Abstract
H-ficolin (Hakata antigen, ficolin-3) activates the lectin pathway of complement similar to mannose-binding lectin. However, its impact on susceptibility to infection is currently unknown. This study investigated whether the serum concentration of H-ficolin at diagnosis is associated with fever and neutropenia (FN) in paediatric cancer patients. H-ficolin was measured by time-resolved immunofluorometric assay in serum taken at cancer diagnosis from 94 children treated with chemotherapy. The association of FN episodes with H-ficolin serum concentration was analysed by multivariate Poisson regression. Median concentration of H-ficolin in serum was 26 mg/l (range 6–83). Seven (7%) children had low H-ficolin (< 14 mg/l). During a cumulative chemotherapy exposure time of 82 years, 177 FN episodes were recorded, 35 (20%) of them with bacteraemia. Children with low H-ficolin had a significantly increased risk to develop FN [relative risk (RR) 2·24; 95% confidence interval (CI) 1·38–3·65; P = 0·004], resulting in prolonged duration of hospitalization and of intravenous anti-microbial therapy. Bacteraemia occurred more frequently in children with low H-ficolin (RR 2·82; CI 1·02–7·76; P = 0·045). In conclusion, low concentration of H-ficolin was associated with an increased risk of FN, particularly FN with bacteraemia, in children treated with chemotherapy for cancer. Low H-ficolin thus represents a novel risk factor for chemotherapy-related infections.
Keywords: bacteraemia, cancer, fever and neutropenia, H-ficolin, innate immunity
Introduction
While advances in multi-modal therapy have significantly improved long-term survival in childhood cancer, chemotherapy-related infections remain a major cause of morbidity and mortality [1]. Infections manifest mainly as fever in severe chemotherapy-induced neutropenia (FN), which is the main reason for non-scheduled hospital admission [1,2]. Although most patients treated with cytotoxic drugs experience periods of severe neutropenia, there is considerable patient-to-patient variability regarding both the frequency and severity of FN [3,4].
Polymorphisms within the innate immune system have been proposed to explain individual susceptibility to infection in this setting [5]. Innate immunity provides defence against a large array of pathogens and is mediated mainly by host proteins recognizing highly conserved pathogen-associated molecular patterns, such as repetitive sugar arrays, present on the surface of many microorganisms but not on mammalian cells [6,7]. The complement system, a mainstay of innate immunity, eliminates microorganisms and enhances the adaptive immune response [8]. Complement activation occurs by the classical, the alternative and the evolutionarily more ancient lectin pathway [9]. The latter consists of soluble pattern recognition molecules containing collagen-like regions, namely mannose-binding lectin (MBL) and L- and H-ficolins [10–12]. MBL has been investigated in various settings. An increased susceptibility to sepsis and chemotherapy-related infections has been demonstrated in MBL-deficient individuals [6,13–16]. In contrast, the role of ficolins in health and disease remains largely undefined [11].
H-ficolin (Hakata antigen, ficolin-3), coded by the FCN-3 gene localized on 1p36.11, is composed of several subunits each containing a collagen-like strand and three C-terminal recognition domains binding to acetyl groups on microbial surfaces. H-ficolin is synthesized both in the liver and in the lung [11,12]. Similar to MBL and L-ficolin, H-ficolin relies on MBL-associated serine protease-2 (MASP-2) for activation of the complement system [12]. The H-ficolin/MASP-2 complex bound to microbial surfaces then cleaves C4 and C2, generating the C3 convertase C4bC2b, which leads to opsonization of pathogens and recruitment of inflammatory cells [7,17] or direct microbial killing. A single nucleotide polymorphism, FCN3 + 1637delC, has been decribed recently and results in decreased H-ficolin concentration in heterozygous individuals; homozygosity for this deletion has not been reported so far [18].
In contrast to several studies with partially controversial results on the impact of MBL deficiency on infection [6,16,19], only one study in 128 adult patients with various malignancies evaluated H-ficolin, and no association with FN was found [20]. The aim of the present study was to determine whether the concentration of H-ficolin in serum from children with cancer is associated with FN.
Materials and methods
Patients and clinical definitions
All children and adolescents up to the age of 17 years diagnosed with a malignancy requiring chemotherapy between 1 July 2002 and 31 October 2005, at the Division of Pediatric Hematology and Oncology, Department of Pediatrics, University of Bern, Switzerland, were eligible for this study. The study was approved by the institutional review board.
