Abstract
South Asian immigrants in western societies exhibit a high burden of diabetes and subsequent vascular complications. Diabetic vascular complications are associated with vascular inflammation. We hypothesize that enhanced complement activation is involved. Therefore, levels of complement C3 and SC5b-9 – the soluble end product of complement activation – in a group of 200 South Asians were compared with an age- and sex-matched control group of native Caucasians. In addition, the association between complement levels and albuminuria, an indicator of renal damage and a cardiovascular risk marker, was assessed in the diabetic South Asian group. Compared with native Caucasians, South Asians had significantly higher levels of both serum C3 and plasma SC5b-9, even when only non-diabetic South Asians were considered. Diabetic South Asians had significantly higher C3 levels compared with non-diabetic South Asians. In diabetic South Asians, higher levels of SC5b-9 were associated with an increased prevalence of albuminuria (odds ratio 5·4, 95% confidence interval 1·8–15·8). These results suggest that enhanced complement activation is part of the unfavourable cardiovascular risk profile in South Asians.
Keywords: cardiovascular, complement, diabetes, South Asian
Introduction
South Asian immigrants in western societies have a high burden of ischaemic heart disease, stroke and diabetes [1,2], and South Asians with diabetes have a 40-fold increased risk of developing end-stage renal disease because of diabetic nephropathy compared with native Caucasians [3]. Traditional cardiovascular risk factors do not explain completely the increased incidence of cardiovascular disease in South Asians [4]. Hence other, genetically determined, factors are likely to be involved.
Atherosclerosis, the pathological substrate of macrovascular disease, is recognized to be an inflammatory process [5–7]. Microvascular disease such as diabetic nephropathy has also been linked to inflammatory markers [8,9]. As a key player in the inflammatory response, the complement system has been implicated in this vascular inflammation [10–12]. Deposition of complement components has been demonstrated in atherosclerotic plaques and in retinae and kidneys of diabetic subjects [13–15].
We hypothesized that in South Asians a highly active innate immune and complement system contributes to the increased susceptibility for vascular disease. We therefore examined complement, as judged by the level of the main component C3 and the level of the final activation product SC5b-9, in South Asian subjects with and without type 2 diabetes living in the Netherlands, and compared these with complement levels in an age- and sex-matched group of native Caucasian volunteers without diabetes. To investigate whether complement levels (C3) and activation (SC5b-9) are involved in cardiovascular disease, we studied the association of these parameters with albuminuria. The presence of albuminuria not only indicates renal damage but also is a risk marker for cardiovascular disease [16,17].
Our results indicate that South Asians have increased complement activation and that albuminuria is associated with increased complement activation. These data support the hypothesis that enhanced complement activation is part of the unfavourable cardiovascular risk profile in South Asians.
Materials and methods
Two hundred South Asians living in the Hague, the Netherlands were studied. They were participants in a previously conducted study [18]. Subjects were recruited from the general practitioner's population, as described earlier. From the original study population, a sample of 100 diabetic and 100 non-diabetic subjects was selected randomly. The study was approved by the Institutional Medical Ethics Committee.
The study protocol has been published previously in detail [18]. Briefly, morning urine and fasting blood samples were taken from all subjects and put immediately into ice. Ethylenediamine tetraacetic acid plasma was obtained after centrifugation (10 min at 550 g at 4°C) and the samples were stored in aliquots at −80°C within 1 h after collection. Serum was prepared by coagulation at room temperature, and after centrifugation the samples were stored at − 80°C. All study participants, except known diabetic subjects, were subjected to an oral glucose tolerance test (75-g glucose load). Diabetes was diagnosed based on the American Diabetes Association 2003 criteria. A brief physical examination (blood pressure, length, weight, hip and waist circumference) was performed. Clinical information concerning medical history, medication and lifestyle was obtained from a questionnaire. Laboratory measurements comprised renal function, fasting lipid profile, glycosylated haemaglobin (HbA1c), C-reactive protein (CRP) and urinary albumin : creatinine ratio. Albuminuria was defined as a urinary albumin : creatinine ratio > 2·5 mg/mmol in men and > 3·5 mg/mmol in women.
