Abstract
A well-recognized characteristic of the autoimmune disease, systemic lupus erythematosus (SLE), is the high level of activated T cells present in the blood. Because of the increased size and granularity of activated T cells, in flow cytometry one might expect to find increased numbers of cells falling outside a standard light-scatter lymphocyte gate, and indeed we now report that the percentage of T lymphocytes in the gate (% TiG) was below the normal range in 23 of 58 (40%) female patients because of increased scatter values. However, the surprising additional observation was made that 18 of 30 (60%) female first-degree relatives of the patients also fell below the normal % TiG range, suggesting the presence of T cell activation in these relatives. This view is strengthened by the strong inverse correlation between plasma total immunoglobulin G(IgG), which was raised in some relatives, and % TiG, as T cell activation is a requirement for IgG production. Conversely, there was no correlation with IgM, which has no comparable link with T cell activation. While a definitive interpretation must await the demonstration of activation antigen expression in relatives, these findings suggest the existence of a T cell activation trait, not harmful in itself, which, however, contributes to the development of disease in patients with SLE.
Keywords: IgG, lymphocyte gate, SLE patients and relatives, T cell activation
Introduction
Determination of the frequency of lymphocyte types in blood samples by flow cytometry generally involves establishing a lymphocyte gate on the basis of forward and side light-scatter [1]. Such a gate will comprise the bulk of all lymphocytes present and, at the same time, exclude monocytes and other non-lymphocyte populations. Forward-scatter (FS) is an indicator of cell size, whereas side-scatter (SS) indicates degree of granularity, and exclusion from the gate will occur where a lymphocyte differs substantially from the norm in one or both of these characteristics. This may occur, for example, when a lymphocyte has been activated leading to increases in both size and granularity [1,2].
One of the characteristics of the autoimmune rheumatic disease, systemic lupus erythematosus (SLE), is the high level of activated T lymphocytes in the blood [3], so it is perhaps not surprising that we have found that the percentage of T cells in the gate (% TiG) in such patients is often below the range observed in control subjects. What was surprising, however, was to find that an even higher proportion of healthy female first-degree relatives of the patients fell below the control TiG range.
The above observations were made during the course of a study of natural killer (NK) and NKT cells in which plasma immunoglobulin (Ig) levels were also addressed [4,5]. In this paper, we present the TiG data for SLE patients, their first-degree relatives and controls and relate this to the previously reported plasma total IgG and IgM levels [5].
Methods
Patients, relatives and controls
Blood samples were obtained from 65 SLE patients (60 female/five male), 45 first-degree relatives of SLE patients (31/14) and 29 healthy control donors (18/11). Patients were attending clinics at St Mary's Hospital and the Centre for Rheumatology, University College London. SLE patients fulfilled the revised criteria of the American College of Rheumatology for classification of this disease [6]. St Mary's Local Research and University College University Trust Ethics Committees approved the study.
Details of age, ethnic background and, for the patients, drug therapy and clinical disease activity have been given elsewhere [4].
Flow cytometry of blood mononuclear cells
Flow cytometry was carried out on peripheral blood mononuclear cells as described previously [4]. For each sample, a lymphocyte gate was established on the basis of linear FS and SS to mark out the area in which the majority of such cells resided (Fig. 2) [1]. Only minor revision of the gate was needed between different donors. While the positioning of a gate carries an element of subjectivity, it was performed ‘blind’ in the sense that the aim was to enclose the bulk of the lymphocyte population and not to study possible light-scatter pattern differences in the subject groups.
Fig. 2.
