Abstract
In this review, we summarize the current understanding of the multiple functions of the mouse lymphoid tissue inducer (LTi) cells in: (i) the development of organized lymphoid tissue, (ii) the generation and maintenance of CD4-dependent immunity in adult lymphoid tissues; and (iii) the regulation of central tolerance in thymus. By contrast with mouse LTi cells, which have been well described, the human equivalent is only just beginning to be characterized. Human LTi-like cells expressing interleukin (IL)-22 have been identified recently and found to differentiate into natural killer (NK) cells. The relationship of LTi cells to NK cells is discussed in the light of several studies reporting a close relationship in the mouse between LTi cells and transcription factor retinoid-related orphan receptor γt-dependent IL-22 producing NK cells in the gut. We also outline our data suggesting that these cells are present in adult human lymphoid tissues.
Keywords: CD4 memory, LTi cells, lymphoid tissue, NK cells
Introduction
CD4 T cells are central to our protection from both intracellular and extracellular pathogens. However, they cannot work alone and are effective only in the context of the help that they provide to other cells. Over the last decade, increasing attention has been paid to the role of lymphoid tissue inducer (LTi) cells, both in fostering the development of the microenvironments within which CD4 T cells initially provide help to other cells [1–6] and also in sustaining CD4 memory, which is essential for our long-term protection [7–10]. Here, we outline the evidence implicating LTi cells in the development of organized lymphoid structures and, in addition, characterize the presence and location of these cells in adult lymphoid tissues. These identify sites where CD4 T cells provide help to B cells, both during priming and for memory T cell-dependent antibody responses. Furthermore, more recent data suggest that these cells also provide signals for other forms of CD4 memory to both intracellular and extracellular pathogens [9].
Memory and lymphoid tissue organization, both LTi cell functions, are relatively recent acquisitions for the vertebrate adaptive immune system [11]. We suppose that an LTi ancestor could have provided relatively primitive functions. Analysis of genes and proteins expressed in both adult and embryonic LTi cells suggests a possible role in innate immunity. They express many surface proteins found on natural killer (NK) cells and produce substantial amounts of the T helper 17 (Th17) cytokine interleukin (IL)-22 and also IL-17 if stimulated with IL-23 [12]. Although they express many NK markers, they do not have cytotoxic function and are associated with the organized white pulp areas of secondary lymphoid tissues [8,13]. Here, we speculate that LTi cells could represent a primitive innate precursor of both NK and CD4 T cells that evolved new functions over time, including the support of the microenvironments for CD4 immune responses.
Finally, we summarize recent data identifying LTi-like cells in humans in both embryonic and adult tissues.
Fetal LTi cells in lymphoid tissue development in mouse
As mouse LTi cells were first identified in fetal lymphoid tissues [2], many groups have focused on the function of LTi cells in lymphoid tissue development [1,4–6,14–16]. The critical function of fetal LTi cells is to induce the development of lymphoid tissues, including lymph nodes and Peyer's patches, by their surface expression of many relevant molecules. The first of these is the tumour necrosis factor superfamily (TNF-SF) member lymphotoxin-alpha (LTα); mice deficient in LTα completely lack lymph nodes and Peyer's patches and also display disrupted splenic architecture [17,18]. A membrane-bound heterotrimer with LTβ (LTα1β2) on LTi cells signals through the LTβ-receptor (LTβR) on stromal cells [4,19–21]. The importance of these signals has been demonstrated in mice deficient in LTβR, which also lack lymph nodes and Peyer's patches [22]. Stromal cells activated through LTβR ligation up-regulate adhesion molecules, leading to tight interaction with LTi cells, and subsequently produce the lymphoid chemokines CXCL13, CCL19 and CCL21 [23,24]. CXCL13, the B zone chemokine, then recruits circulating B cells to what becomes the B cell area of lymphoid tissues, and the T zone chemokines, CCL19 and CCL21, attract T and dendritic cells to shape the T cell area [25,26].
In addition to LTα and LTβ, many other molecules expressed by LTi cells are involved in lymph node and Peyer's patch development, including CD127 (IL-7Rα), Ikaros, retinoid-related orphan receptor gamma (RORγ), Id2 (helix-loop-helix protein), Janus kinase 3 and CD132 (common cytokine receptor γ chain). Mice deficient in any of these molecules show lack of Peyer's patches and impaired lymph node development [1,4,14,15,27–29]. Although mice deficient in TNF-related activation-induced cytokine (TRANCE, TNF-SF11) or receptor activator of nuclear factor kappa B (RANK, TNF-RSF11A), which are also expressed on LTi cells, have normal Peyer's patches, they have certain defects in lymph node development, suggesting an important role for signals from LTi cells through these molecules [5,30].
