Abstract
Hepatic fibrosis is the end-stage consequence of chronic liver disease, affecting many people worldwide. Unlike the anti-fibrotic effect of natural killer (NK) cells, CD8 and NK T subsets are considered as profibrogenic subsets. Padma Hepaten is a multi-compound herbal preparation derived from Tibetan medicine and has proven efficacy in some clinical trials and tests at the cellular level. In this study, we evaluate the immune efficacy of Padma Hepaten administered intraperitoneally (i.p.) and/or orally in a mice model of hepatic fibrosis. Hepatic fibrosis was induced by 6 weeks of biweekly i.p. carbon tetrachloride (CCl4) injections in male C57Bl6 mice. There were four groups, including naive mice, non-treated fibrotic mice and fibrotic mice treated by Padma Hepaten at weeks 5–6 of fibrosis induction either orally or by i.p. injections. Padma Hepaten was prepared at 10 mg/ml in saline and 250 µl (2·5 mg) were administered four times per week. After week 6, animals were killed. To isolate a Padma Hepaten-associated effect on lymphocytes, splenocytes were harvested from either naive or Padma Hepaten-treated non-fibrotic donors. Isolated splenocytes were therefore reconstituted into two groups of irradiated recipients. Recipients were then administered the same CCl4 regimen. Hepatic fibrosis was determined by sirius red staining of liver sections and by assessment of alpha smooth muscle actin expression compared with β-actin (both by mRNA as well as the protein liver extract western blotting). Hepatic fibrosis and alanine aminotransferase serum levels were decreased significantly in both Padma Hepaten-treated groups compared with the non-treated fibrotic group. Padma Hepaten treatment was associated with attenuation of lymphocyte subsets in both treated groups. Using a chemiluminescence technique to assess total anti-oxidant capacities (TAC), it was found that both the plasmas and livers of mice treated by CCl4 had significantly higher TAC compared with controls. However, the levels of TAC in animals treated either by CCl4 alone or CCl4 with Padma Hepaten were similar. Adoptive transfer of Padma Hepaten-treated lymphocytes was associated with fibrosis amelioration compared with recipients with naive lymphocytes. CCl4 generates higher levels of anti-oxidant capacities, probably as a response to oxidative stress. Padma Hepaten administration attenuated hepatic fibrogenesis significantly, accompanied by attenuation of lymphocyte but not anti-oxidant capacities.
Keywords: cirrhosis, hepatic fibrosis, lymphocytes, Padma Hepaten, stellate cell, Tibetan medicine
Introduction
Hepatic fibrosis refers to the accumulation of interstitial or ‘scar’ extracellular matrix after either acute or chronic liver injury. Cirrhosis is the end-stage of progressive fibrosis, characterized by septum formation and rings of scar tissue surrounding hepatocyte nodules [1]. Hepatic fibrosis is a highly integrated cellular response to tissue injury [2], characterized essentially by activation of hepatic stellate cells (HSC), secretion and accumulation of extracellular matrix proteins [3]. The cellular basis of hepatic fibrosis involves the interplay of many factors and cells, including HSC, hepatocytes, Küpffer cells, endothelial cells, platelets and lymphocytes [4]. In liver injury, T helper type 2 (Th2) lymphocytes, characterized by elevated interleukin (IL)-4, appear to promote fibrogenesis relative to Th1 lymphocytes that express high levels of interferon (IFN)-γ[5–13]. Liver lymphocytes interact directly with activated HSC by adhesion [14]. In mice fibrotic models, fibrosis is mediated mainly by direct activation of HSC by CD8 lymphocytes and decreased CD4/CD8 ratio [15]. Natural killer (NK) cells have anti-fibrotic properties in animal models, as they become stimulated to kill activated HSC that have lost major histocompatibility complex class I expression, a self-recognition marker [16,17]. Therefore, immune modulation of lymphocytes has been suggested as an anti-fibrotic strategy via CD8 alleviation and NK support achieved by glatiramer acetate [18] and β-glucosylceramide [19]. β-glucosylceramide administration, however, also decreased liver NK T cells while alleviating fibrosis, suggesting rodent NK T cells as a profibrogenic subset.
