Abstract
In our recent publication,1 we have shown that a T-DNA insertion in Arabidopsis CIPK6 gene encoding a CBL-interacting protein kinase caused reduction in expression of the gene and emergence of lateral roots. The change in phenotype in the mutant line was likely due to reduction in shoot-to-root acropetal and the root tip basipetal auxin transport. Here we report identification of a homozygous knockout line of AtCIPK6 (atcipk6) with no detectable expression of the gene in normal growth condition. The knockout line exhibited considerable decrease in growth rate of the taproot as well as in emergence of lateral roots. The mutant line also showed reduction in the root tip basipetal and shoot-to-root acropetal auxin transport. Relative rate of auxin transport and the root phenotype of the atcipk6 closely matched with those of pgp4-1, an Arabidopsis line mutated in PGP4. This gene encodes an ABC integral membrane transporter, which functions in polar auxin transport. These observations strengthen our earlier proposal that CIPK6 is probably involved in polar auxin transport and indicate that it may function through the PGP4 auxin transporter.
Key words: Arabidopsis, CIPK6, auxin, MDR4/PGP4, root
Root cells are first to respond to change in soil conditions. Continuous change in root system architecture is the vital part of the developmental plasticity that allows the plant to adjust in altered environment. Phytohormone auxin is the most important regulator of all the stages of root development. For example, mutation in Aberrant Lateral Root Formation3 (ALF3) gene results in arrest of lateral root development, which can be rescued by exogenous application of auxin.2 Mutation in PGP4 that functions in polar auxin transport causes reduction in root length.3 Recently, an unprecedented role of stress-related phytohormone abscisic acid (ABA) in regulation of lateral root formation is emerging. A gene encoding ABA-biosynthesis enzyme 9-cis-epoxycarotenoid dioxygenase 9 (NCED9) was reported to express in the pericycle cells surrounding the lateral root initiation sites.4 ABI5, an ABA-induced transcription factor encoding gene is expressed in the tip of the lateral root5 and abi8 mutant showed defects in root meristem maintenance.6 While lateral root arrest after emergence of primordia in alf3 mutant can be rescued by exogenous application of auxin; the same treatment cannot rescue ABA-induced arrest of lateral root development.7 Therefore, it appears that both the phytohormones auxin and ABA play coordinated but distinct roles in lateral root development. Interestingly, in agreement with possible cross talks between the signaling pathways regulated by these two hormones the ABA-responsive mutants, abi3, lrd2 and aba2-1 show reduced response to polar auxin transport inhibitors.8 We reported recently that a mutant Arabidopsis line (atcipk6kd: SALK_080951) compromised in expression of CBL-interacting protein kinase 6 (CIPK6) showed reduction in polar auxin transport. CIPK6 gene expression in chickpea is induced by ABA and atcipk6kd line is relatively more sensitive to high salinity.1 This observation reinforces the concept of coordinated function of auxin and ABA signaling in lateral root development and indicates that CIPK6 may be a nodal point of those two signaling pathways.