Levels of complement C3, which plays a pivotal role in complement activation, and SC5b-9, the soluble end product of complement activation, were measured and compared with levels in an age- and sex-matched group of native Caucasians without diabetes, recruited from healthy personnel from the dialysis ward and laboratory (n = 60). Complement levels in diabetic South Asians with albuminuria were compared with complement levels in diabetic South Asians without albuminuria, and South Asians with diabetes were compared with South Asians without diabetes.
Quantification of SC5b-9
The SC5b-9 levels were assessed by sandwich enzyme-linked immunosorbent assay (ELISA). In brief, 96-well ELISA plates were coated with the monoclonal antibody aE11 (mouse IgG2a anti-C5b-9 3 mg/ml), described in detail previously [19]. Plasma samples were diluted 1/5 and 1/20 and incubated in the coated wells. Bound SC5b-9 was detected with a biotin-labelled monoclonal anti-C6 antibody produced in our laboratory (9C4), followed by detection with streptavidin–poly horseradish peroxidase (Sanquin, Amsterdam, the Netherlands). Enzyme activity was detected using 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (Sigma Chemical Co., St Louis, MO, USA). All assays were performed on ice. The optical density was measured at 415 nm using a microplate biokinetics reader (EL312e; Biotek Instruments, Winooski, VT, USA). A calibration line was produced using zymosan-activated serum with a known concentration of SC5b-9 of 1000 U/ml.
Quantification of C3
Serum C3 was quantified using radial immunodiffusion according to Mancini, using a polyclonal rabbit anti-human C3 anti-serum as described earlier [20].
Statistics
Normally distributed variables are expressed as mean ± standard deviation and skewed distributed variables as median with interquartile range. Differences between groups were assessed with the independent-samples t-test or Mann–Whitney U-test as appropriate. Correlations between normally and skewed distributed variables were calculated with Pearson's correlation and Spearman's correlation respectively. Associations between complement levels and the presence of albuminuria were assessed with logistic regression. All tests were two-sided and the level of significance was set at 0·05. All analyses were performed using spss Statistical Software Package version 15·0 (SPSS Inc., Chicago, IL, USA).
Results
Two hundred South Asians aged 30–75 years were studied. Of the 100 diabetic subjects, 77 were already known with diabetes at the time of the study, and 23 subjects were newly diagnosed with diabetes based on oral glucose tolerance testing. In the diabetes group 28 subjects had albuminuria, and in the non-diabetes group two subjects had albuminuria. Age (mean age in South Asians 48 ± 9 years versus 46 ± 8 years in Caucasians, P = 0·245) and sex (43% male in the South Asian group versus 45% in the Caucasian group, P = 0·879) were not different between the South Asian and Caucasian group. Characteristics of the South Asian study population are shown in Table 1.
Table 1.
Baseline characteristics of the study population.
