Abstract
Piriformospora indica is a mutualistic root-colonising basidiomycete that tranfers various benefits to colonized host plants including growth promotion, yield increases as well as abiotic and biotic stress tolerance. The fungus is characterized by a broad host spectrum encompassing various monocots and dicots.1,2 Our recent microarray-based studies indicate a general plant defense suppression by P. indica and significant changes in the GA biosynthesis pathway.3 Furthermore, barley plants impaired in GA synthesis and perception showed a significant reduction in mutualistic colonization, which was associated with an elevated expression of defense-related genes. Here, we discuss the importance of plant hormones for compatibility in plant root-P. indica associations. Our data might provide a first explanation for the colonization success of the fungus in a wide range of higher plants.
Key words: compatibility, plant defense, gibberellic acid, symbiosis, plant hormones
Introduction
As sessile organisms plants have to cope with their environment and have developed efficient strategies to face harmful abiotic or biotic challenges. The underlying molecular network is extremely complex due to the multitude of perception and signaling systems combined with a multilateral crosstalk. Phytohormones are embedded in these signaling events and are well known integrators of stress responses as observed by their challenge-responsive synthesis and signaling.4–8 Salicylic acid (SA), jasmonate (JA) and ethylene are the best characterized phytohormones in terms of averting invasions by plant pathogens. In a simplified model, SA is seen as a resistance mediator against biotrophic organisms, while JA and ethylene are involved in effective defense responses against necrotrophic pathogens.9,10 Other studies could also decipher the significance of abscisic acid (ABA) and gibberellins (GAs) for the outcome of plant-microbe interactions by modulating defense responses.4,11–14 Plant hormones are known to have antagonistic acitivity in plant developmental processes and this antagonism is also observed in plant defense responses (e.g., SA-JA-, GA-ABA antagonism).5,8,15,16 In contrast, other hromones (e.g., JA and ethylene) act mostly synergistically in defense processes.17,18 Hence, it is not further astonishing that microbes have evolved sophisticated strategies to efficiently establish compatible interactions by synthesising and mimicking phytohormones, or directly manipulating hormone signaling.4,5,19,20
The mutualistic root colonising fungus Piriformospora indica has been characterized as a exceptionally efficient organism as indicated by its ability to colonize a broad variety of monocot and dicot plant species.1,2 Interestingly, a nonhost has not been identified. Related to our recent studies, we discuss GA and other hormones as significant components for the colonization success of this mutualist.3
Hormone Synthesis and Signaling during Mutualistic Root Colonization
The molecular and biochemical events activated in plants in response to P. indica colonisation are mostly unknown. First cytological studies draw a more complex picture on these mutualistic interactions as was initially believed. In Arabidopsis, the fungus was shown to colonize root cells by an initial biotrophic phase followed by a later cell death-dependent colonization phase (Schäfer P and Zechmann B, unpublished data).1,21 At the biotrophic phase the plasma membranes of colonized cells is invaginated and the cell is alive. A similar infection strategy is expected for barley roots. Hence, the fungus is not simply colonising dead root cells or killing cells prior to or during penetration. Provided that the fungus is certainly recognized by plasma membrane localized pattern recognition receptors, which perceive microbe-derived molecules (e.g., chitin), the fungus should activate innate immune signaling. Interestingly, our microarray studies revealed defense suppression by P. indica during barley root colonization. As this corrumption obviously supports fungal establishment, it remains to be determined by which means the fungus suppresses plant defense.