The major outcome event, FN, was defined as an axillary temperature ≥ 38·5°C persisting for ≥ 2 h, or a single axillary temperature ≥ 39°C [21] during severe chemotherapy-induced neutropenia (absolute neutrophil count < 500/µl). Emergency hospitalization and empirical intravenous therapy with broad-spectrum antibiotics were routine measures for FN during the study period [21].
Fever at diagnosis of cancer was defined as an axillary temperature ≥ 38·5°C requiring intravenous anti-microbial therapy within 48 h after admission. Chemotherapy was classified into four levels of intensity according to the expected duration of severe neutropenia [16,22]. Chemotherapy exposure time was defined as the cumulative duration of chemotherapy plus 2 weeks, because neutropenia may persist after cessation of chemotherapy. The cumulative duration of neutropenia was defined as the sum of weeks with severe neutropenia.
Outcome measures and data assessment
The total number of FN episodes was analysed as the primary end-point. Secondary end-points were the cumulative duration of hospitalization and of intravenous anti-bacterial therapy because of FN, and the number of FN episodes with bacteraemia, with microbiologically confirmed viral infection and without microbiologically confirmed aetiology. Bacteraemia was defined as the recovery of bacterial pathogens from a blood culture obtained during FN.
Characteristics of the patient, duration of chemotherapy and severe neutropenia and outcome measures were extracted from patient records. Serum concentration of C-reactive protein (CRP) was determined routinely at diagnosis. Parameters of cell turnover were determined routinely in patients with acute leukaemia (serum concentration of uric acid and lactate-dehydrogenase, blast and monocyte count in peripheral blood, blast percentage in bone marrow smear).
Quantification of H-ficolin serum concentration
Serum samples were obtained at time of cancer diagnosis, before starting chemotherapy, and stored at −20°C until analysis. Serum concentration of H-ficolin was measured by a time-resolved immunofluorometric assay, a sandwich assay similar to enzyme-linked immunosorbent assay (ELISA). The assay was carried out as described [23], with minor modifications. The wells of FlouroNunc microtitre plates (Nunc, Kamstrup, Denmark) were coated by incubating with 100 ng of monoclonal anti-H-ficolin antibody [17] (4H5; HyCult Biotechnology, Uden, the Netherlands) in 100 µl of phosphate-buffered saline (PBS) overnight at 4°C in a humid chamber. The wells were blocked with 200 µg of human serum albumin (HSA) in 200 µl triethanolamine-buffered saline (TBS) for 1 h at room temperature, followed by three washes with TBS with 10 mM CaCl2 and 0·05% Tween 20. Serum samples were diluted 1000-fold in 1 M salt buffer [20 mM Tris-HCl, 1 M NaCl, 0·05% Triton X-100 (Sigma-Aldrich, St Louis, MO, USA), 10 mM CaCl2, 0·1% (w/v) HSA]. Of the diluted sample, 100 µl was added to duplicate wells. The plates were incubated overnight at 4°C, washed as described, and incubated with 100 µl of TBS with 10 mM CaCl2 and 0·05% Tween 20 containing 25 ng monoclonal anti-H-ficolin antibody biotinylated with 33 µg of biotinyl-N-hydroxy-succinimide (Sigma-Aldrich) per mg of protein. The plates were incubated for 2 h at room temperature and washed three times. Next, 10 ng of europium-labelled streptavidin (Perkin Elmer, Waltham, MA, USA) in 100 µl of TBS containing 0·05% (v/v) Tween 20 and 25 µM ethylenediamine tetraacetic acid was added per well. After incubation at room temperature for 1 h and washing, 200 µl of enhancement solution (Wallac, Turku, Finland) was added, and europium was quantified by time-resolved fluorometry with a 1232 Delfia fluorometer (Wallac). Normal human standard serum with known content of H-ficolin (20 mg/l) was used to construct the standard curve with 3·5-fold dilutions from 1/20 on every plate. The concentration of H-ficolin in the standard serum was determined using a highly purified preparation of the protein as primary standard [12,24]. Reproducibility of this assay was assessed by 25-fold replication in three different control sera (coefficients of variation, 9·6% for 9·8 mg/l, 8·2% for 16·8 mg/l, 11·8% for 24·1 mg/l).
Statistical analysis
Because data were non-normally distributed, medians, interquartile ranges (IQR) and unconditional exact 95% confidence intervals (CI) were calculated for descriptive statistics. Exact Fisher's, Fisher–Freeman–Halton, Wilcoxon–Mann–Whitney and Kruskal–Wallis tests were used for analytical statistics [25]. Spearman's rank correlation test, repeated 10 000 times with jittering because of tied data, was used to analyse potential associations of H-ficolin serum concentration with laboratory parameters at diagnosis.