Padma Hepaten is a formula derived from traditional Tibetan medicine, which is produced according to international quality guidelines in Switzerland by Padma Inc. In Tibetan it is called the ‘three fruits’ or ‘Bras bu 3 (pronounced Deh-boo 3) and also called myrobalans [20–22]. Padma Hepaten is composed of the fruits of chebulic myrobalan (Terminalia chebula Retz.), amla fruit (Phyllanthus emblica L.) and belleric myrobalan (Terminalia bellerica Roxb.) in the ratio 2:1:1 [20–22]. A similar formula of the same three fruits in an equiproportional mixture is known in ayurvedic medicine as Triphala (also recognized as Padma-Liver, P-Liver or Padma-28) and is derived from traditional Tibetan medicine [23,24]. These formulas were administered to a large number of patients in Europe and have revealed several promising results in atherosclerosis [25] and chronic hepatitis B infection [26]. Padma Hepaten and Triphala have each been shown to provide hepatoprotective and anti-oxidative effects [27–32]. It has been suggested that Padma Hepaten plays a role in modulating immune system functions, e.g. influencing the production and secretion of cytokines [33]. Triphala inhibits immunobiological effects in stimulated human peripheral blood mononuclear cells in vitro. The suppression of neopterin production and tryptophan degradation suggests a specific influence on biochemical pathways induced by Th1-type cytokine IFN-γ[34]. As there is some literature on the ayurvedic Triphala but not on the Tibetan formula ‘Bras bu 3 (Padma Hepaten), we analysed the effect of this formula in a mouse liver fibrosis model. The initial aim of this study was to determine whether fibrosis induction in an animal model is affected by the anti-oxidative capacity of Padma Hepaten administration. The rationale for the study arises because oxidative stress is involved in liver injury progressing to fibrogenesis, and Padma Hepaten has been shown to be a potent anti-oxidant preparation.
Materials and methods
Animals
C57BL/6 male mice received care according to National Institutes of Health guidelines. Mice were purchased and housed in a barrier facility. At the end of each experiment, animals were killed and harvested 3 days after the final dose of CCl4. At the time of killing, mice were weighed and anaesthetized intramuscularly with 0·1 ml of ketamine : xylazine : acepromazine (4:1:1) per 30 g of body weight, and blood samples were collected from the inferior vena cava. Determination of serum alanine aminotransferase (ALT) was performed using an automated enzymatic assay by the Vistros Chemistry Systems 950 (Johnson & Johnson, Rochester, NY, USA). Whole livers were harvested and weighed. Liver samples were harvested from both liver lobes to reduce sampling variability among experimental and control mice.
Fibrosis models
The carbon tetrachloride (CCl4; Sigma, C-5331, St Louis, MO, USA) fibrosis model was diluted 1:9 in corn oil and induced by intraperitoneal (i.p.) injections at 5 µl pure CCl4/g body weight twice each week for 6 weeks.
Treatments
Padma Hepaten was obtained from Padma Inc. (Schwerzenbach, Switzerland) as a powder and prepared to 10 mg/ml in saline; 250 µl (2·5 mg) was administered four times/week. Oral gavage and i.p. Padma Hepaten were administered at weeks 5–6 of fibrosis induction. Doses of Padma Hepaten were selected according to previously reported experience of Triphala20 [21,22,27–30].
Total-body irradiation model
To isolate the effect of Padma Hepaten on lymphocytes, splenocytes were harvested from either naive or Padma Hepaten-treated non-fibrotic donors. Isolated splenocytes were therefore reconstituted into two groups of irradiated recipients (eight rodents each). Cells (1 × 106) were reconstituted by i.p. injections once a week for 2 weeks. Recipients were then administered the same CCl4 regimen for 6 weeks without direct administration of Padma Hepaten. Sublethal irradiation was achieved with a single total-body dose of 700 cGy from a dual Cs-source (dose rate of 62 cGy/min) before induction of fibrosis by CCl4. Sublethal doses were used to suppress lymphocyte proliferation while maintaining the animal in an apparently healthy state [35]. The experiment was repeated three times.
The ALT measurements
To measure the serum ALT levels, commercial kits were used with an automatic analyser.