| Diabetic SA | Non-diabetic SA | P-value | |
|---|---|---|---|
| Age (years) | 49·8 ± 10·1 | 46·0 ± 7·3 | 0·003 |
| Sex (% male) | 40·0 | 45·0 | 0·477 |
| Current or former smoker (%) | 42·4 | 32·0 | 0·142 |
| Total cholesterol (mmol/l) | 5·06 ± 0·97 | 5·31 ± 0·97 | 0·067 |
| HDL-cholesterol (mmol/l) | 1·25 ± 0·34 | 1·36 ± 0·40 | 0·033 |
| Fasting triglycerides (mmol/l)† | 1·52 (1·19–2·23) | 1·21 (0·83–1·89) | < 0·001 |
| Systolic blood pressure (mm Hg) | 135·4 ± 28·3 | 127·6 ± 16·7 | 0·018 |
| Diastolic blood pressure (mm Hg) | 81·9 ± 14·4 | 80·9 ± 9·5 | 0·545 |
| Waist to hip ratio | 0·98 ± 0·074 | 0·93 ± 0·079 | < 0·001 |
| Body mass index | 28·1 ± 4·6 | 26·0 ± 4·0 | 0·001 |
| Cockroft clearance (ml/min/1.73 m2) | 88·8 ± 25·6 | 85·4 ± 12·7 | 0·233 |
| C-reactive protein (mg/l)† | 5·0 (2·0–8·5) | 2·0 (0·1–7·0) | 0·001 |
| Albuminuria (%) | 28·0 | 2·0 | <0·001 |
| Serum C3 (µg/ml) | 934 ± 156 | 854 ± 139 | <0·001 |
| Plasma SC5b-9 (U/ml) | 0·390 ± 0·13 | 0·362 ± 0·13 | 0·132 |
Normally distributed variables are expressed as means ± 1 standard deviation, skewed distributed parameters
expressed as median (interquartile range in brackets). HDL, high density lipoprotein; SA, South Asians.
Complement levels in South Asians compared with native Caucasians
To test our hypothesis, complement levels in South Asians were compared with native Caucasians.
South Asians had significantly higher levels of serum C3 compared with native Caucasians (895 ± 153 µg/ml versus 580 ± 128 µg/ml, P < 0·001) (Fig. 1a). When considering only non-diabetic South Asians, they still had higher C3 levels than native Caucasians (854 ± 139 µg/ml versus 580 ± 128 µg/ml, P < 0·001).
Fig. 1.
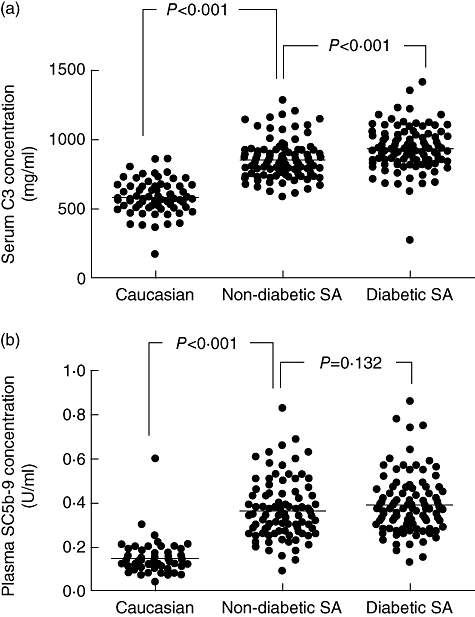
(a) Serum concentration of C3 in native Caucasians (n = 60) compared with South Asians (SA), the latter divided into non-diabetic (n = 100) and diabetic (n = 100) groups. The C3 concentration is significantly higher in the non-diabetic SA group compared with the Caucasian group, and significantly higher in the diabetic SA than in the non-diabetic SA group. (b) Plasma concentration of SC5b-9 in native Caucasians (n = 60) compared with South Asian (SA) immigrants, the latter divided into non-diabetic (n = 100) and diabetic (n = 100) groups. The SC5b-9 concentration is significantly higher in the non-diabetic SA group compared with the Caucasian group. There is no statistically significant difference between the diabetic SA and non-diabetic SA group.
In addition to C3 levels, South Asians also had significantly higher plasma levels of SC5b-9, the soluble end product of complement activation (mean 0·376 ± 0·13 U/ml versus 0·149 ± 0·07 U/ml, P < 0·001) (Fig. 1b). This was also the case when considering only non-diabetic South Asians (0·362 ± 0·13 U/ml versus 0·149 ± 0·07 U/ml, P < 0·001).