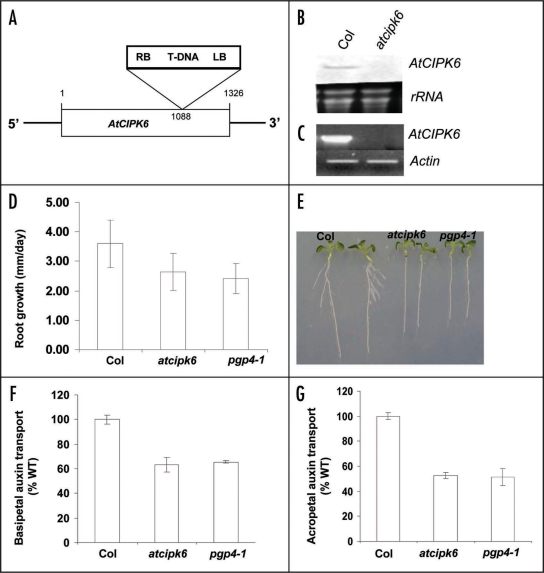
To further investigate the biological function of AtCIPK6, a T-DNA insertion line (GK-448C12-024532) available through the European Arabidopsis stock Center at Nottingham University (NASC: http://arabidopsis.info/) was procured and the seeds obtained were screened for the homozygous T-DNA insertion. In order to confirm the exact position of the T-DNA insertion the junction of AtCIPK6 and T-DNA was amplified using RP GABI 5′-GAA GAA AGG ATA CGA CGG AGC-3′ and 08409 5′-ATA TTG ACC ATC ATA CTC ATT GC-3′ (http://arabidopsis.info/) and sequenced. The sequence revealed that the T-DNA insertion in this homozygous line (atcipk6) is after the 1088th base from the translation start site of AtCIPK6 gene (Fig. 1A). Expression of AtCIPK6 gene was analyzed in this mutant line in normal growth condition. No full-length ORF-specific product was detected after reverse transcription followed by PCR amplification (RT-PCR) up to 30 cycles (Fig. 1C) or by northern analysis with full-length cDNA as probe (Fig. 1B). For morphological phenotype analysis growth rate of primary root was measured in vertically grown seedlings between the period 4-days to 7-days after germination. Growth rate of primary roots of atcipk6 seedlings was 29% (p < 0.005) less when compared with wild type seedlings (Col) of same stage (Fig. 1D). Unlike the wild type seedlings, the mutant seedlings did not exhibit any lateral root emergence up to 10-day after germination (Fig. 1E). In our previous report, we mentioned that atcipk6kd mutant had defects in lateral root emergence and showed reduced polar auxin transport. Based on the observation we proposed a hypothesis that AtCIPK6 may regulate an auxin efflux transporter in Arabidopsis. Two membrane-bound transporter proteins have been identified as substrates of two other CIPKs.9,10 Exploring the possibility we searched the literature for Arabidopsis lines having mutation in an auxin transporter gene and showing similar phenotypes as atcipk6. We found that an Arabidopsis T-DNA insertion line pgp4-1, mutated in a gene MDR4/PGP4, which functions in the basipetal redirection of auxin transport from the root tip, has almost similar phenotypes like atcipk6 when 10-day old (after germination) seedlings were compared (Fig. 1E). pgp4-1 exhibited reduced basipetal auxin transport in roots and a small decrease in shoot-to-root transport.3 When compared with atcipk6, almost similar root phenotype was observed for pgp4-1 mutants showing 34.5% (p < 0.0003) reduction in growth rate of primary root in comparison to the wild type (Col) between the period 4-days to 7-days after the germination of the seedlings (Fig. 1E). To further correlate the physiology, root basipetal and shoot to root acropetal auxin transport was measured in pgp4-1 and atcipk6 seedlings as described previously.11 Significant decreases of 36.7% and 34.4% from the wild type in root tip basipetal transport of radiolabeled indole acetic acid (IAA) was observed in atcipk6 and pgp4-1 seedlings respectively (Fig. 1F). Also, a similar decrease of 47.36% (p < 0.0002) and 48.68% (p < 0.002) from the wild type (Col) in the root-shoot junction to root acropetal auxin transport was noticed in atcipk6 and pgp4-1 seedlings respectively (Fig. 1G) (for experimental methods11). RNA gel blot analysis showed that MDR4/PGP4 expression in stem was relatively less than that in root,3 while AtCIPK6 primarily expresses in stem and leaf; and during dehydration and high-salinity also in root. IAA (50 µM) and ABA (100 µM) moderately induced expression of chickpea CIPK6 gene.1 MDR4/PGP4 is a late auxin response gene and ABA treatment caused an oscillatory pattern of expression of this gene.3 All these correlative data strongly indicate that both CIPK6 and MDR4/PGP4 may operate in the same pathway, functioning together in the polar shoot-to-root auxin transport. Cooperative function of these two proteins in the polar root auxin transport may be relevant for root system plasticity under abiotic stress situations.