Tissue RNA extraction and real-time polymerase chain reaction analysis
Total cellular RNA was extracted from target tissues using Trizol reagent® (Gibco-BRL, Life Technologies, Rockville, MD, USA), followed by DNase-I digestion. Purified RNA was then used as a template for reverse transcription into single-strand cDNA using the reverse transcription system (Promega Corporation, Madison, WI, USA). Synthesis of cDNA was performed using 2 µg of total RNA per sample with random primers and reagents contained in the reverse transcription system kit, according to the manufacturer's protocol (Promega Corporation). The reverse transcriptase product was diluted 20× in nuclease-free H2O and 5 µl of each sample was loaded into 96-well plates for real-time polymerase chain reaction (PCR) in an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). β-actin served as an internal control and H2O served as a negative control. Amplification reactions included oligonucleotide primers for each target gene and for β-actin as well as platinum Taq polymerase and SYBR Green DNA-binding dye. Fluorescence signals were analysed during each of 40 cycles (denaturation 15 s at 95°C, annealing 15 s at 56°C and extension 40 s at 72°C). Denaturation curves of target genes and β-actin, performed at the end of the PCR, and detection of the PCR products by agarose gel electrophoresis confirmed the homogeneity of the DNA products. Relative quantification was calculated using the comparative threshold cycle (CT) method (as described in the User Bulletin #2, ABI PRISM 7700 Sequence Detection System, Foster City, CA, USA). CT indicates the fractional cycle number at which the amount of amplified target genes reaches a fixed threshold within the linear phase of gene amplification, and it is related inversely to the abundance of mRNA transcripts in the initial sample. Mean CT of duplicate measurements was used to calculate ΔCT as the difference in CT for target and reference. ΔCT for each sample was compared with the corresponding control CT and expressed as ΔCT. Relative quantity of product was expressed as fold-induction or repression of the target gene compared with the control primers, according to the formula 2−ΔCT. The sequence of mouse β-actin (as a housekeeping gene) was as follows – forward: 5′-GAT GAG ATT GGC ATG GCT TT-3′, reverse: 5′-AGA GAA GTG GGG TGG CTT TT-3′; and α-smooth muscle actin (α-SMA) – forward: 5′-TCC TCC CTG GAG AAG AGC TAC-3′, reverse: 5′-TAT AGG TGG TTT CGT GGA TGC-3′[15,16,18,19].
Histological assessment of liver injury
The posterior one-third of the liver was fixed in 10% formalin for 24 h and then paraffin-embedded in an automated tissue processor. Seven-millimetre sections were cut from the livers of each animal. Sections (15 µm) were stained in 0·1% sirius red F3B in saturated picric acid (both from Sigma).
Fibrosis quantitation
Relative fibrosis area (expressed as percentage of the total liver area) was assessed based on 36 fields from nine sirius red-stained liver sections/animal. Each field was acquired at 10× magnification and then analysed using a computerized Bioquant® morphometry system (R & M Biometrics, Nashville, TN, USA). To evaluate the relative fibrosis area, the measured collagen area was divided by the net field area and then multiplied by 100. Subtraction of the vascular luminal area from the total field area yields the final calculation of the net fibrosis area [15,16,18,19].
The α-SMA immunoblot
Immunoblot analysis of α-SMA in liver extracts was performed with modifications as described previously [14–16,18,19]. Whole liver or HSC protein extracts were prepared in liver homogenization buffer [50 mmol/l Tris-HCl (pH 7·6), 0·25% Triton-X 100, 0·15 M NaCl, 10 mM CaCl2 and complete mini-ethylenediamine tetraacetic acid free protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany)]. Next, proteins (30 µg per lane) were resolved on a 10% (wt/vol) sodium dodecyl sulphate-polyacrylamide gel (Novex, Groningen, the Netherlands) under reducing conditions. For immunoblotting, proteins were transferred to a Protran membrane (Schleicher & Schuell, Dassel, Germany). Blots were incubated overnight at 4°C in a blocking buffer containing 5% skimmed milk and then incubated with either anti-α-SMA (Dako; cat.# M0851, Carpinteria, CA, USA) or β-actin (Sigma) mouse monoclonal antibody, diluted 1/2000 for 2 h at room temperature, and subsequently with peroxidase-conjugated goat anti-mouse immunoglobulin G (P.A.R.I.S., Compiègne, France), diluted 1/10 000 for 1 h at room temperature. Immunoreactivity was revealed by enhanced chemiluminescence using an ECL kit (Amersham Pharmacia Biotech, Les Ulis, France).
Protein determination
The Bradford method [36] modified for the Micro Titer Bio-Rad Assay (BioRad, Hercules, CA, USA) was used to quantify the amount of protein present in the cell extracts. A standard curve was generated utilizing bovine serum albumin (BSA). Absorbance was read using a Bio-tek PowerWave 340 microplate scanning spectrophotometer (Bio-tek Instruments, Winooski, VT, USA) at a wavelength of 595 nm.