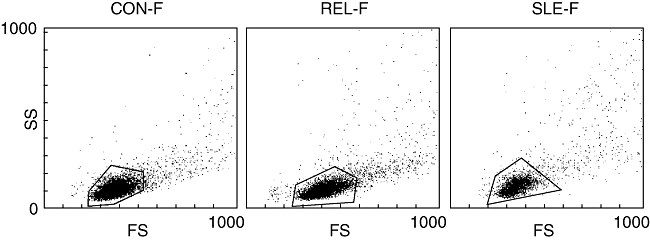
Distribution of T cell events in relation to the lymphocyte gate in forward/side-scatter plots from a representative female control donor (90·7% of T cells within the gate), female relative of a systemic lupus erythematosus (SLE) patient (82·8%) and a female SLE patient (76·8%). The number of T cell events analysed was, respectively, 8600, 10 500 and 3900. A total of 20 000 events was examined from each peripheral blood mononuclear cell sample.
For this paper, T lymphocytes are defined as the CD3+CD56− population, thus excluding CD3+CD56+ NKT cells. The lymphocyte gate also encompassed B cells defined as CD19+ and the NK/NKT population of CD56+ cells. Values for the percentage of T cells found within the gate (% TiG) were recorded.
To determine the percentage of T cells outside the gate but relatively close to it, a new quadrilateral region was established with the original gate in the bottom left corner and sides extending vertically and horizontally to points mid-way between the end of the light-scatter scales and the original gate. The cells close to the gate are thus obtained by subtracting those within the gate. Similarly, the percentage of events far from the gate was found by extending the quadrilateral to the end of the scales.
As will be discussed below, some of the T cells outside the gate are likely to be artefacts. For this reason, where appropriate, the term ‘T cell events’ will be used.
Plasma total IgG and IgM
Data on plasma total IgG and IgM in the above donor groups have been taken from Green et al.[5] and are now plotted against corresponding values of % TiG. Total IgG or IgM was determined by enzyme-linked immunosorbent assay and is expressed as mg/ml plasma.
Statistical evaluation of results
Values for the percentage of T cells within the gate in patients with SLE or their relatives were compared with control values using the Mann–Whitney U-test. Correlation analysis was carried out using Spearman's rank test.
Results
The percentage of T cells, defined as cells expressing CD3 but not CD56, that were contained within the FS/SS lymphocyte gate (% TiG) are shown in Fig. 1 for patients with SLE, first-degree relatives of SLE patients and control subjects, with each category being divided into female and male groups. Individual values of % TiG are shown together with the median for each group. The female control subjects formed a tightly knit group with values ranging from 86% to 96%. By contrast, the female relatives covered a much wider range (72–93%) with 60% of the group falling below the lowest female control (% TiG = 86), representing a significant reduction at P < 0·0001. Nearly 40% of the female SLE patients also fell below that level (P = 0·0005), including two with extremely low values.
Fig. 1.
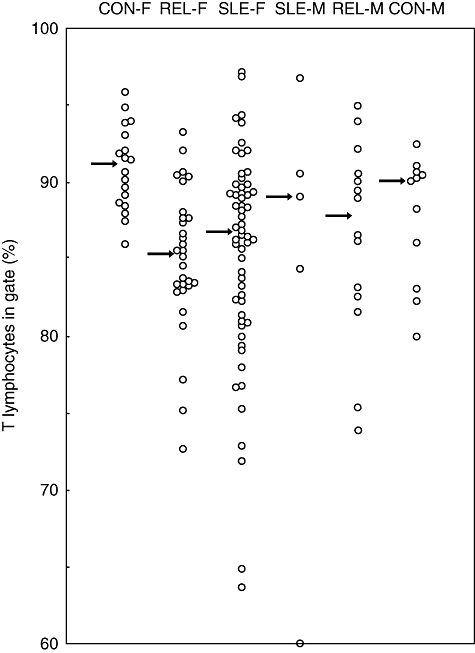
Percentage of blood T cells within the lymphocyte gate in systemic lupus erythematosus (SLE) patients, their first-degree relatives and control subjects. Arrows indicate the median value for each group. The circle on the baseline in the column headed SLE-M represents a value of 57·9%. CON-F, female controls; REL-F, female relatives of SLE patients; SLE-F, female SLE patients; CON-M, male controls; REL-M, male relatives of SLE patients; SLE-M, male SLE patients.