H-ficolin serum concentration was categorized for analysis of associations with outcome measures, as a linear association could not be assumed. As no data on normal H-ficolin concentration in children are available, the adjacent categories method [26] based on multivariate Poisson analysis in patients without fever at diagnosis was applied. It started with seven categories defined by limits of 7 mg/l as the lower normal limit in adults [11] and its multiples (14, 21, 28, 35, 42). The two resulting categories were defined by concentrations < 14 mg/l (furthermore called low H-ficolin) and ≥ 14 mg/l (normal H-ficolin), respectively.
The assumption that outcome measures had a constant incidence over time was confirmed graphically and by the Kolmogorov–Smirnov goodness-of-fit test (data not shown). The association of low H-ficolin with outcome measures was thus assessed using Poisson regression with chemotherapy duration as exposure time, and stratified on chemotherapy intensity, which is known to influence heavily the risk of FN [1,16]. For multivariate analysis, sex, age at diagnosis (four categories), cancer type (acute lymphoblastic leukaemia versus other), fever at diagnosis and central venous line were included as covariates [25].
Two sensitivity analyses were performed: the first used duration of severe neutropenia instead of duration of chemotherapy as exposure time. The second included only patients without fever at diagnosis.
Two-sided tests were used throughout, and P-values below 0·05 were considered significant. Cytel Studio 6·3·0 (Cytel Software Corporation, Cambridge, MA, USA) was used for exact calculations, and R 2·5·1 (R Foundation for Statistical Computing, Vienna, Austria) for the remaining statistical analyses.
Results
Patients and episodes of FN
Of 103 eligible children, serum samples taken at diagnosis were not available in nine patients. Table 1 summarizes the characteristics of the remaining 94 (91%) patients studied. The age of the patients ranged from 2 months to 16 years with a median of 6·8 years. During a cumulative chemotherapy exposure time of 81·7 patient years, 177 episodes of FN were recorded in 63 patients. Bacteraemia was detected in 35 (20%) episodes, invasive fungal infection in six (3%) and proven viral infection in 14 (8%), while no microbial aetiology was identified in 114 (64%). The most common isolates in bacteraemia were alpha-haemolytic streptococci and coagulase-negative staphylococci in 10 episodes each, Escherichia coli in six and Pseudomonas aeruginosa in four. Five children were admitted to the paediatric intensive care unit during FN; three of these died. Two of the patients who died (tumour-related thoracic inlet obstruction in one child and coagulopathy due to disseminated adenovirus infection in the other child) had low H-ficolin serum concentration (< 14 mg/l). Further clinical details of these patients have been published elsewhere [16,27].
Table 1.
Patient characteristics and H-ficolin serum concentration.
| Patients by H-ficolin status† |
H-ficolin serum concentration (mg/l) |
||
|---|---|---|---|
| Characteristic | Low | Normal | Median (interquartile range) |
| Gender | Fisher‡ = 3·48, P = 0·11 | WMW§ = 1237, P = 0·32 | |
| Female | 1 (14%) | 45 (52%) | 29·1 (21·7–39·1) |
| Male | 6 (86%) | 42 (48%) | 25·6 (17·9–39·2) |
| Age at diagnosis | FFH¶ = 3·51, P = 0·32 | KW** = 1·92, P = 0·59 | |
| < 4 years | 2 (29%) | 25 (29%) | 24·1 (18·4–31·9) |
| 4–8 years | 0 | 23 (26%) | 25·2 (21·8–40·6) |