In South Asians, serum C3 levels correlated with CRP, SC5b-9, fasting triglycerides, lipoprotein(a), body mass index, hip circumference and waist circumference (Table 2). C3 levels also correlated with renal function. However, when diabetic and non-diabetic subjects were analysed separately, the correlation was apparent only in diabetic subjects (Spearman's r = 0·288, P = 0·004), even after adjusting for blood pressure and CRP (Spearman's r = 0·280, P = 0·006).
Table 2.
C3 level and its correlations (Spearman's ρ).
| P-value | ||
|---|---|---|
| Plasma CRP | 0·253 | < 0·001 |
| Plasma SC5b-9 | 0·160 | 0·024 |
| Total cholesterol | 0·103 | 0·149 |
| Fasting HDL cholesterol | 0·034 | 0·634 |
| Fasting triglycerides | 0·155 | 0·030 |
| Lipoprotein (a) | 0·174† | 0·015 |
| Body mass index | 0·234 | 0·001 |
| Waist circumference | 0·229 | 0·001 |
| Hip circumference | 0·253 | < 0·001 |
| Waist : hip ratio | 0·108 | 0·133 |
| Cockroft clearance/1.73 m | 0·168† | 0·018 |
| Systolic blood pressure | 0·165† | 0·021 |
| Diastolic blood pressure | 0·170† | 0·017 |
Pearson's correlation. CRP, C-reactive protein; HDL, high density lipoprotein.
Diabetic versus non-diabetic South Asians
To assess the influence of diabetes on complement levels, diabetic South Asians were compared with non-diabetic South Asians.
Diabetic South Asians had significantly higher C3 levels than non-diabetic South Asians (934 ± 156 versus 854 ± 139, P < 0·001, Fig. 1). SC5b-9 levels were also higher in diabetic South Asians compared with non-diabetic South Asians, although this difference did not reach statistical significance (0·390 ± 0·13 U/ml versus 0·362 ± 0·13 U/ml, P = 0·132).
Complement levels and albuminuria in diabetic South Asians
To investigate whether increased complement levels might be associated with renal damage and cardiovascular risk, complement levels in diabetic South Asians with albuminuria were compared with diabetic South Asians without albuminuria. In this analysis, only diabetic South Asians were included, because in the non-diabetic group only two subjects had albuminuria.
The C3 levels were not different between diabetic South Asians with albuminuria and diabetic South Asians without albuminuria (929 ± 106 µg/ml versus 936 ± 172 µg/ml, P = 0·232), whereas the mean level of SC5b-9 was significantly higher in diabetic South Asians with albuminuria (0·42 ± 0·14 U/ml versus 0·37 ± 0·12 U/ml, P = 0·045). SC5b-9 levels and log transformed albumin : creatinine ratio correlated positively (r = 0·217, P = 0·030). A SC5b-9 level above the median (0·36 U/l) was associated with a 5·4-fold increased risk for the presence of albuminuria (odds ratio 5·4, 95% confidence interval 1·8–15·8).
Discussion
In a group of South Asians living in a western society we found significantly higher levels of complement C3 and SC5b-9, the soluble end product of complement activation, compared with native Caucasians. In addition, in diabetic South Asians higher levels of SC5b-9 were associated with the presence of albuminuria, which is – besides being an indicator of renal damage – a risk marker for cardiovascular disease [16,17].
Atherosclerosis, the pathological substrate of macrovascular disease, is considered to be a vascular inflammatory process. The complement system has been implicated in this vascular inflammation [10–12]. Activation of the complement cascade, via either the classical, alternative or lectin pathway, results in activation of C3, and ultimately in the formation of the terminal complement complex, C5b-9. The soluble form of this complex, SC5b-9, can be measured in plasma and its levels have been found to reflect in vivo complement activation [21,22].
Our hypothesis – a highly active complement system which contributes to the increased susceptibility for vascular disease in South Asian immigrants – was tested by comparing C3 and SC5-9 levels in South Asians with native Caucasians. We found significantly higher serum C3 levels in South Asians compared with native Caucasians, which is in line with the findings of Somani and Ajjan [23,24]. In addition, we found for the first time that SC5b-9 is also increased in South Asians compared with native Caucasians, supporting our hypothesis that complement activation is increased in South Asians.