The TiG data for male control subjects ranged slightly lower than for female controls (80–92%). Unlike for females, only two of the male relatives had values below the normal male range. The small group of male SLE patients showed one abnormally low value.
Because the female relative and control groups were not matched closely for age [4], we have also compared these groups after exclusion of subjects older than 44 years. The mean age of the remaining controls [± standard deviation (s.d.)] was 30 ± 7 years (17 subjects) and of the relatives 29 ± 10 (15 subjects); the difference in TiG values [median: 91·5% (controls), 86·2% (relatives)] remained highly significant (P < 0·002).
Apart from the varying proportions found within the gate, it is difficult to distinguish visually between the FS/SS plots of T cell events in the different donor groups (Fig. 2). In each group the out-of-gate events have the appearance of the tail of a comet, stretching to higher FS/SS values. While increased size and granularity is suggestive of T cell activation, many of the out-of-gate events, particularly those further from the gate, may represent CD3+CD56− clumps of cells, aggregates of cell fragments or other artefacts.
In an analysis of six representative female controls and the same numbers of patients and relatives with abnormally low values of percentage TiG, it was found that the percentage of T cells close to the gate was enhanced significantly in patients (P < 0·005) or relatives (P < 0·05), but this was also true of far events (P < 0·005 in each case). Mean percentage values ± s.d. for the controls were 4·1 ± 1·2 (near), 3·8 ± 1·6 (far); SLE patients gave corresponding values of 9·0 ± 2·4 and 11·4 ± 3·0 and female relatives of 7·1 ± 2·8 and 11·7 ± 4·3.
Nevertheless, because T cell activation is expected in patients [3], it seems likely that the TiG data overall reflect activation in both patients and relatives. This view is supported by the strong inverse correlation seen in female relatives between plasma IgG levels and % TiG (r = −0·50, P < 0·005) (Fig. 3a) as production of IgG, which is at a high level in some relatives [5], is dependent on T cell activity [7]. Male relatives showed a lesser degree of IgG enhancement, yet an inverse relationship between IgG and % TiG approached significance (r = −0·48, P = 0·08). By contrast, no such correlation was found for the female control group (r = −0·06, P > 0·05) (Fig. 3b). There were also few male controls for this analysis, but combining male and female control data revealed no significant association (r = +0·03, P > 0·05). The correlation was also not apparent in female SLE patients (r = −0·04, P > 0·05) (Fig. 3c).
Fig. 3.
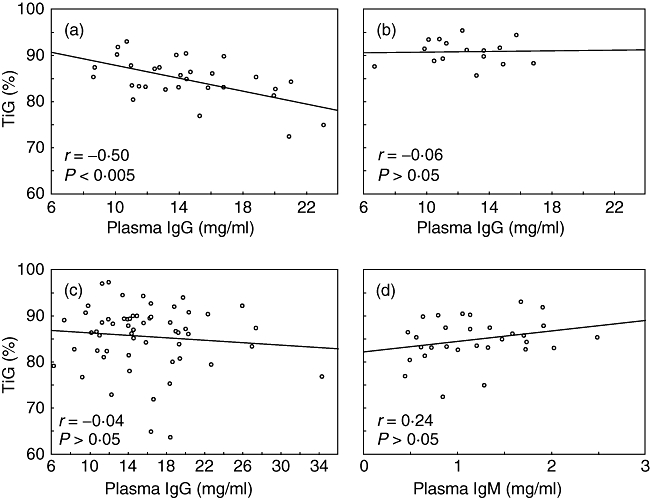
Relationship between percentage of T cells within the lymphocyte gate (% TiG) and total plasma immunoglobulin (Ig)G or IgM. (a) IgG relationship in female relatives of systemic lupus erythematosus (SLE) patients; (b) IgG relationship in female controls; (c) IgG relationship in female SLE patients; (d) IgM relationship in female relatives. The best-fit lines (linear regression) are shown.