Taken together, the previous studies on the developing immune system have proved that fetal LTi cells are crucial for lymphoid tissue development.
Adult LTi cells in memory responses in mouse
Our group has identified the adult equivalent of fetal LTi cells in mouse secondary lymphoid tissues including spleen and lymph nodes, implicating them in multiple functions in the immune system [7,31–33]. The phenotypic difference between adult and fetal LTi cells is the expression of OX40-ligand (L) (TNF-SF4) and CD30L (TNF-SF8), which is critical for memory CD4 T cell generation [34]. The evidence for this is provided by mice deficient in both OX40 and CD30, which have impaired CD4 T cell-dependent memory antibody responses in spleen and gut [9,10,32]. Recently, we also showed that the maintenance of CD4 Th1 memory against bacterial infection depends on OX40 and CD30 signals [9]. The lack of expression of OX40L and CD30L on fetal LTi cells could explain the phenomenon of tolerance rather than immunity to foreign antigens in the periphery that occurs in neonatal mammals [34].
Adult LTi cells in spleen are located mainly in white pulp, particularly at the junction of B- and T-zones and in the follicle. LTi cells also occur in the T cell area, where they interact tightly with stromal cells, providing LTα1β2 signals to them [7,8,26]. The engagement of LTβR on stromal cells stimulates their production of CXCL13, CCL19 and CCL21, maintaining the segregation of B and T cell areas [8].
The importance of cross-talk between LTi cells and stromal cells in white pulp structures has been also been demonstrated in mice deficient in CD30 [13]. Although these mice have normal expression of CCL19 and CCL21, their splenic architecture shows impaired B- and T-zone segregation. This is due to lack of CD30-signalling to stromal cells from LTi cells and failure to express podoplanin (gp38), a mucin-type transmembrane protein.
This body of data implicates adult LTi cells convincingly in both the maintenance of segregated B- and T-zones in secondary lymphoid tissues and the provision of survival signals for T helper memory cells. Because disorganized lymphoid architecture supports neither high-affinity antibody responses nor memory generation [35], these two LTi functions are linked.
Thymic LTi cells in central tolerance in mouse
In addition to their functions in the secondary lymphoid tissues, LTi cells have been shown to be required for a vital part of the education of developing T cells in the thymus [33]. Although phenotypically the same as lymphoid LTi cells, thymic LTi cells have a distinct role, defined by their expression of TRANCE. TRANCE on thymic LTi cells interacts with its ligand RANK on the medullary epithelial cells that promote the expression of Aire. Up-regulation of Aire controls the expression of self-tissue-restricted antigens on thymic medullary epithelial cells. This is the heart of the negative selection process in the thymus, which is crucially important in providing central tolerance to self-antigens [33].
This result shows that LTi cells in the primary lymphoid organ, thymus, regulate central tolerance, and in secondary lymphoid organs, spleen and lymph nodes, control peripheral immunity. Ancestral LTi cells are therefore likely to have functioned in both tolerance and immunity before the adaptive immune system was developed.
Origin of LTi cells
Mouse fetal LTi cells were reported initially to be able to differentiate into antigen-presenting cells (APCs), NK cells and follicular cells but not T or B cells [2]. Their expression of CD45 implies that they are derived from haematopoietic stem cells. When we dissected many organs in mouse, we found LTi cells in liver, spleen, lymph nodes and thymus but not bone marrow. LTi cells are detected from mouse embryonic day 12 (E12) liver, and a report has shown that IL-7Rα+Sca-1lowCD117low cells in E14 liver give rise to LTi cells (Fig. 1) [36]. Their development does not require recombinase-activating genes, indicating their distinction from T and B cells. Luther et al. showed that CD4+CD3−IL-7Rα+ cells are found in neonatal blood, implying that they circulate [37]. Fetal LTi cells express gut-homing integrin α4β7 and migrate to the intestine to induce the Peyer's patches [2,38–40]. Yoshida et al. showed that approximately 16% of fetal LTi cells can differentiate into NK1·1+ cells in the presence of IL-15 and approximately 17% of the cells into CD11c+ dendritic cells in stimulation with IL-3, TNF-α and stem cell factor, suggesting that fetal intestinal LTi cells are at an intermediate stage before NK cells and dendritic cells (Fig. 1) [38].