| 8–12 years | 3 (43%) | 17 (20%) | 29·1 (24·4–39·7) |
| ≥ 12 years | 2 (29%) | 22 (23%) | 36·1 (19·8–41·2) |
| Diagnostic group | FFH¶ = 5·56, P = 0·21 | KW** = 28·6, P < 0·001 | |
| Acute lymphoblastic leukaemia | 1 (14%) | 31 (36%) | 39·6 (29·2–49·7) |
| Acute myeloid leukaemia | 0 | 9 (10%) | 38·1 (22·8–42·3) |
| Lymphoma | 3 (43%) | 11 (13%) | 24·5 (15·0–33·3) |
| Brain tumour | 2 (29%) | 13 (15%) | 23·0 (15·7–25·5) |
| Sarcoma | 1 (14%) | 13 (15%) | 22·1 (17·5–25·7) |
| Other | 0 | 10 (11%) | 23·4 (21·1–26·4) |
| Relapse status | Fisher‡ = 1·69, P = 0·25 | WMW§ = 533, P = 0·95 | |
| No relapse | 5 (71%) | 76 (87%) | 25·8 (18·8–39·3) |
| Relapse | 2 (29%) | 11 (13%) | 29·5 (23·9–38·1) |
| Fever at diagnosis | Fisher‡ = 0·09, P = 1·00 | WMW§ = 1156, P = 0·036 | |
| No | 5 (71%) | 62 (71%) | 24·4 (18·4–36·5) |
| Yes | 2 (29%) | 25 (29%) | 37·3 (24·6–39·9) |
| Central venous line | Fisher‡ = 0·74, P = 0·43 | WMW§ = 519, P < 0·001 | |
| No | 1 (14%) | 28 (32%) | 34·8 (25·7–49·5) |
| Yes | 6 (86%) | 59 (68%) | 23·8 (17·9–37·3) |
| Chemotherapy intensity†† | FFH¶ = 4·48, P = 0·16 | KW** = 1·71, P = 0·64 | |
| Minimally myelosuppressive | 4 (57%) | 32 (37%) | 33·4 (20·4–41·6) |
| Briefly myelosuppressive | 4 (57%) | 70 (80%) | 26·3 (21·2–39·2) |
| Strongly myelosuppressive | 3 (43%) | 12 (14%) | 25·6 (17·0–39·3) |
| Myeloablative | 1 (14%) | 6 (7%) | 24·4 (22·8–29·6) |
Seven (7%) patients with low H-ficolin (serum concentration < 14 mg/l); 87 (93%) patients with normal concentration (≥ 14 mg/l). Exact test statistic of
Fisher's test,
Wilcoxon–Mann–Whitney (WMW) test,
Fisher–Freeman–Halton (FFH) test and
Kruskal–Wallis (KW) test;
n = 132, because 38 patients were treated with chemotherapy of two different intensities.
H-ficolin serum concentration
H-ficolin serum concentration ranged from 5·7 to 83 mg/l (median 26; IQR 20–39). The distribution of H-ficolin concentrations was non-normal (Shapiro–Wilks statistic, 0·945; P < 0·001), while the distribution of the logarithmized concentrations was approximately normal (0·970; P = 0·030; Fig. 1). Seven (7%) patients had low H-ficolin with a serum concentration < 14 mg/l. Serum storage time was not associated significantly with H-ficolin concentration when assessed graphically and with linear regression (P = 0·90).
Fig. 1.
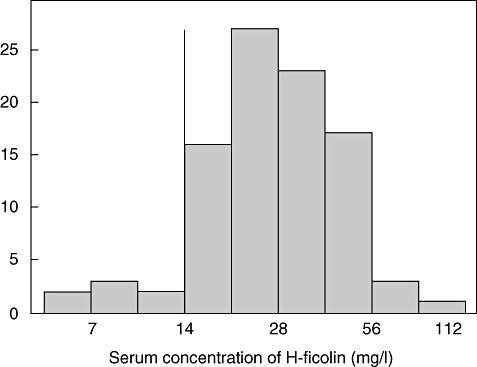
Serum concentration of H-ficolin in 94 children with cancer. The solid vertical line indicates the limit between low and normal H-ficolin concentrations (14 mg/l).
H-ficolin serum concentration and clinical characteristics
There were no significant differences of clinical characteristics between patients with low versus normal H-ficolin (Table 1). However, patients with fever at diagnosis had higher H-ficolin concentrations than those without fever (Table 1). The 41 patients with acute leukaemia had significantly higher H-ficolin compared to all other patients (median, 39 versus 23 mg/l; P < 0·001). This difference remained highly significant when restricting the analysis to patients without fever at diagnosis (median, 39 versus 24 mg/l; P < 0·001).
There was a significant positive correlation between serum concentrations of H-ficolin and CRP in the entire sample (rho = 0·27; 95% CI, 0·25–0·30; P = 0·014, Fig. 2). No significant correlation of H-ficolin with cell turnover markers was found in patients with acute leukaemia (data not shown).
Fig. 2.
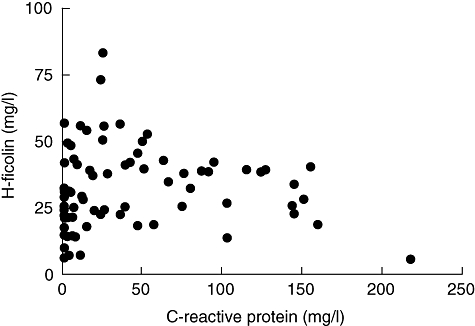
Serum concentrations of H-ficolin and C-reactive protein.