There was no inverse correlation in relatives between IgM and % TiG (female relatives: r = +0·24, P > 0·05; male relatives: r = +0·05, P > 0·05 – female relatives shown in Fig. 3d). Similar results were obtained for the patient and control groups. IgM levels were depressed significantly in female relatives, whereas the patients showed examples of both depressed and enhanced levels [5].
Discussion
It appears from these results that many female SLE patients and female relatives of patients differ from normal controls in that a greater proportion of their T cells exhibit values of FS and SS that are higher than the normal range. The larger size and greater granularity that this implies is compatible with T cell activation and, as such activation occurs in SLE, it seems reasonable to suspect that it is also occurring in the relatives. This view is strengthened by the inverse correlation between % TiG and IgG levels in relatives because of the IgG dependency on T cell activation [7]. There was no correlation with IgM, which has no such dependency. It is perhaps not surprising that no correlation was seen for IgG in patients, bearing in mind the complexity of the disease process and the potency of the immunosuppressive drugs that are used [4,5].
Most information on T cell activation in SLE relates to the expression by the cells of the class II histocompatibility antigen HLA-DR [3,8]. By this token, it appears that a mean of approximately 15% of CD8+ cells are activated in active SLE, with less effect with respect to CD4 cells. Activated CD8 cells would be expected to develop granules in addition to enlargement with consequent increases in scatter values. It seems that the CD4/CD8 ratio is often < 1 in SLE [3], although total T cell numbers are low [4]. In these studies of HLA-DR expression in SLE, no indication is given as to whether changes to a standard lymphocyte gate were needed.
The foregoing interpretation of the results cannot be regarded as definitive until activation antigens such as HLA-DR have been demonstrated on T cells from relatives. Such data were not obtained because the T cell gate results were an unexpected side-product of a project not related directly to the question of T cell activation. In the absence of this direct confirmation, the possibility remains that we are seeing differences between donor groups in artefact numbers rather than changes within lymphocytes. That artefacts must be taken seriously is demonstrated by the finding that many of the increased T cell events were found to be far away from the gate and could well represent cell clumps. The nearer events could then be smaller clumps or activated cells. A further complication arises, however, as activated cells will be expressing adhesion molecules likely to encourage clump formation. Although we cannot be certain at this stage whether or not T cell activation is occurring in relatives, it is undoubtedly the case that female relatives differ from controls immunologically in showing weaker regulation of Ig production [9].
An explanation of low TiG values based on artefacts would need to account for the observed relationship between percentage TiG and IgG in the female relatives. It could be simply that the number of T cell clumps reflects the degree of T cell activation which, in turn, bears on the level of IgG production. Up to 20% of events outside the gate were apparently mixed T/B lymphocyte clumps as they expressed both CD3 and CD19. However, it seems unlikely that these B lymphocytes have any special significance. Clearly, B cell activation will be occurring at sites of antibody production, and in patients there was a reduction in the percentage of B cells in the lymphocyte gate comparable to that seen for T cells. However, no such reduction from control level was observed for relatives.
A further artefact scenario, described previously in cytometry [10], involves monocytes binding to T cells through Fcγ receptors. Antibodies against T lymphocytes are found in the majority of SLE patients and, whereas these are generally detected as cold-reactive IgM, there is also evidence for the presence of IgG antibodies [11]. Evidence for cold-reactive antibodies in relatives is conflicting [12], but if IgG antibodies were present bound to T cells this could result in the formation of monocyte–T cell aggregates. A link between IgG on T cells and total IgG level could conceivably provide an explanation for the TiG/IgG relationship.