Fig. 1.
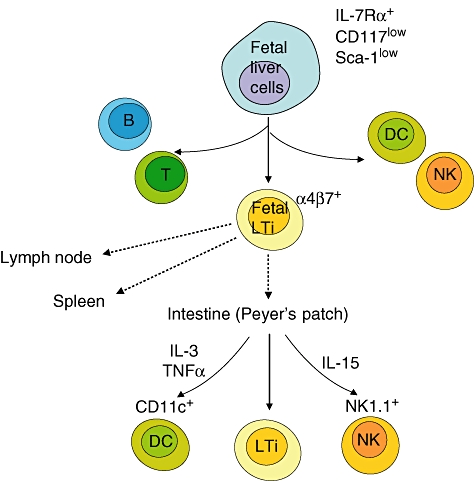
Development from interleukin (IL)-7Rα+ CD117low Sca-1low cells found in the fetal liver to lymphoid tissue inducer (LTi) cells in mouse. The fetal liver cells give rise to lymphoid cells including T, B, natural killer (NK) and dendritic cells and integrin α4β7-expressing LTi cells. Fetal LTi cells migrate to lymph node and spleen anlagen and intestine. In the intestine, LTi cells induce Peyer's patch formation. LTi cells differentiate into CD11c+ dencritic cells (DCs) in the presence of IL-3, tumour necrosis factor (TNF)-α and stem cell factor and into NK1·1+ cells in the presence of IL-15.
Our group has detected α4β7-expressing LTi cells in the earliest identifiable mouse embryonic spleen (E12). When they were cultured with IL-7, the number of LTi cells was augmented markedly. In addition, we found not only CD4+ LTi cells but also LTi cells, which shared the same phenotype but were CD4−[41]. There was no evidence that CD4− LTi cells acquire CD4 expression or vice versa.
The LTi cells are a unique population which is distinct from NK cells. They do not express the NK surface marker, NK1·1 and pan-NK DX5 or the cytokine interferon (IFN)-γ and have not been shown to kill cells in cytotoxicity assays [42]. In addition, functional LTi cells are found in mice deficient in NK cells [41,43]. However, our preliminary data of LTi cells indicate that they have mRNA expression for NK characteristics, including CD96, CD244 (NK cell activation-inducing ligand) and CD160 (NK1, NK28). It is plausible that LTi cells share closer common lymphoid progenitors with NK cells than they do with B or T cells. Recent studies showed that a subset of RORγt-expressing NK cells in mouse gut produce high levels of IL-22, which is a cytokine known previously to be produced by Th17 cells [44–46]. Luci et al. showed that one-third of RORγt+ cells in mouse cryptopatch express NKp46 (CD335), which is a receptor expressed on NK cell surface [44]. By their developmental dependence on RORγt, the authors proposed that these RORγt+NKp46+ NK cells are a subset of LTi cells or could be derived from LTi cells. The same result was reported by Sanos et al., showing that RORγt+NKp46+ NK cells found in the intestinal lamina propria do not have cytotoxic function; rather, they produce large amounts of IL-22 [45]. Although RORγt+NKp46− LTi-like cells in gut do not produce IL-22, both results indicate that RORγt+NKp46+ NK cells are related closely to LTi cells.
The LTi cells in human
Identifying the human equivalent of murine LTi cells has been a major issue. In 1986, Spencer et al. reported that CD4+CD3− cells were found in gut-associated lymphoid tissue in the terminal ileum of human fetal intestine [47]. Mouse LTi cells express CD4, but not CD3 and CD11c. Therefore, to identify a human equivalent, the focus was placed on cells phenotyped as CD4+CD3−. However, our group has identified CD4−CD3− LTi cells in mouse secondary lymphoid tissues, indicating that LTi cells are heterogeneous [41]. A recent publication by Cupedo et al. showed that human LTi cells are IL-17- and IL-22-producing CD4−CD56−IL-7Rα+ retinoic acid receptor-related orphan receptor C (RORC)+ NK-like cells, negative for CD4, and found in fetal mesentery [48]. The major phenotypic difference between mouse and human LTi cells was CD4 expression (Table 1). In comparison with mouse LTi cells, which are either CD4+ or CD4−, human LTi cells are CD4− or CD4low, although CD4 is expressed widely in human cells. In the case of CD7, which is a T and NK cell marker, 50–70% human LTi cells express CD7 and we have detected CD7 mRNA expression in mouse LTi cells (unpublished data).