Figure 1.
Phenotype characterization of the Arabidopsis AtCIPK6 knockout mutant. (A) Genomic structure of AtCIPK6 T-DNA insertion sites in atcipk6 (GK-448C12-024532). Rectangle represent exon and lines represent untranslated regions of the gene. T-DNA, represented by triangles, is not drawn to scale. RB and LB represent left border and right border of T-DNA. (B) RNA gel blot analysis of AtCIPK6 gene expression. Ten micrograms of total RNA of wild-type (Col) or mutant (atcipk6) seedlings were probed with 32P-labeled full length AtCIPK6 cDNA (C) RT-PCR (30 cycles) analysis showing AtCIPK6 gene expression in wild-type (Col) and atcipk6 mutant plants. (D) Comparison of growth rates of the primary root of the seedlings of wild type (Col), atcipk6 and pgp4-1 Arabidopsis lines between the period of 4–7 days after germination. The means of three measurements of 20 seedlings each are shown. (E) Morphology of vertically grown 10-day-old (after germination) seedlings of wild type (Col), atcipk6 and pgp4-1 Arabidopsis lines. (F) Basipetal root auxin transport in a root segment 2 mm above the site of 3H-IAA application at the root apex in wild type (Col), atcipk6 and pgp4-1 mutant lines (G) Acropetal root auxin transport from the root-shoot junction to root measured 2 mm bellow the site of 3H-IAA application. Data are presented as percentage of auxin transport, relative to wild type (Col) (n = 3 group of 7 seedlings each).
Acknowledgements
The project was funded by the Department of Biotechnology, Government of India. V.T. acknowledges fellowship from National Institute of Plant Genome Research. N.S. acknowledges fellowship from Indian Council of Medical Research.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/9002
References
- 1.Tripathi V, Parasuraman B, Laxmi A, Chattopadhyay D. CIPK6, a CBL-interacting protein kinase is required for development and salt tolerance in plant. Plant J. 2009;58:778–790. doi: 10.1111/j.1365-313X.2009.03812.x. [DOI] [PubMed] [Google Scholar]
- 2.Celenza JL, Jr, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- 3.Terasaka K, Blakeslee JJ, Titapiwatanakun B, Peer WA, Bandyopadhyay A, Makam SN, et al. PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell. 2005;17:2922–2939. doi: 10.1105/tpc.105.035816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003;35:44–56. doi: 10.1046/j.1365-313x.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 5.Brocard IM, Lynch TJ, Finkelstein RR. Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar and stress response. Plant Physiol. 2002;129:1533–1543. doi: 10.1104/pp.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brocard-Gifford I, Lynch TJ, Garcia ME, Malhotra B, Finkelstein RR. The Arabidopsis thaliana ABSCISIC ACID-INSENSITIVE8 encodes a novel protein mediating abscisic acid and sugar responses essential for growth. Plant Cell. 2004;16:406–421. doi: 10.1105/tpc.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, Zhang H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003;33:543–555. doi: 10.1046/j.1365-313x.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 8.De Smet I, Zhang H, Inzé D, Beeckman T. A novel role for abscisic acid emerges from underground. Trends Plant Sci. 2006;11:434–439. doi: 10.1016/j.tplants.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA. 2002;99:8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Kim BG, Cheong YH, Pandey GK, Luan S. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:12625–12630. doi: 10.1073/pnas.0605129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin H, Shin HS, Guo Z, Blancaflor EB, Masson PH. Complex regulation of Arabidopsis AGR1/PIN2-mediated root gravitropic response and basipetal auxin transport by cantharidin-sensitive protein phosphatases. Plant J. 2000;42:188–200. doi: 10.1111/j.1365-313X.2005.02369.x. [DOI] [PubMed] [Google Scholar]