H-ficolin serum concentration and the risk of FN
In multivariate analysis, accounting for other patient characteristics associated with FN, low serum concentration of H-ficolin was associated with a significantly higher risk to develop FN during chemotherapy (multivariate rate ratio, 2·24; 95% CI, 1·38–3·65; P = 0·004; Table 2). The increased risk of FN resulted in significantly longer cumulative durations of hospitalization and intravenous anti-bacterial therapy due to FN. Patients with low H-ficolin experienced episodes of FN with bactaeremia three times more frequently than those with normal H-ficolin (multivariate rate ratio, 2·82; 95% CI, 1·02–7·76; P = 0·045; Table 2). No significant difference regarding bacterial aetiology (Gram-positive versus Gram-negative) was found (data not shown).
Table 2.
Associations of low serum concentration of H-ficolin with outcome measures.
| H-ficolin status† |
Univariate analysis‡ |
Multivariate analysis‡ |
||||
|---|---|---|---|---|---|---|
| Outcome measure | Low (%) | Normal (%) | Rate ratio (95% CI) | P | Rate ratio (95% CI) | P |
| Total number of FN§ episodes | 23 (13) | 154 (87) | 1·60 (0·97–2·51) | 0·06 | 2·24 (1·38–3·65) | 0·004 |
| Duration of hospitalization due to FN§,¶ | 202 (13) | 1355 (87) | 1·31 (1·12–1·52) | < 0·001 | 1·78 (1·51–2·10) | < 0·001 |
| Duration of i.v. anti-microbial therapy¶ | 198 (14) | 1266 (86) | 1·45 (1·25–1·69) | < 0·001 | 2·02 (1·71–2·38) | < 0·001 |
| FN episodes with bacteraemia | 7 (20) | 28 (80) | 3·84 (1·24–10·9) | 0·019 | 2·82 (1·02–7·76) | 0·045 |
| FN episodes with confirmed viral infection | 2 (14) | 12 (86) | 1·39 (0·15–6·59) | 0·92 | 1·36 (0·25–7·48) | 0·720 |
| FN episodes with no identified aetiology | 13 (11) | 101 (89) | 1·57 (0·80–2·83) | 0·19 | 2·08 (1·10–3·94) | 0·025 |
Seven (7%) patients with low H-ficolin (serum concentration < 14 mg/l); 87 (93%) patients with normal concentration (≥ 14 mg/l).
Results of Poisson regression stratified on chemotherapy intensity, with duration of chemotherapy as exposure time, with sex, age at diagnosis (four categories), fever at diagnosis, cancer type (acute lymphoblastic leukaemia versus other) and central venous line as covariates.
Fever in severe chemotherapy-induced neutropenia.
Cumulative duration indicated in days. CI, confidence interval; FN, fever and neutropenia; i.v., intravenous.
Sensitivity analyses
Both sensitivity analyses, i.e. replacing chemotherapy exposure time by duration of neutropenia, on one hand, and restricting analysis to patients without fever at cancer diagnosis on the other hand, confirmed these results fully (details not shown).
Discussion
The results of this study indicate that H-ficolin deficiency in children treated with chemotherapy for cancer was associated with a twofold increased risk to develop FN, which resulted in a significantly prolonged cumulative duration of hospitalization and of antibiotic treatment due to FN. This increase was caused in part by more frequent FN episodes with bacteraemia. To the best of our knowledge, this is the first report of a clinical relevance for H-ficolin in vivo.
Contrary to the rarity of most congenital complement deficiencies, single nucleotide polymorphisms within the lectin pathway of complement activation are frequent [11]. Under normal circumstances, redundant pathways within the innate immune system provide adequate immunological function [28]. However, in severe secondary immunodeficiency such as chemotherapy-associated neutropenia, patients rely on functional innate defence mechanisms. While deficiency of MBL, MASP-2 or ficolins is clinically inapparent in the healthy host, an increased susceptibility to chemotherapy-related infections has been shown for patients deficient in MBL [13,14,16,19] and of MASP-2 [27,29]. Despite close functional and structural similarities of ficolins with MBL [30], their role has received little attention so far. The results of this study indicate that, similarly to MBL and MASP-2 deficiency, low H-ficolin concentration confers an increased susceptibility to infection in children with chemotherapy-induced neutropenia. Kilpatrick et al. assessed H-ficolin in adults receiving chemotherapy for cancer and did not find an association with FN [20]. However, follow-up periods were shorter, and the majority of patients had received myeloablative chemotherapy. After myeloablative chemotherapy, the relevance of lectin-mediated opsonization may be minimized because phagocytes are virtually absent [5]. Also, children may depend more upon innate defence mechanisms than adults because of the immaturity of their adaptive immune system [14].