Measurements of anti-oxidant capacities by a chemiluminescence-inducing cocktail [37,38]
Luminol-dependent chemiluminescence (LDCL) induced by the glucose oxidase (GO) cocktail was employed to measure the total anti-oxidant capacity of the liver extracts (100 µg protein) and plasma samples (10 µl). This cocktail is comprised of a combination of luminol (10 µM), GO from Aspergillus niger (1·46 units) (H2O2 generator), sodium selenite (IV) (2 mM) and Cobalt (II) (10 µM) in a final volume of 1 ml of Hanks' balanced salt solution. This cocktail generates a very rapid and steep peak of LDCL. Liver extracts at 100 µg protein (calculated following protein analysis) and 10 µl of plasma were mixed with the cocktail, vortexed briefly and the degree of light quenching was monitored for several minutes. Light generation, which persisted for about 10 min at a high steady level, was measured in a LUMAC type 2500 luminometer attached to a PC computer (Landgraaf, the Netherlands).
Cell isolation, staining and flow cytometric analysis
Intrahepatic lymphocytes were isolated by perfusion of the liver with digestion buffer [3 ml medium (in 1 min) containing collagenase (2 mg/10 ml) and DNAse I (0·2 mg/10 ml) at 37°C]. After perfusion, the liver was homogenized with an additional 10 ml of digestion buffer and completed to 40 ml by RPMI-1640 + 5% fetal bovine serum, then incubated under constant shaking (Hot Shaker, 1 cycle/s) at 37°C for 30 min. The digested liver cell suspension was centrifuged at 30 g for 3 min at 4°C to remove hepatocytes and cell clumps. The supernatant was then centrifuged to obtain a pellet of cells free of hepatocytes, to a final volume of 1 ml. Lymphocytes were isolated from this cell suspension using 24% metrizamide gradient separation. Cells were counted and adjusted to 2 × 107/ml in staining buffer (in saline containing 1% BSA). A total of 50 µl of the cell suspension was incubated with antibodies on ice for 30 min, washed with staining buffer and fixed with 2% paraformaldehyde. The cells were then analysed by fluorescence-activated cell sorter (FACScalibur; Becton Dickinson, Immunofluorometry Systems, Mountain View, CA, USA). Cells were passed at a rate of about 1000/s, using saline as the sheath fluid. A 488 nm argon laser beam was used for excitation. Antibodies used for staining were mouse anti-CD4, CD8, CD3, CD45 and pan-NK antibodies, conjugated by fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll and allophycocyanin respectively. For every antibody assay, unstained cells and anti-mouse immunoglobulin G-negative controls were used. Instrument calibration and settings were performed using CaliBRITE™-3 beads (Becton Dickinson). Antibodies were purchased from BD Biosciences, Transduction Laboratories. FACS data were analysed using CellQuest software (BD Biosciences, San Jose, CA, USA) [14–16,18,19].
Statistical methods
Results are presented as mean ± standard deviation. Student's t-test and anova were used for statistically significant correlations.
Results
Hepatic fibrosis was ameliorated by Padma Hepaten administration
Hepatic fibrosis was ameliorated significantly by both oral and i.p. Padma Hepaten administration (Figs 1–4). Sirius red staining showed no fibrotic septa in naive mice (Fig. 1a) that became prominent following fibrosis induction (Fig. 1b). Both oral and i.p. Padma Hepaten administration decreased the hepatic fibrosis (Fig. 1c and d respectively). Bioquant® assessment of sirius red-stained slides revealed the same pattern (Fig. 1e) and presented as relative fibrosis area, increasing from 0·42 ± 0·05% in naive mice to 8·4 ± 0·9 following fibrosis induction. Oral and i.p. Padma Hepaten administration decreased the relative fibrosis area significantly to 4·1 ± 1·1 and 3 ± 1 respectively (P < 0·001).
Fig. 1.