It has been shown previously that high C3 levels cluster with type 2 diabetes and the metabolic syndrome. However, the pathophysiological mechanism linking C3 to type 2 diabetes is unknown. Because C3 is synthesized mainly by the liver, increased C3 levels might be caused by increased hepatic synthesis. As C3 levels correlate with hyperlipidaemia [25], increased hepatic C3 production may be linked to an altered hepatic lipoprotein metabolism, which is a well-known feature of the metabolic syndrome and type 2 diabetes [26]. Alternatively, because C3 levels correlate with CRP levels, increased hepatic C3 synthesis might be part of an acute phase response, as was suggested previously [27].
As well as being produced in the liver, C3 production by adipocytes and macrophages in atherosclerotic plaques has also been reported [28]. Because the metabolic syndrome and type 2 diabetes are linked to visceral obesity, increased C3 production by adipocytes is an appealing hypothesis. However, this remains speculative, as the contribution of adipocyte-derived C3 to the total serum C3 levels has not been quantified.
An important question is, of course, whether increased C3 levels, regardless of the underlying mechanism, have any pathophysiological meaning or are merely an epiphenomenon. In a study with hypertriglyceridaemic subjects [29], C3 levels correlated positively with the activity of the complement system as measured by the haemolytic complement activity assay (CH50), indicating that increased C3 levels indeed have functional significance.
In contrast to C3 levels, SC5b-9 levels were associated not with diabetes but with albuminuria, the presence of which indicates renal damage but also is a risk marker for cardiovascular disease. These data are in agreement with the known role of complement in organ damage and cardiovascular disease.
In addition to a high prevalence of diabetic nephropathy, South Asians also have a high incidence of renal failure of undetermined origin [30,31]. It is intriguing to speculate that complement-related mechanisms are involved. In our diabetic South Asians, C3 levels correlated with renal function. Experimental data demonstrate an important role for complement-mediated renal damage in both diabetic and non-diabetic models [32,33]. As a central molecule in the complement cascade, C3 has been found to play an important role in tubulointerstitial damage and C3a has been shown recently to mediate epithelial to mesenchymal transition of renal tubular cells [34–36]. However, prospective human data concerning C3 levels and renal failure are currently lacking.
In conclusion, high C3 levels might render South Asians more susceptible to complement activation. The increased levels of SC5b-9, the end product of complement activation and the association of SC5b-9 with albuminuria, both suggest that enhanced complement activation is part of the unfavourable cardiovascular risk profile in South Asians.
Acknowledgments
We thank Dr M. Mallat for assisting with statistical analysis and critically reviewing the manuscript.
Disclosures
None.