The increased frequency of FN in children with low H-ficolin was due in part to a threefold increased incidence of bacteraemia in these patients. This finding suggests that H-ficolin exerts important functions in innate defence against invasive bacterial infections in the immunocompromised host. While L-ficolin has been shown to promote phagocytosis of group B streptococci [31], knowledge about the action of H-ficolin on microorganisms is limited [11]. Significant binding of H-ficolin to bacteria has so far only been revealed for Aerococcus viridans[23], a bacterium encountered only rarely in oncology. Compared with other ficolins, MBL or surfactant protein D, H-ficolin has strong resistance against bacterial collagenases, which may provide an advantage in the elimination of microorganisms secreting collagenase [30].
H-ficolin concentrations were significantly higher in patients presenting with fever at diagnosis, indicating that H-ficolin has acute-phase properties. Additional support for this new finding arises from the observed significant correlation between serum concentrations of H-ficolin and C-reactive protein. Because ficolins recognize deposited C-reactive protein on bacteria, H-ficolin may enhance the inflammatory response during acute-phase reactions by interacting with C-reactive protein, as has been shown for L-ficolin and M-ficolin [32].
The concentrations of H-ficolin measured in the present cohort are in good agreement with published data from adult patients with median values of 25–30 mg/l, and were not correlated with age [11,33,34]. In our cohort, patients with acute leukaemia had significantly higher H-ficolin serum concentration than the remaining patients. Part of this difference may be caused by acute-phase reactions in the context of febrile infection or chronic malignancy-associated inflammation, which are frequent at diagnosis of acute leukaemia, but the difference remained significant in patients without fever at diagnosis. Theoretically, extrahepatic synthesis of H-ficolin, which has been shown for epithelial cells [35] and a glioma cell line [36], might also account for higher H-ficolin concentration in these patients. However, we observed no association between H-ficolin and several markers of haematological malignancy turnover.
This study has certain limitations, most of them related to its retrospective design. Because fewer than 10% of eligible patients were excluded, a selection bias seems unlikely. Until now, no limit for H-ficolin deficiency has been defined. Using ‘optimal’ cut-points in the evaluation of prognostic factors may be problematic [37]. However, the limit of 14 mg/l resulting from application of the adjacent categories method seems plausible in view of the known range of adult normal values [11,33,34]. The study focused solely upon phenotype, i.e. H-ficolin concentration in serum at the time of cancer diagnosis, without examining the FCN3 genotype. Lower H-ficolin concentrations have been shown for individuals heterozygous for the FCN3 + 1637delC mutation, which occurs at an allelic frequency of 1·1% in Danish Caucasians [18]. However, recent data on the H-ficolin genotype–phenotype relationship indicate that the 10-fold interindividual variation in serum concentrations arises in part from post-transcriptional and/or epigenetic events [30].
The strengths of this study include the fact that the patients studied reflect the entire spectrum of paediatric malignancies. The statistical model applied, i.e. Poisson regression stratified for chemotherapy intensity with chemotherapy duration as exposure time, has been shown to be valid for analysis of FN in this population treated with different chemotherapy regimens [16,27]. The sensitivity analyses performed confirmed the results of the main analysis.
In conclusion, we report that low H-ficolin serum concentration at cancer diagnosis was associated with an increased risk of FN in paediatric cancer patients. The risk of bacteraemia was increased threefold in children with low H-ficolin. Although independent replication by prospective cohorts is needed, the results of this study indicate that low H-ficolin needs to be considered as a novel risk factor for chemotherapy-associated infections, comparable to MBL or MASP-2 deficiency. Future work on the role of the lectin pathway of complement should study the ficolins, MBL and MASP-2 in combination, as analysis of an isolated parameter may be too simplistic. The perspective of using innate immunity factors to predict individual susceptibility to infection is promising, especially when considering out-patient management of FN [38–40]. Whether different strategies regarding prophylaxis or treatment of FN are needed for these patients remains to be evaluated in prospective trials. Considering the very limited understanding of the biological function of H-ficolin, further studies should aim at defining its kinetics, bacterial specificity and the clinical significance of low H-ficolin concentrations for infections in children and adults.
Acknowledgments
We thank Andrea Wasem and Nadine Beusch, study nurses, for their help in data extraction from patient records; Margrith Otth, for handling the serum samples, and the Institute for Infectious Diseases, University of Bern, Switzerland, for providing us with the serum samples. This work was supported by a grant from the Professor E. Rossi Foundation for Research in Pediatrics, Department of Pediatrics, University of Bern, Switzerland.
Disclosure
None.