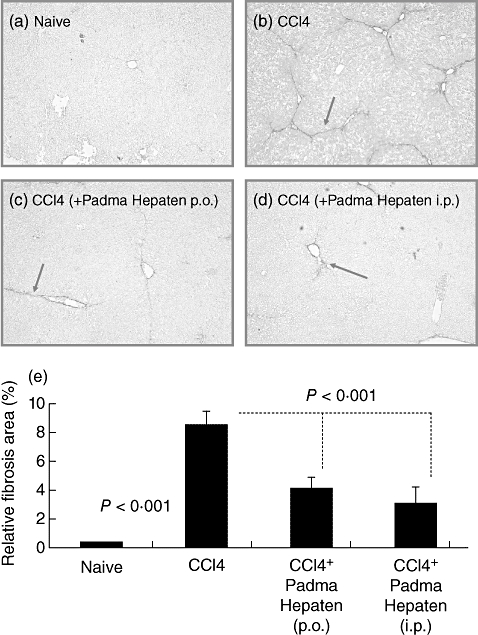
Hepatic fibrosis was ameliorated by Padma Hepaten administration: following fibrosis induction for 6 weeks, tissue sections were stained with sirius red, as described in Materials and methods. Representative tissue sections from naive and fibrotic animals are shown (a,b respectively). Fibrotic septa that are well established in fibrotic animals are highlighted by arrows (magnification 10×). Oral and intraperitoneal (i.p.) Padma Hepaten administration during the final 2 of 6 CCl4 weeks decreased the presence of red fibrotic septa (c,d respectively). Relative fibrosis area (e) in the livers of Padma Hepaten-treated animals was lower than that seen in non-Padma Hepaten-treated fibrotic mice. Results are presented as the mean relative area of all animal groups ± standard deviation. The findings are representative of at least three different experiments, with the same number of eight animals in each subgroup.
Fig. 4.
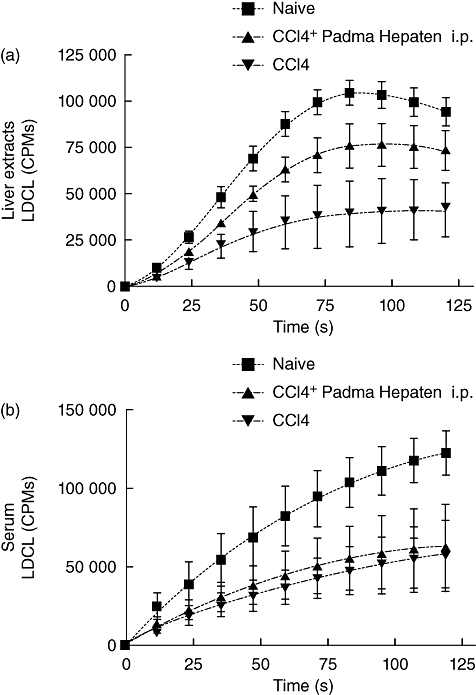
Padma Hepaten anti-fibrotic effect was not due to an anti-oxidative property: (a) a luminol-dependent chemiluminescence (LDCL) induced by the glucose oxidase (GO) cocktail, using liver extracts (100 µg protein) obtained from untreated animals ( ), animals treated intra-peritoneally with CCl4 (
), animals treated intra-peritoneally with CCl4 ( ) and from animals treated with a combination of CCl4 and Padma Hepaten extract (
) and from animals treated with a combination of CCl4 and Padma Hepaten extract ( ). The intensity of light, generated by the GO cocktail alone (not shown) was at a constant level of 250 000 counts per million. Data represent mean ± standard error (s.e.) from six animals per group. (b) LDCL induced by the GO cocktail, using 10 µl amounts of plasma obtained from untreated animals (
). The intensity of light, generated by the GO cocktail alone (not shown) was at a constant level of 250 000 counts per million. Data represent mean ± standard error (s.e.) from six animals per group. (b) LDCL induced by the GO cocktail, using 10 µl amounts of plasma obtained from untreated animals ( ), animals treated intra-peritoneally with CCl4 (
), animals treated intra-peritoneally with CCl4 ( ) and from animals treated with a combination of CCl4 and Padma Hepaten extract (
) and from animals treated with a combination of CCl4 and Padma Hepaten extract ( ). The intensity of light, generated by the GO cocktail alone (not shown) was at a constant level of 250 000 counts per million. Data represent mean ± s.e. from six animals per group.
). The intensity of light, generated by the GO cocktail alone (not shown) was at a constant level of 250 000 counts per million. Data represent mean ± s.e. from six animals per group.