In recent studies, ABA and GA were shown to affect Arabidopsis colonization by fungal and bacterial pathogens. ABA was shown to suppress basal defense in Arabidopsis thereby facilitating leaf colonization by Pseudomonas syringae pv. tomato.13 In addition, Arabidopsis mutants blocked in GA signaling showed enhanced susceptibility against Pseudomonas syringae DC3000 and enhanced resistance against two necrotrophic pathogens (Alternaria brassicicola, Botrytis cinerea).4 In contrast, quadruple DELLA mutants with a constitutive GA signaling phenotype were more resistant against the bacterium but highly susceptible against both necrotrophs. Interestingly, the quadruple mutant displayed enhanced levels of free SA after P. syringae DC3000 attack, which was also reflected by elevated PR1 and PR2 transcripts, while the JA/ethylene-responsive PDF1.2 exhibited a delayed expression.4 This indicates a direct connection of GA signaling with SA and JA responses.
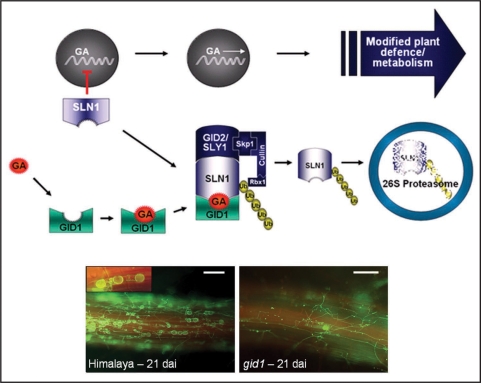
Barley root colonization was also accompanied by changes in plant hormone metabolism. During extracellular fungal development (1 day after inoculation, dai) the expression of ABA-responsive genes was induced. This, however, changed at penetration/early colonization (3 dai) and progressed colonization stages (7 dai). At these time points GA synthesis was observed to be obviously elevated as indicated by the induction of almost all genes of the non-mevalonate pathway and two putative kaurene synthases. In contrast, SA and JA-related defense genes only showed a weak and transient induction pattern or were even suppressed.3 It is tempting to speculate that ABA might be recruited by P. indica to suppress defense at pre-penetration stages while GA is taking over this job at subsequent interaction stages (3, 7 dai). However, our studies showed the GA-dependence of barley root colonisation by P. indica as a GA synthesis mutant and the GA receptor mutant gid1 were significantly less colonized by the mutualist. Moreover, PR gene expression was significantly elevated by P. indica in both mutants compared to wild type roots at 3 dai.3 Subsequent, cytological studies showed a substantial reduction of extracellular fungal growth and of extra-und intracellular sporulation in gid1 compared to wild type Himalaya (Fig. 1). In gid1, fungal colonization might be stopped at the penetration stage. The resulting restriction in nutrient acquisition might explain its impaired extracelullar development and sporulation.
Figure 1.
Impaired GA perception reduces barley root colonisation by P. indica. GA-responsive gene transcription is inhibited by SLENDER1 (SLN1) in the absence of GA (upper). After GA synthesis, GA gets attached to the GA receptor GID1. Thereafter, SLN1 binds to GID1, which results in SLN1 ubiqutination and its proteasomal degradation.22 By removing SLN1, GA-responsive transcription is initiated that is thought to modify plant defense signaling and metabolism (upper). In the barley mutant gid1, GA-responsive transcritption is inhibited and P. indica root colonisation is markedly reduced at 21 dai (lower right) compared to parent line Himalaya (lower left). Intracellular sporulation was almost absent in gid1 (inset, lower right). The fungus was stained with wheat germ agglutinin-Alexa Fluor 488 (WGA-AF 488) and visualized by fluorescence microscopy (Bars = 20 µm).
In summary, plant hormone signaling is obviously recruited by P. indica in order to manipulate plant defense and most probably plant metabolism. Plant hormones might further be a key to explain the broad host spectrum of P. indica. Current studies are directed to decipher the phytohormonal state and signaling during plant colonization by P. indica.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft, DFG research group FOR666 to K.H.K., P.S., F.W. and U.S.