Table 1.
Similarities and differences between mouse and human lymphoid tissue inducer (LTi) cells.
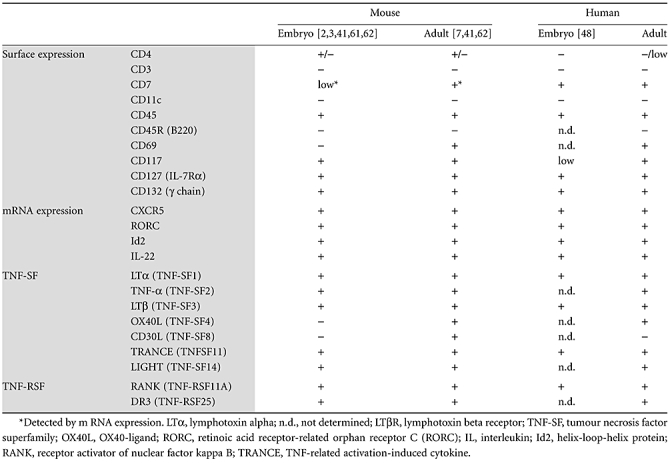 |
Because human LTi cells express NK cell markers including CD7 and CD161, it seems that human LTi cells are related closely to a subset of IL-7Rα+ NK cells. In support of this idea, for differentiation into both NK and LTi cells, the Id2 molecule is required [49]. In addition to Id2, another transcription factor, RORC (RORγt in mice), is required for LTi cell differentiation (Fig. 2) [50].
Fig. 2.
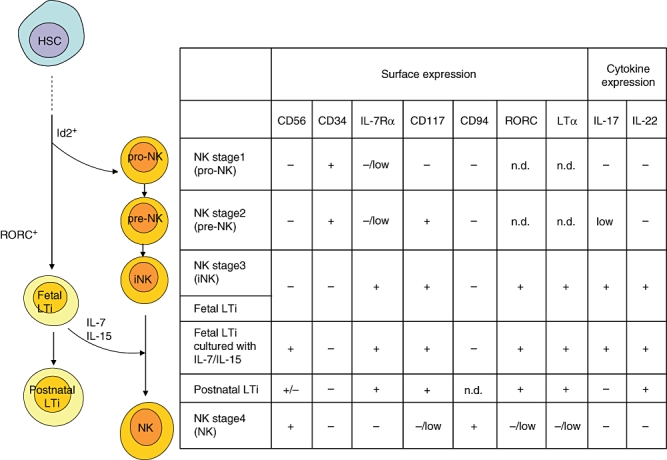
Comparison of human fetal and adult lymphoid tissue inducer (LTi) cells with natural killer (NK) cells which are divided into four developmental stages [pro-NK, pre-NK, immature committed NK (iNK) and NK] by the surface and cytokine expression. For differentiation into fetal LTi cells, helix-loop-helix protein (Id2) and retinoic acid receptor-related orphan receptor C (RORC) are required. Fetal LTi cells in human have a similar phenotype to iNK (stage 3 NK cells) and can differentiate into CD56+ cells in the presence of interleukin (IL)-7 and IL-15. Postnatal LTi-like cells are similar to between stage 3 and stage 4 NK cells. HSC, haematopoietic stem cell; n.d., not determined.
Caligiuri's group has characterized four stages of NK cell development according to their expression of CD56, CD34, CD117 and CD94 in human lymph nodes and tonsils (Fig. 2) [51,52]. Human LTi cells are very similar to stage 3 CD56−IL-7Rα+ NK cells, called immature committed NK cells. Half of these cells differentiate into CD56+IL-7Rα+ cells, which are the intermediate stage between stage 3 (CD56−IL-7Rα+) and stage 4 (CD56+IL-7Rα−) in the presence of IL-7 or IL-15 but fail to differentiate into T cells or dendritic cells [48]. Although it has not been proved whether these human LTi cells can or cannot differentiate into stage 4 NK cells, which have cytotoxic function, it is apparent that LTi and NK cells have closely related developmental lineages.