Hepatic fibrosis was then determined by western blotting of α-SMA in protein liver extracts (Fig. 2a, lower panel). All animals were included, but only representative samples are shown. Both oral and i.p. Padma Hepaten-treated groups showed decreased hepatic fibrosis compared with the non-treated fibrotic group. Because of the variability of β-actin, we standardized results by calculating the ratio of α-SMA to β-actin following the band measurement densities. Our results support further the decrease of α-SMA expression in both Padma Hepaten-treated groups (Fig. 2a, upper panel). The α-SMA/β-actin ratio increased significantly (P < 0·0001) from 0·16 ± 0·02 in naive mice to 4·3 ± 1·9 following fibrosis induction. Oral and i.p. Padma Hepaten administration decreased significantly the α-SMA/β-actin ratio to 1·3 ± 0·4 and 1·2 ± 0·2 respectively (P < 0·001).
Fig. 2.
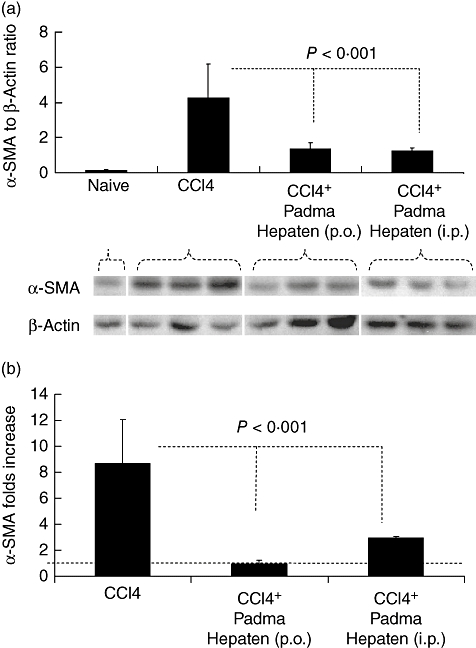
Whole liver protein lysates were analysed by western blotting for alpha smooth muscle actin (α-SMA) and β-actin (a, lower panel). Increased α-SMA expression (upper lane) was found in lysates of fibrotic mice receiving CCl4, compared with naive animals. To obtain standardization of β-actin/α-SMA expression of all wells tested, bands were scanned and quantified as described in Methods. Results were expressed as a ratio (a, upper panel). Real-time polymerase chain reaction was performed to quantify mRNA expression for α-SMA and β-actin, as described in Materials and methods. There was reduced expression of fibrogenic mRNAs in Padma Hepaten-treated mice compared with non-Padma Hepaten-treated fibrotic rodents (b). The threshold at value 1 representing naive controls is illustrated by a horizontal line. Data depicted represent triplicate values, with similar results obtained in two separate experiments. Results are presented as the mean relative area of all animal groups ± standard deviation. The findings are representative of at least three different experiments, with the same number of eight animals in each subgroup.
The assessment of α-SMA mRNA expression compared with β-actin also confirmed this pattern (Fig. 2b). Compared with naive mice, the α-SMA mRNA expression increased to 8·6 ± 3·4-fold following fibrosis induction. Oral and i.p. Padma Hepaten administration decreased α-SMA mRNA expression significantly to 1 ± 0·3 (P = 0·002) and 2·9 ± 0·1 (P = 0·4) respectively.
Padma Hepaten also improved liver injury but was not due to an anti-oxidative effect
Serum-ALT levels in the naive wild type were 17·3 ± 14·4 U/l, which was raised significantly (P = 0·003) to 70 ± 33·4 following fibrosis induction by CCl4. Both oral and i.p. administration of Padma Hepaten decreased ALT serum levels significantly to 32·3 ± 16·6 (P = 0·02) and 19 ± 5·8 U/l (P = 0·002) respectively (Fig. 3). Both treated groups had almost similar levels to the non-treated fibrotic group (P = not significant). Compared with oral Padma Hepaten administration, however, i.p. Padma Hepaten administration revealed lower levels (P = 0·05). ALT and fibrosis results suggested oxidative stress activity; therefore, anti-oxidant capacity was assessed using the LDCL (as described in Methods). The intensity of light readings is presented by mean values and standard error (Fig. 4). The intensity of luminescence correlated linearly with the generation of radicals, and anti-oxidants therefore resulted in a decline of radicals and decreased intensity of luminescence [30,31]. The intensity of light, generated by the GO cocktail alone (not shown), was at a constant level of 250 000 counts per minute (cpm). Compared with naive animals (Fig. 4a, upper curve), liver extracts obtained from CCl4 mice increased significantly in the total anti-oxidant capacities as measured by the GO cocktail (Fig. 4a, lower two curves). Although a slight increase in LDCL was observed in liver extracts derived from animals treated i.p. with Padma Hepaten, this was not statistically significant.
