redox homeostasis. Loss of TRX-m3, or oxidative stress, alters this redox equilibrium and affects metabolic balance, which induces a “defense” response that diverts UDP-Glc to the synthesis of callose by callose synthases (CALS). Alternatively, ROS or other signaling molecules produced in the plastid might modulate the activity of proteins, the synthesis of starting metabolites or the expression of genes that participate in callose metabolism. Callose deposition constricts the PD channel and blocks the unloading of essential molecules into the cell, which adversely affects meristem development.
Concluding Remarks
Our results indicate that GAT1 (TRX-m3) is necessary and sufficient to control intercellular PD transport, most likely via a redox dependent mechanism that modulates callose production and deposition in PD channels. Loss of GAT1 or its ectopic expression resulted in severe developmental phenotypes, indicating that this mechanism is critical for normal plant development. The recent identification of PD-localized proteins that bind callose and regulate its deposition in the channel8,28 also support the role of callose as a key component in PD regulation. The redox regulation of callose metabolism by GAT1/TRX-m3 appears to be essential to maintain cell-cell communication in meristems. Further efforts will be required to identify TRX-m3 target proteins and to clone other trafficking mutants exhibiting defective redox phenotypes in order to elucidate additional players in this novel regulatory mechanism.
Acknowledgements
We thank Fritz Kragler, John Lunn and all members of Dave Jackson laboratory for helpful comments on the manuscript.
Abbreviations
- PD
plasmodesmata
- ROS
reactive oxygen species
- gat
‘gfp arrested trafficking’
- TRX
thioredoxin
- CC
companion cells
- SE
sieve elements
- AGPase
ADP-glucose pyrophosphorylase
- SUC2
SUCROSE-H+SYMPORTER2
- rml1
root meristemless 1
- NADP-MDH
NADP-malate dehydrogenase
- FBPase
fructose-1,6-bisphosphate phosphatase
- 2-Cys Prx
2-Cys peroxiredoxin
- FNR
ferredoxin-NADP+ reductase
- FTR
ferredoxin-thioredoxin reductase
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8992
References
- 1.Benitez-Alfonso Y, Cilia M, San Roman A, Thomas C, Maule A, Hearn S, et al. Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc Natl Acad Sci USA. 2009;106:3615–3620. doi: 10.1073/pnas.0808717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford KM, Zambryski PC. Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol. 2001;125:1802–1812. doi: 10.1104/pp.125.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas WJ, Lee JY. Plasmodesmata as a supracellular control network in plants. Nat Rev Mol Cell Biol. 2004;5:712–726. doi: 10.1038/nrm1470. [DOI] [PubMed] [Google Scholar]
- 4.Benitez-Alfonso Y, Cantrill L, Jackson D. Plasmodesmata: Cell-Cell Channels in Plants. In: Baluska F, Volkmann D, Barlow PW, editors. Cell-Cell Channels. Austin, TX: Landes Bioscience; 2006. [Google Scholar]
- 5.Oparka KJ, Cruz SS. The great escape: phloem transport and unloading of macromolecules. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:323–347. doi: 10.1146/annurev.arplant.51.1.323. [DOI] [PubMed] [Google Scholar]
- 6.Kim I, Hempel FD, Sha K, Pfluger J, Zambryski PC. Identification of a developmental transition in plasmodesmatal function during embryogenesis in Arabidopsis thaliana. Development. 2002;129:1261–1272. doi: 10.1242/dev.129.5.1261. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K, Otegui MS, Krishnakumar S, Mindrinos M, Zambryski P. INCREASED SIZE EXCLUSION LIMIT 2 encodes a putative DEVH box RNA helicase involved in plasmodesmata function during Arabidopsis embryogenesis. Plant Cell. 2007;19:1885–1897. doi: 10.1105/tpc.106.045666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson C, Thomas C, Findlay K, Bayer E, Maule AJ. An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell. 2009;21:581–594. doi: 10.1105/tpc.108.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucher GL, Tarina C, Heinlein M, Di Serio F, Meins F, Jr, Iglesias VA. Local expression of enzymatically active class I beta-1, 3-glucanase enhances symptoms of TMV infection in tobacco. Plant J. 2001;28:361–369. doi: 10.1046/j.1365-313x.2001.01181.x. [DOI] [PubMed] [Google Scholar]