Addendum to: Schäfer P, Pfiffi S, Voll LM, Zajic D, Chandler PM, Waller F, et al. Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica. Plant J. 2009 doi: 10.1111/j.1365-313X.2009.03887.x. In press.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/9038
References
- 1.Schäfer P, Kogel KH. The sebacinoid fungus Piriformospora indica: an orchid mycorrhiza which may increase host plant reproduction and fitness. In: Deising HB, Esser K, editors. The Mycota, Vol. 5, Plant Relationships. Heidelberg: Springer-Verlag; 2009. pp. 99–112. [Google Scholar]
- 2.Varma A, Verma S, Sudha, Sahay N, Bütehorn B, Franken P. Piriformospora indica, a cultivable plant-growthpromoting root endophyte. Appl Environ Microbiol. 1999;65:2741–2744. doi: 10.1128/aem.65.6.2741-2744.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schäfer P, Pfiffi S, Voll LM, Zajic D, Chandler PM, Waller F, et al. Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica. Plant J. 2009 doi: 10.1111/j.1365313X.2009.03887.x. In press. [DOI] [PubMed] [Google Scholar]
- 4.Navarro L, Bari R, Achard P, Lison P, Nemri A, Harberd NP, Jones JDG. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol. 2008;18:650–655. doi: 10.1016/j.cub.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 5.Robert-Seilaniantz A, Navarro L, Bari R, Jones JDG. Pathological hormone imbalances. Curr Opin Plant Biol. 2007;10:372–379. doi: 10.1016/j.pbi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;3:348–351. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Zhang WH, Qin CB, Zhao J, Wang XM. Phospholipase D alpha1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA. 2004;101:9508–9513. doi: 10.1073/pnas.0402112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, et al. Integration of plants responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- 9.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Ann Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 10.Loake G, Grant M. Salicylic acid in plant defence-the players and protagonists. Curr Opin Plant Biol. 2007;10:466–472. doi: 10.1016/j.pbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, et al. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;16:3460–3479. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asselbergh B, De Vleesschauwer D, Höfte M. Global switches and fine-tuning—ABA modulates plant pathogen defence. Mol Plant-Microbe Interact. 2008;21:709–719. doi: 10.1094/MPMI-21-6-0709. [DOI] [PubMed] [Google Scholar]
- 13.De Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Egea PR, et al. Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signaling pathway to cause disease. EMBO J. 2007;26:1434–1443. doi: 10.1038/sj.emboj.7601575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohr PG, Cahill DM. Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv. tomato. Funct Integr Genomics. 2007;7:181–191. doi: 10.1007/s10142-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 15.Lopez MA, Bannenberg G, Castresana C. Controlling hormone signaling is a plant and pathogen challenge for growth and survival. Curr Opin Plant Biol. 2008;11:1–8. doi: 10.1016/j.pbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Razem FA, Baron K, Hill RD. Turning on gibberellin and abscisic acid signaling. Curr Opin Plant Biol. 2006;9:454–459. doi: 10.1016/j.pbi.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Broekaert WF, Delauré SL, De Bolle MF, Cammue BP. The role of ethylene in host-pathogen interactions. Annu Rev Phytopathol. 2006;44:393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- 18.Ellis C, Turner JG. The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell. 2001;13:1025–1033. doi: 10.1105/tpc.13.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaguè V, Elad Y, Barakat R, Tudzynski P, Sharon A. Ethylene biosynthesis in Botrytis cinerea. FEMS Microbial Ecol. 2002;40:143–149. doi: 10.1111/j.1574-6941.2002.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol. 2008;59:225–251. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 21.Deshmukh S, Hückelhoven R, Schäfer P, Imani J, Sharma M, Weiss M, et al. The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proc Natl Acad Sci USA. 2006;103:18450–18457. doi: 10.1073/pnas.0605697103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueguchi-Tanaka M, Nakajima M, Motoyuki A, Matsuoka M. Gibberellin receptor and its role in gibberellin signaling in plants. Annu Rev Plant Biol. 2007;58:183–198. doi: 10.1146/annurev.arplant.58.032806.103830. [DOI] [PubMed] [Google Scholar]