Human LTi-like cells in postnatal secondary lymphoid tissue
We have searched for human LTi cells in postnatal secondary lymphoid tissues and have identified CD4−CD3−CD11c−CD45low and CD117+ LTi-like cells, which produce IL-22 in lymph node, spleen, gut and tonsil (unpublished data). The phenotype of these cells is similar to the intermediate stage between stage 3 and stage 4 NK cells (Fig. 2). The really interesting feature of these cells was high levels of OX40L expression. OX40L expression has been reported in APCs, such as activated monocytes, dendritic cells, B cells [53–55] and NK cells following the simultaneous cross-linking of killer cell immunoglobulin-like receptor DS2 (KIR2DS2) and NKG2D receptors [55]. The latter showed that up-regulation of OX40L on peripheral blood NK cells was induced through stimulation with IL-2 with activation by an NK receptor, but not through IL-2 stimulation alone. In comparison, OX40L expression on LTi-like cells in tissues showed constitutive expression with no requirement for any stimulation (unpublished data). A study by Zingoni et al. showed that OX40L and B7 engagement with OX40+CD28+ T cells leads to T cell activation and IFN-γ production [55], implicating LTi-like cells in the Th1 response.
Because they have a similar phenotype to NK cells, we looked for their expression of functional genes related to cytotoxicity. They expressed low levels of mRNA for IFN-γ, perforin and granzyme B (unpublished data), although it is unknown whether the expression of these molecules is up-regulated upon activation or if they are functional.
The Th17 cells have been identified as a major source of IL-22 [56–58]. We have also found high levels of expression of mRNA for IL-22 in adult mouse LTi cells [59], and a recent study by Takatori et al. showed that splenic adult LTi cells are the source of the IL-22 contributing to innate immunity against yeast zymosan [12]. Further support for the idea that the cells we have identified are LTi cells is their strong expression of IL-22 in human adult (our personal observations) and embryo [48].
Potential implications for LTi cell functions in Th17 responses are depicted in Fig. 3. Proinflammatory IL-6 and the immunosuppressive transforming growth factor-β are required for the differentiation of CD4 T cells towards Th17 cells, which produce IL-17 and IL-22. Postnatal LTi cells produce IL-22 without simulation and IL-17 after stimulation with IL-23 [12,48]. The cytokines IL-17 and IL-22 induce chemokines to protect against extracellular bacteria and fungi but are also involved in the pathogenic process of tissue inflammation leading to organ-specific autoimmunity [60].
Fig. 3.
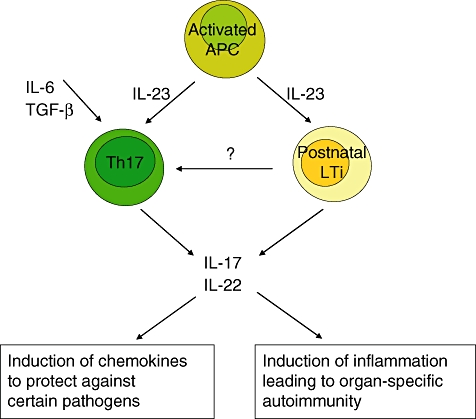
Potential implications for lymphoid tissue inducer (LTi) cell functions in T helper type 17 (Th17) responses. The cytokines interleukin (IL)-6 and transforming growth factor (TGF)-β are required for the differentiation of CD4 T cells towards Th17 cells which produce IL-17 and IL-22. Postnatal LTi cells produce IL-22 without simulation and IL-17 after stimulation. The cytokines IL-17 and IL-22 induce chemokines to protect against certain pathogens, but also involve in the pathogenic process of tissue inflammation leading to organ-specific autoimmunity.
In summary, our work on human LTi-like cells indicates many more similarities than differences with their murine equivalents, and it will be extremely interesting to dissect the contribution of these cells to human diseases, especially those involving CD4 T cells.
Conclusion
In this paper, we have reviewed the data on embryonic and adult LTi cells in mice and human beings. The key point to remember about these cells is that they form the microenvironments within which CD4 T cells provide help for effector immune responses and then sustain primed CD4 T cells for memory responses. In addition, recent data suggest an ancestral role in innate immunity through expression of the Th17 cytokine, IL-22. The future lies in dissecting the role of these cells in human diseases; this will require good markers that work in conventional paraffin embedded tissues in routine histopathology laboratories.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007-313-C00564).