If T cell activation is taking place in relatives, this would represent an important advance in understanding the aetiology of SLE. First, it would imply that such activation is not secondary to the disease process/drugs but is likely to be a key factor in disease causation. Second, it would suggest the existence of a T cell activation trait, not harmful in itself, but which on coming together with other abnormalities may lead to the disease of SLE. In patients, such activation would lead to high levels of pathogenic IgG autoantibodies, whereas in relatives the raised level of IgG antibodies would have specificities restricted largely to recall antigens [13]. The presence of activation in relatives may also be useful in indicating a predisposition to the development of SLE. Because of the potential importance of this activation concept, confirmation of T cell activation in relatives of SLE patients is urgently required.
Acknowledgments
We thank Angelina Mosley, Niga Nawroly and Frederic Toulza for advice on the analysis of flow cytometric data, and Charles Bangham and Keith Gould for critical reading of the manuscript. The work was supported by LUPUS UK, NW Nicholls Trust of St Mary's Hospital and the TR Golden Charitable Trust.
References
- 1.Loken MR, Brosnan JM, Bach BA, Ault KA. Establishing optimal lymphocyte gates for immunophenotyping by flow cytometry. Cytometry. 1990;11:453–9. doi: 10.1002/cyto.990110402. [DOI] [PubMed] [Google Scholar]
- 2.Krömer E, Grossmüller F. Light scatter based lymphocyte gate – helpful tool or source of error? Cytometry. 1994;15:87–9. doi: 10.1002/cyto.990150115. [DOI] [PubMed] [Google Scholar]
- 3.Maeda N, Sekigawa I, Iida N, Matsumoto M, Hashimoto H, Hirose S. Relationship between CD4+/CD8+ T cell ratio and T cell activation in systemic lupus erythematosus. Scand J Rheumatol. 1999;28:166–70. doi: 10.1080/03009749950154248. [DOI] [PubMed] [Google Scholar]
- 4.Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer cell activity in families of patients with systemic lupus erythematosus: demonstration of a killing defect in patients. Clin Exp Immunol. 2005;41:165–73. doi: 10.1111/j.1365-2249.2005.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer T cells in families of patients with systemic lupus erythematosus: their possible role in regulation of IgG production. Arthritis Rheum. 2007;56:303–10. doi: 10.1002/art.22326. [DOI] [PubMed] [Google Scholar]
- 6.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 7.Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559–64. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya N, Mitamura T, Goto M, et al. 2-dimensional flow cytometric analysis of peripheral blood T lymphocytes from patients with systemic lupus erythematosus: preferential expression of HLA-DR antigen on the surface of Leu 2a+ cells. J Rheumatol. 1988;15:946–51. [PubMed] [Google Scholar]
- 9.Clark J, Bourne T, Salaman MR, Seifert MH, Isenberg DA. B lymphocyte hyperactivity in families of patients with systemic lupus erythematosus. J Autoimmun. 1996;9:59–65. doi: 10.1006/jaut.1996.0008. [DOI] [PubMed] [Google Scholar]
- 10.Gratama JW, van der Linden R, van der Holt B, Bolhuis RL, van de Winkel JG. Analysis of factors contributing to the formation of mononuclear cell aggregates (‘escapees’) in flow cytometric immunophenotyping. Cytometry. 1997;29:250–60. [PubMed] [Google Scholar]
- 11.Osman C, Swaak AJ. Lymphocytotoxic antibodies in SLE: a review of the literature. Clin Rheumatol. 1994;13:21–7. doi: 10.1007/BF02229861. [DOI] [PubMed] [Google Scholar]
- 12.Miles S, Isenberg D. A review of serological abnormalities in relatives of SLE patients. Lupus. 1993;2:145–50. doi: 10.1177/096120339300200303. [DOI] [PubMed] [Google Scholar]
- 13.Dar O, Salaman MR, Seifert MH, Isenberg DA. B lymphocyte activation in systemic lupus erythematosus: spontaneous production of IgG antibodies to DNA and environmental antigens in cultures of blood mononuclear cells. Clin Exp Immunol. 1988;73:430–5. [PMC free article] [PubMed] [Google Scholar]