References
- 1.Anand SS, Yusuf S. Risk factors for cardiovascular disease in Canadians of South Asian and European origin: a pilot study of the Study of Heart Assessment and Risk in Ethnic Groups (SHARE) Clin Invest Med. 1997;20:204–10. [PubMed] [Google Scholar]
- 2.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–6. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 3.Chandie Shaw PK, Vandenbroucke JP, Tjandra YI, et al. Increased end-stage diabetic nephropathy in Indo-Asian immigrants living in the Netherlands. Diabetologia. 2002;45:337–41. doi: 10.1007/s00125-001-0758-5. [DOI] [PubMed] [Google Scholar]
- 4.Forouhi NG, Sattar N, Tillin T, McKeigue PM, Chaturvedi N. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia. 2006;49:2580–8. doi: 10.1007/s00125-006-0393-2. [DOI] [PubMed] [Google Scholar]
- 5.Binder CJ, Chang MK, Shaw PX, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–26. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–91. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Mora C, Navarro JF. Inflammation and diabetic nephropathy. Curr Diab Rep. 2006;6:463–8. doi: 10.1007/s11892-006-0080-1. [DOI] [PubMed] [Google Scholar]
- 9.Navarro JF, Mora C, Maca M, Garca J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis. 2003;42:53–61. doi: 10.1016/s0272-6386(03)00408-6. [DOI] [PubMed] [Google Scholar]
- 10.Hansen TK. Mannose-binding lectin (MBL) and vascular complications in diabetes. Horm Metab Res. 2005;37(Suppl. 1):95–8. doi: 10.1055/s-2005-861372. [DOI] [PubMed] [Google Scholar]
- 11.Niculescu F, Rus H. The role of complement activation in atherosclerosis. Immunol Res. 2004;30:73–80. doi: 10.1385/IR:30:1:073. [DOI] [PubMed] [Google Scholar]
- 12.Ostergaard J, Hansen TK, Thiel S, Flyvbjerg A. Complement activation and diabetic vascular complications. Clin Chim Acta. 2005;361:10–19. doi: 10.1016/j.cccn.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Falk RJ, Sisson SP, Dalmasso AP, Kim Y, Michael AF, Vernier RL. Ultrastructural localization of the membrane attack complex of complement in human renal tissues. Am J Kidney Dis. 1987;9:121–8. doi: 10.1016/s0272-6386(87)80089-6. [DOI] [PubMed] [Google Scholar]
- 14.Gerl VB, Bohl J, Pitz S, Stoffelns B, Pfeiffer N, Bhakdi S. Extensive deposits of complement C3d and C5b-9 in the choriocapillaries of eyes of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2002;43:1104–8. [PubMed] [Google Scholar]
- 15.Rus HG, Niculescu F, Vlaicu R. Co-localization of terminal C5b-9 complement complexes and macrophages in human atherosclerotic arterial walls. Immunol Lett. 1988;19:27–32. doi: 10.1016/0165-2478(88)90115-0. [DOI] [PubMed] [Google Scholar]
- 16.Schmieder RE, Schrader J, Zidek W, et al. Low-grade albuminuria and cardiovascular risk. Clin Res Cardiol. 2007;96:247–57. doi: 10.1007/s00392-007-0510-3. [DOI] [PubMed] [Google Scholar]
- 17.Tillin T, Forouhi N, McKeigue P, Chaturvedi N. Microalbuminuria and coronary heart disease risk in an ethnically diverse UK population: a prospective cohort study. J Am Soc Nephrol. 2005;16:3702–10. doi: 10.1681/ASN.2005060584. [DOI] [PubMed] [Google Scholar]
- 18.Chandie Shaw PK, van Es LA, Paul LC, Rosendaal FR, Souverijn JH, Vandenbroucke JP. Renal disease in relatives of Indo-Asian Type 2 diabetic patients with end-stage diabetic nephropathy. Diabetologia. 2003;46:618–24. doi: 10.1007/s00125-003-1095-7. [DOI] [PubMed] [Google Scholar]
- 19.Mollnes TE, Lea T, Froland SS, Harboe M. Quantification of the terminal complement complex in human-plasma by an enzyme-linked immunosorbent-assay based on monoclonal-antibodies against a neoantigen of the complex. Scand J Immunol. 1985;22:197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 20.Kohler PF, Müller-Eberhard HJ. Immunochemical quantitation of third, fourth and fifth components of human complement – concentrations in serum of healthy adults. J Immunol. 1967;99:1211–16. [PubMed] [Google Scholar]