Fig. 3.
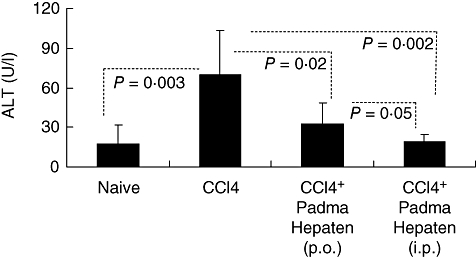
Padma Hepaten improved liver injury: serum alanine aminotransferase (ALT) levels followed the same pattern seen in hepatic fibrosis, as Padma Hepaten-treated mice showed a significant decline. Results are presented as the mean value (U/l) of each animal group ± standard deviation.
Figure 6b shows the LDCL inhibition patterns induced by serum from the three groups of fibrotic animals. As can be seen, compared with controls (Fig. 4b, upper curve) sera from animals treated with either CCl4 or CCl4–Padma Hepaten combinations (Fig. 4b, lower curve) achieved a significantly higher inhibitory effect on LDCL (higher anti-oxidant capacities). However, the total anti-oxidant capacities of both CCl4 and CCl4–Padma Hepaten treatments were similar. No significant changes in the anti-oxidant capacities of liver extracts as well as serum samples from animals treated by oral or i.p. Padma Hepaten were observed (data not shown).
Fig. 6.
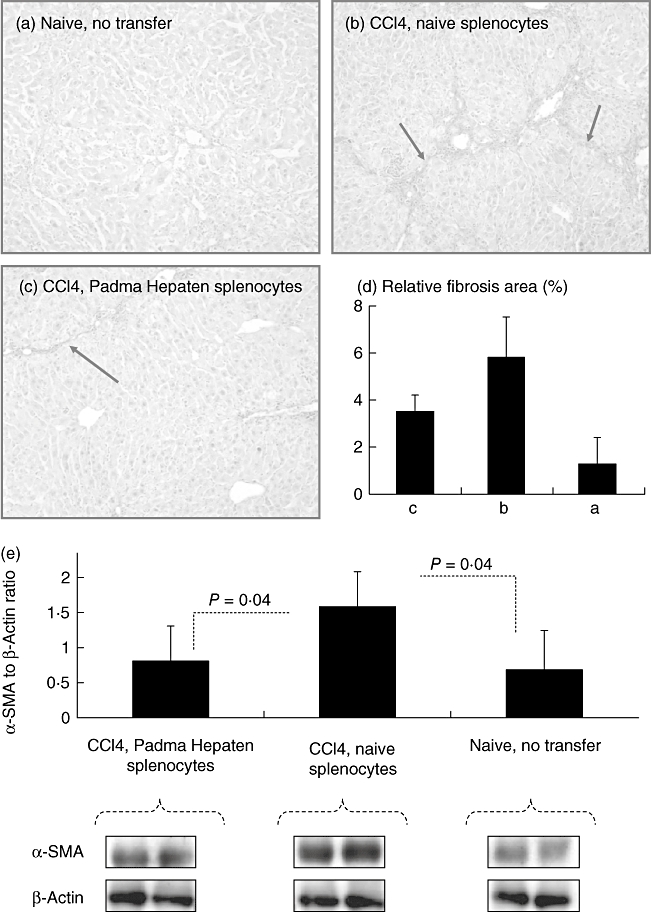
Adoptive transfer of naive versus Padma Hepaten-treated lymphocytes: to isolate the net effect of Padma Hepaten on lymphocytes in the liver fibrogenesis we used the adoptive transfer model, as explained in Methods. Following fibrosis induction for 6 weeks, tissue sections were stained with sirius red, as described in Materials and methods. Representative tissue sections from naive and fibrotic animals are shown (a,b,c). (d) The relative fibrosis area of the three groups; compared with naive animals, increased fibrosis was found in fibrotic mice receiving naive splenocytes. Fibrosis was reduced significantly while receiving Padma Hepaten-treated splenocytes. Protein expression of α-smooth muscle actin (α-SMA) and β-actin was assessed by western blotting (e, lower panel). The β-actin/α-SMA ratio (e, upper panel) shows a similar pattern. The findings are representative of two different experiments with the same number of six animals in each subgroup.