References
- 1.Walsh TJ, Roilides E, Groll AH, et al. Infectious complications in pediatric cancer patients. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 5th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 1269–329. [Google Scholar]
- 2.Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin Infect Dis. 2005;40(Suppl. 4):S240–5. doi: 10.1086/427329. [DOI] [PubMed] [Google Scholar]
- 3.Timmer-Bonte JN, de Boo TM, Smit HJ, et al. Prevention of chemotherapy-induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony-stimulating factor in small-cell lung cancer: a Dutch randomized phase III study. J Clin Oncol. 2005;23:7974–84. doi: 10.1200/JCO.2004.00.7955. [DOI] [PubMed] [Google Scholar]
- 4.Wicki S, Keisker A, Aebi C, et al. Risk prediction of fever in neutropenia in children with cancer: a step towards individually tailored supportive therapy? Pediatr Blood Cancer. 2008;51:778–83. doi: 10.1002/pbc.21726. [DOI] [PubMed] [Google Scholar]
- 5.Neth OW, Bajaj-Elliott M, Turner MW, et al. Susceptibility to infection in patients with neutropenia: the role of the innate immune system. Br J Haematol. 2005;129:713–22. doi: 10.1111/j.1365-2141.2005.05462.x. [DOI] [PubMed] [Google Scholar]
- 6.Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37:1496–505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen R, Thiel S, Jensenius JC. Mannan-binding-lectin-associated serine proteases, characteristics and disease associations. Springer Semin Immunopathol. 2005;27:299–319. doi: 10.1007/s00281-005-0006-z. [DOI] [PubMed] [Google Scholar]
- 8.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 9.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway – its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 10.Holmskov U, Thiel S, Jensenius JC. Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–78. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 11.Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44:3875–88. doi: 10.1016/j.molimm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita M, Kuraya M, Hamasaki N, et al. Activation of the lectin complement pathway by H-ficolin (Hakata antigen) J Immunol. 2002;168:3502–6. doi: 10.4049/jimmunol.168.7.3502. [DOI] [PubMed] [Google Scholar]
- 13.Peterslund NA, Koch C, Jensenius JC, et al. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 14.Neth O, Hann I, Turner MW, et al. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614–18. doi: 10.1016/S0140-6736(01)05776-2. [DOI] [PubMed] [Google Scholar]
- 15.Klein NJ. Mannose-binding lectin: do we need it? Mol Immunol. 2005;42:919–24. doi: 10.1016/j.molimm.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Schlapbach LJ, Aebi C, Otth M, et al. Serum levels of mannose-binding lectin and the risk of fever in neutropenia pediatric cancer patients. Pediatr Blood Cancer. 2007;49:11–16. doi: 10.1002/pbc.21097. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto R, Yae Y, Akaiwa M, et al. Cloning and characterization of the Hakata antigen, a member of the ficolin/opsonin p35 lectin family. J Biol Chem. 1998;273:20721–7. doi: 10.1074/jbc.273.33.20721. [DOI] [PubMed] [Google Scholar]
- 18.Munthe-Fog L, Hummelshoj T, Ma YJ, et al. Characterization of a polymorphism in the coding sequence of FCN3 resulting in a Ficolin-3 (Hakata antigen) deficiency state. Mol Immunol. 2008;45:2660–6. doi: 10.1016/j.molimm.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Klein NJ, Kilpatrick DC. Is there a role for mannan/mannose-binding lectin (MBL) in defence against infection following chemotherapy for cancer? Clin Exp Immunol. 2004;138:202–4. doi: 10.1111/j.1365-2249.2004.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilpatrick DC, McLintock LA, Allan EK, et al. No strong relationship between mannan binding lectin or plasma ficolins and chemotherapy-related infections. Clin Exp Immunol. 2003;134:279–84. doi: 10.1046/j.1365-2249.2003.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ammann RA, Hirt A, Ridolfi Lüthy A, et al. Predicting bacteremia in children with fever and chemotherapy-induced neutropenia. Pediatr Infect Dis J. 2004;23:61–7. doi: 10.1097/01.inf.0000106782.30100.4f. [DOI] [PubMed] [Google Scholar]
- 22.Kern WV, Cometta A, de Bock R, et al. Oral versus intravenous empirical antimicrobial therapy for fever in patients with granulocytopenia who are receiving cancer chemotherapy. International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med. 1999;341:312–18. doi: 10.1056/NEJM199907293410502. [DOI] [PubMed] [Google Scholar]