- 21.Mollnes TE, Lea T, Harboe M. Detection and quantification of the terminal C5b-9 complex of human complement by a sensitive enzyme-linked immunosorbent assay. Scand J Immunol. 1984;20:157–66. doi: 10.1111/j.1365-3083.1984.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 22.Mollnes TE, Froland SS, Harboe M. Increased plasma levels of the terminal complement complex in patients with evidence of complement activation. Complement. 1985;2:175–84. doi: 10.1159/000467858. [DOI] [PubMed] [Google Scholar]
- 23.Ajjan R, Carter AM, Somani R, Kain K, Grant PJ. Ethnic differences in cardiovascular risk factors in healthy Caucasian and South Asian individuals with the metabolic syndrome. J Thromb Haemost. 2007;5:754–60. doi: 10.1111/j.1538-7836.2007.02434.x. [DOI] [PubMed] [Google Scholar]
- 24.Somani R, Grant PJ, Kain K, Catto AJ, Carter AM. Complement C3 and C-reactive protein are elevated in South Asians independent of a family history of stroke. Stroke. 2006;37:2001–6. doi: 10.1161/01.STR.0000231649.56080.6d. [DOI] [PubMed] [Google Scholar]
- 25.Muscari A, Massarelli G, Bastagli L, et al. Relationship between serum C3 levels and traditional risk factors for myocardial infarction. Acta Cardiol. 1998;53:345–54. [PubMed] [Google Scholar]
- 26.Vergès B. New insights into the pathophysiology of lipid abnormalities in type 2 diabetes. Diabetes Metab. 2005;31:429–39. doi: 10.1016/s1262-3636(07)70213-6. [DOI] [PubMed] [Google Scholar]
- 27.Prasad UK, Grant PJ, Carter AM. Associations of C-reactive protein and complement C3 levels in patients with myocardial infarction. J Vasc Res. 2005;42:69. [Google Scholar]
- 28.Morgan BP, Gasque P. Extrahepatic complement biosynthesis: where, when and why? Clin Exp Immunol. 1997;107:1–7. doi: 10.1046/j.1365-2249.1997.d01-890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uza G, Cristea A, Cucuianu MP. Increased level of the complement C3-protein in endogenous hypertriglyceridemia. J Clin Lab Immunol. 1982;8:101–5. [PubMed] [Google Scholar]
- 30.Ball S, Lloyd J, Cairns T, et al. Why is there so much end-stage renal failure of undetermined cause in UK Indo-Asians? Q J Med. 2001;94:187–93. doi: 10.1093/qjmed/94.4.187. [DOI] [PubMed] [Google Scholar]
- 31.Lightstone L, Rees AJ, Tomson C, Walls J, Winearls CG, Feehally J. High-incidence of end-stage renal-disease in Indo-Asians in the UK. Q J Med. 1995;88:191–5. [PubMed] [Google Scholar]
- 32.Abbate M, Zoja C, Corna D, et al. Complement-mediated dysfunction of glomerular filtration barrier accelerates progressive renal injury. J Am Soc Nephrol. 2008;19:1158–67. doi: 10.1681/ASN.2007060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostergaard J, Thiel S, Gadjeva M, Hansen TK, Rasch R, Flyvbjerg A. Mannose-binding lectin deficiency attenuates renal changes in a streptozotocin-induced model of type 1 diabetes in mice. Diabetologia. 2007;50:1541–9. doi: 10.1007/s00125-007-0686-0. [DOI] [PubMed] [Google Scholar]
- 34.Sacks S, Zhou WD. New boundaries for complement in renal disease. J Am Soc Nephrol. 2008;19:1865–9. doi: 10.1681/ASN.2007101121. [DOI] [PubMed] [Google Scholar]
- 35.Sheerin NS, Risley P, Abe K, et al. Synthesis of complement protein C3 in the kidney is an important mediator of local tissue injury. FASEB J. 2008;22:1065–72. doi: 10.1096/fj.07-8719com. [DOI] [PubMed] [Google Scholar]
- 36.Tang Z, Bao L, Hatch E, Sacks SH, Sheerin NS. C3a mediates epithelial-to-mesenchymal transition in proteinuric nephropathy. J Am Soc Nephrol. 2009;20:593–603. doi: 10.1681/ASN.2008040434. [DOI] [PMC free article] [PubMed] [Google Scholar]