References
- 1.Fukuyama S, Hiroi T, Yokota Y, et al. Initiation of NALT organogenesis is independent of the IL-7r, LTbetaR, and NIK signaling pathways but requires the Id2 gene and CD3(−)CD4(+)CD45(+) cells. Immunity. 2002;17:31–40. doi: 10.1016/s1074-7613(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 2.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 3.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, Naito A, Inoue J, et al. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer's patches. Immunity. 2002;17:823–33. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Mebius RE, MacMicking JD, et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467–78. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finke D, Acha-Orbea H, Mattis A, Lipp M, Kraehenbuhl J. CD4+CD3- cells induce Peyer's patch development: role of alpha4beta1 integrin activation by CXCR5. Immunity. 2002;17:363–73. doi: 10.1016/s1074-7613(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 7.Kim MY, Gaspal FM, Wiggett HE, et al. CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–54. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 8.Kim MY, McConnell FM, Gaspal FM, et al. Function of CD4+CD3- cells in relation to B- and T-zone stroma in spleen. Blood. 2007;109:1602–10. doi: 10.1182/blood-2006-04-018465. [DOI] [PubMed] [Google Scholar]
- 9.Gaspal F, Bekiaris V, Kim MY, et al. Critical synergy of CD30 and OX40 signals in CD4 T cell homeostasis and Th1 immunity to Salmonella. J Immunol. 2008;180:2824–9. doi: 10.4049/jimmunol.180.5.2824. [DOI] [PubMed] [Google Scholar]
- 10.Gaspal FM, Kim MY, McConnell FM, Raykundalia C, Bekiaris V, Lane PJ. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol. 2005;174:3891–6. doi: 10.4049/jimmunol.174.7.3891. [DOI] [PubMed] [Google Scholar]
- 11.Lane PJ, Gaspal FM, Kim MY. Two sides of a cellular coin: CD4(+)CD3- cells regulate memory responses and lymph-node organization. Nat Rev Immunol. 2005;5:655–60. doi: 10.1038/nri1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takatori H, Kanno Y, Watford WT, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekiaris V, Withers D, Glanville SH, et al. Role of CD30 in B/T segregation in the spleen. J Immunol. 2007;179:7535–43. doi: 10.4049/jimmunol.179.11.7535. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida H, Honda K, Shinkura R, et al. IL-7 receptor alpha+ CD3(−) cells in the embryonic intestine induces the organizing center of Peyer's patches. Int Immunol. 1999;11:643–55. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 15.Adachi S, Yoshida H, Honda K, et al. Essential role of IL-7 receptor alpha in the formation of Peyer's patch anlage. Int Immunol. 1998;10:1–6. doi: 10.1093/intimm/10.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Hashi H, Yoshida H, Honda K, et al. Compartmentalization of Peyer's patch anlagen before lymphocyte entry. J Immunol. 2001;166:3702–9. doi: 10.4049/jimmunol.166.6.3702. [DOI] [PubMed] [Google Scholar]
- 17.Banks TA, Rouse BT, Kerley MK, et al. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–93. [PubMed] [Google Scholar]
- 18.De Togni P, Goellner J, Ruddle NH, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–7. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 19.Cupedo T, Vondenhoff MF, Heeregrave EJ, et al. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol. 2004;173:2968–75. doi: 10.4049/jimmunol.173.5.2968. [DOI] [PubMed] [Google Scholar]
- 20.Adachi S, Yoshida H, Kataoka H, Nishikawa S. Three distinctive steps in Peyer's patch formation of murine embryo. Int Immunol. 1997;9:507–14. doi: 10.1093/intimm/9.4.507. [DOI] [PubMed] [Google Scholar]
- 21.Rennert PD, James D, Mackay F, Browning JL, Hochman PS. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 1998;9:71–9. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 22.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 23.Dejardin E, Droin NM, Delhase M, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–35. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 24.Honda K, Nakano H, Yoshida H, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer's patch organogenesis. J Exp Med. 2001;193:621–30. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol. 2005;174:21–5. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 26.Scandella E, Bolinger B, Lattmann E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–75. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 27.Wang JH, Nichogiannopoulou A, Wu L, et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–49. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 28.Sun Z, Unutmaz D, Zou YR, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–73. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 29.Park SY, Saijo K, Takahashi T, et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–82. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 30.Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MY. Roles of embryonic and adult lymphoid tissue inducer cells in primary and secondary lymphoid tissues. Yonsei Med J. 2008;49:352–6. doi: 10.3349/ymj.2008.49.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane P, Kim MY, Withers D, et al. Lymphoid tissue inducer cells in adaptive CD4 T cell dependent responses. Semin Immunol. 2008;20:159–63. doi: 10.1016/j.smim.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Rossi SW, Kim MY, Leibbrandt A, et al. RANK signals from CD4+3- inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204:1267–72. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MY, Anderson G, White A, et al. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3- inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J Immunol. 2005;174:6686–91. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- 35.Fu YX, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD. Lymphotoxin-alpha (LTalpha) supports development of splenic follicular structure that is required for IgG responses. J Exp Med. 1997;185:2111–20. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mebius RE, Miyamoto T, Christensen J, et al. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3- cells, as well as macrophages. J Immunol. 2001;166:6593–601. doi: 10.4049/jimmunol.166.11.6593. [DOI] [PubMed] [Google Scholar]
- 37.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–8. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida H, Kawamoto H, Santee SM, et al. Expression of alpha(4)beta(7) integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J Immunol. 2001;167:2511–21. doi: 10.4049/jimmunol.167.5.2511. [DOI] [PubMed] [Google Scholar]
- 39.Nishikawa S, Honda K, Vieira P, Yoshida H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 2003;195:72–80. doi: 10.1034/j.1600-065x.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 40.Ivanov II, Diehl GE, Littman DR. Lymphoid tissue inducer cells in intestinal immunity. Curr Top Microbiol Immunol. 2006;308:59–82. doi: 10.1007/3-540-30657-9_3. [DOI] [PubMed] [Google Scholar]
- 41.Kim MY, Rossi S, Withers D, et al. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology. 2008;124:166–74. doi: 10.1111/j.1365-2567.2007.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lane PJ, Kim MY, Gaspal FM, McConnell FM. CD4+CD3- cells regulate the organization of lymphoid tissue and T-cell memory for antibody responses. Int J Hematol. 2006;83:12–6. doi: 10.1532/IJH97.05117. [DOI] [PubMed] [Google Scholar]
- 43.Hollander GA, Wang B, Nichogiannopoulou A, et al. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 1995;373:350–3. doi: 10.1038/373350a0. [DOI] [PubMed] [Google Scholar]
- 44.Luci C, Reynders A, Ivanov II, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 45.Sanos SL, Bui VL, Mortha A, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Spencer J, MacDonald TT, Finn T, Isaacson PG. The development of gut associated lymphoid tissue in the terminal ileum of fetal human intestine. Clin Exp Immunol. 1986;64:536–43. [PMC free article] [PubMed] [Google Scholar]
- 48.Cupedo T, Crellin NK, Papazian N, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 49.Yokota Y, Mansouri A, Mori S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–6. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 50.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 51.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 52.Freud AG, Yokohama A, Becknell B, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–43. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weatherill AR, Maxwell JR, Takahashi C, Weinberg AD, Vella AT. OX40 ligation enhances cell cycle turnover of Ag-activated CD4 T cells in vivo. Cell Immunol. 2001;209:63–75. doi: 10.1006/cimm.2001.1783. [DOI] [PubMed] [Google Scholar]
- 54.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 55.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40–OX40 ligand interactions. J Immunol. 2004;173:3716–24. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 56.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;19:353–61. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 58.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Lane PJ. The architects of B and T cell immune responses. Immunity. 2008;29:171–2. doi: 10.1016/j.immuni.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol. 2007;19:362–71. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim MY, Toellner KM, White A, et al. Neonatal and adult CD4+ CD3- cells share similar gene expression profile, and neonatal cells up-regulate OX40 ligand in response to TL1A (TNFSF15) J Immunol. 2006;177:3074–81. doi: 10.4049/jimmunol.177.5.3074. [DOI] [PubMed] [Google Scholar]
- 62.Lane PJ, Kim MY, Gaspal FM, McConnell FM. CD4(+)CD3(−) Cells regulate the organization of lymphoid tissue and T-cell memory for antibody responses. Int J Hematol. 2006;83:12–6. doi: 10.1532/IJH97.05117. [DOI] [PubMed] [Google Scholar]