- 23.Krarup A, Sorensen UB, Matsushita M, Jensenius JC, Thiel S. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect Immun. 2005;73:1052–60. doi: 10.1128/IAI.73.2.1052-1060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colley KJ, Beranek MC, Baenziger JU. Purification and characterization of the core-specific lectin from human serum and liver. Biochem J. 1988;256:61–8. doi: 10.1042/bj2560061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman DG. Practical statistics for medical research. London: Chapman & Hall; 1991. [Google Scholar]
- 26.Byar DP. Identification of prognostic factors. In: Buyse ME, Staquet MJ, Sylvester RJ, editors. Cancer clinical trial methods in practice. Oxford: Oxford Medical Publications; 1988. pp. 423–43. [Google Scholar]
- 27.Schlapbach LJ, Aebi C, Otth M, et al. Deficiency of mannose-binding lectin-associated serine protease-2 associated with increased risk of fever and neutropenia in pediatric cancer patients. Pediatr Infect Dis J. 2007;26:989–94. doi: 10.1097/INF.0b013e31811ffe6a. [DOI] [PubMed] [Google Scholar]
- 28.Roos A, Garred P, Wildenberg M, et al. Antibody-mediated activation of the classical pathway of complement may compensate for mannose-binding lectin deficiency. Eur J Immunol. 2004;34:2589–98. doi: 10.1002/eji.200324401. [DOI] [PubMed] [Google Scholar]
- 29.Granell M, Urbano-Ispizua A, Suarez B, et al. Mannan-binding lectin pathway deficiencies and invasive fungal infections following allogeneic stem cell transplantation. Exp Hematol. 2006;34:1435–41. doi: 10.1016/j.exphem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Hummelshoj T, Fog LM, Madsen HO, et al. Comparative study of the human ficolins reveals unique features of Ficolin-3 (Hakata antigen) Mol Immunol. 2008;45:1623–32. doi: 10.1016/j.molimm.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Aoyagi Y, Adderson EE, Min JG, et al. Role of L-ficolin/mannose-binding lectin-associated serine protease complexes in the opsonophagocytosis of type III group B streptococci. J Immunol. 2005;174:418–25. doi: 10.4049/jimmunol.174.1.418. [DOI] [PubMed] [Google Scholar]
- 32.Ng PM, Le Saux A, Lee CM, et al. C-reactive protein collaborates with plasma lectins to boost immune response against bacteria. EMBO J. 2007;26:3431–40. doi: 10.1038/sj.emboj.7601762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ytting H, Christensen IJ, Thiel S, et al. Biological variation in circulating levels of mannan-binding lectin (MBL) and MBL-associated serine protease-2 and the influence of age, gender and physical exercise. Scand J Immunol. 2007;66:458–64. doi: 10.1111/j.1365-3083.2007.01991.x. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen RG, Vind I, Munkholm P, et al. Genetic polymorphisms of mannan binding lectin (MBL), serum levels of MBL, the MBL associated serine protease and H-ficolin in patients with Crohn's disease. Gut. 2007;56:311–12. doi: 10.1136/gut.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akaiwa M, Yae Y, Sugimoto R, et al. Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J Histochem Cytochem. 1999;47:777–86. doi: 10.1177/002215549904700607. [DOI] [PubMed] [Google Scholar]
- 36.Kuraya M, Matsushita M, Endo Y, et al. Expression of H-ficolin/Hakata antigen, mannose-binding lectin-associated serine protease (MASP)-1 and MASP-3 by human glioma cell line T98G. Int Immunol. 2003;15:109–17. doi: 10.1093/intimm/dxg008. [DOI] [PubMed] [Google Scholar]
- 37.Altman DG, Lausen B, Sauerbrei W, et al. Dangers of using ‘optimal’ cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86:829–33. doi: 10.1093/jnci/86.11.829. [DOI] [PubMed] [Google Scholar]
- 38.Mullen CA. Which children with fever and neutropenia can be safely treated as outpatients? Br J Haematol. 2001;112:832–7. doi: 10.1046/j.1365-2141.2001.02632.x. [DOI] [PubMed] [Google Scholar]
- 39.Ammann RA, Simon A, de Bont ES. Low risk episodes of fever and neutropenia in pediatric oncology: is outpatient oral antibiotic therapy the new gold standard of care? Pediatr Blood Cancer. 2005;45:244–7. doi: 10.1002/pbc.20287. [DOI] [PubMed] [Google Scholar]
- 40.Klastersky J, Paesmans M, Georgala A, et al. Outpatient oral antibiotics for febrile neutropenic cancer patients using a score predictive for complications. J Clin Oncol. 2006;24:4129–34. doi: 10.1200/JCO.2005.03.9909. [DOI] [PubMed] [Google Scholar]