- 10.Radford JE, Vesk M, Overall RL. Callose deposition at plasmodesmata. Protoplasma. 1998;201:30–37. [Google Scholar]
- 11.Scheible WR, Pauly M. Glycosyltransferases and cell wall biosynthesis: novel players and insights. Curr Opin Plant Biol. 2004;7:285–295. doi: 10.1016/j.pbi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, et al. The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot. 2002;53:1367–1376. [PubMed] [Google Scholar]
- 13.Kurek I, Kawagoe Y, Jacob-Wilk D, Doblin M, Delmer D. Dimerization of cotton fiber cellulose synthase catalytic subunits occurs via oxidation of the zinc-binding domains. Proc Natl Acad Sci USA. 2002;99:11109–11114. doi: 10.1073/pnas.162077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma DP, Hong Z. Plant callose synthase complexes. Plant Mol Biol. 2001;47:693–701. doi: 10.1023/a:1013679111111. [DOI] [PubMed] [Google Scholar]
- 15.Hendriks JH, Kolbe A, Gibon Y, Stitt M, Geigenberger P. ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol. 2003;133:838–849. doi: 10.1104/pp.103.024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geigenberger P, Kolbe A, Tiessen A. Redox regulation of carbon storage and partitioning in response to light and sugars. J Exp Bot. 2005;56:1469–1479. doi: 10.1093/jxb/eri178. [DOI] [PubMed] [Google Scholar]
- 17.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 18.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 19.Pitzschke A, Forzani C, Hirt H. Reactive oxygen species signaling in plants. Antioxid Redox Signal. 2006;8:1757–1764. doi: 10.1089/ars.2006.8.1757. [DOI] [PubMed] [Google Scholar]
- 20.Vieira Dos Santos C, Rey P. Plant thioredoxins are key actors in the oxidative stress response. Trends Plant Sci. 2006;11:329–334. doi: 10.1016/j.tplants.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Gelhaye E, Rouhier N, Navrot N, Jacquot JP. The plant thioredoxin system. Cell Mol Life Sci. 2005;62:24–35. doi: 10.1007/s00018-004-4296-4. [DOI] [PubMed] [Google Scholar]
- 22.Stadler R, Wright KM, Lauterbach C, Amon G, Gahrtz M, Feuerstein A, et al. Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. Plant J. 2005;41:319–331. doi: 10.1111/j.1365-313X.2004.02298.x. [DOI] [PubMed] [Google Scholar]
- 23.Cairns NG, Pasternak M, Wachter A, Cobbett CS, Meyer AJ. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol. 2006;141:446–455. doi: 10.1104/pp.106.077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collin V, Issakidis-Bourguet E, Marchand C, Hirasawa M, Lancelin JM, Knaff DB, et al. The Arabidopsis plastidial thioredoxins: new functions and new insights into specificity. J Biol Chem. 2003;278:23747–23752. doi: 10.1074/jbc.M302077200. [DOI] [PubMed] [Google Scholar]
- 25.Riou-Khamlichi C, Menges M, Healy JM, Murray JA. Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol. 2000;20:4513–4521. doi: 10.1128/mcb.20.13.4513-4521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolbe A, Oliver SN, Fernie AR, Stitt M, van Dongen JT, Geigenberger P. Combined transcript and metabolite profiling of Arabidopsis leaves reveals fundamental effects of the thiol-disulfide status on plant metabolism. Plant Physiol. 2006;141:412–422. doi: 10.1104/pp.106.081208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schurmann P, Buchanan BB. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid Redox Signal. 2008;10:1235–1274. doi: 10.1089/ars.2007.1931. [DOI] [PubMed] [Google Scholar]
- 28.Levy A, Erlanger M, Rosenthal M, Epel BL. A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. Plant J. 2007;49:669–682. doi: 10.1111/j.1365-313X.2006.02986.x. [DOI] [PubMed] [Google Scholar]