Padma Hepaten anti-fibrotic effect was associated with lymphocyte alterations
The FACS analysis of intrahepatic lymphocytes is illustrated in Fig. 5a and presented as a percentage of total CD45+ cells as a panleukocyte marker. CD4 T cells were 13·3 ± 1·5% in naive mice and decreased significantly to 11·3 ± 1·3 (P = 0·01) following fibrosis induction, 11·2 ± 2·1 (P = 0·02) in the oral Padma Hepaten-treated group and 8 ± 1·4 (P < 0·001) in the i.p. Padma Hepaten-treated animals. While oral Padma Hepaten did not affect the CD4 count, the i.p. administration decreased it further (P = 0·004). The CD8 T cells, however, increased following fibrosis induction from 10·7 ± 1·5% to 13·5 ± 2·4% of the CD45+ cells (P = 0·02). While oral Padma Hepaten treatment decreased CD8 content significantly to 11 ± 3% (P = 0·05), i.p. administration did not. NK T cells increased significantly from 1·8 ± 0·6% to 4·5 ± 2·2% of CD45+ cells (P < 0·0001) following fibrosis induction. Padma Hepaten treatment also depressed the NK T cells significantly in both treated groups to 2·9 ± 0·8% (P = 0·0003) and 2·5 ± 0·5% (P = 0·0002) in the oral and i.p. Padma Hepaten therapy respectively. NK cells followed the same pattern seen with NK T cells; they increased significantly following fibrosis induction from 8·5 ± 1·2% of CD45+ cells to 20 ± 2·2% (P < 0·0001). Oral and i.p. Padma Hepaten treatment attenuated the NK content significantly to 15·8 ± 3·4% (P = 0·003) and 12 ± 2·3% (P < 0·001) respectively. The same NK and NK T pattern was also seen in the calculated total number of liver lymphocyte subsets (Fig. 5b). However, calculated total numbers also show a significant attenuation of CD4 and CD8 subsets following Padma Hepaten treatment. Reduction in the profibrotic CD8 cells suggests an additional explanation for the anti-fibrotic response.
Fig. 5.
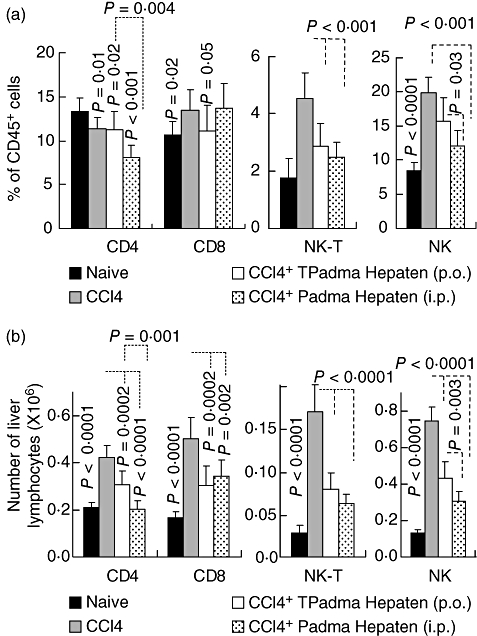
Padma Hepaten anti-fibrotic effect was associated with lymphocyte alterations: using fluorescence-activated cell sorting (FACS), intrahepatic lymphocytes were isolated and their composition analysed to define patterns that correlated with reduced fibrosis. Results from FACS analysis of intrahepatic lymphocytes are presented in (a) as a percentage of CD45+ cells (a panleukocyte marker) and in (b) as number of liver lymphocytes. The natural killer (NK) and NK T attenuation were the most prominent findings in the Padma Hepaten-treated mice in (a). However, in examining the total number of liver lymphocytes we found attenuation of all lymphocytes. The findings are representative of at least three different experiments with the same number of eight animals in each subgroup.
Adoptive transfer of naive versus Padma Hepaten-treated lymphocytes
To isolate the net effect of Padma Hepaten on lymphocytes in liver fibrogenesis, we used the adoptive transfer model, as mentioned previously in Materials and methods. Hepatic fibrosis was ameliorated significantly among fibrotic recipients that were reconstituted by i.p. administration of splenocytes from Padma Hepaten-treated donors compared with the non-Padma Hepaten-treated splenocytes (Fig. 6a–d). Sirius red staining showed no fibrotic septa in naive mice (Fig. 6a) that became prominent following fibrosis induction in the case of non-Padma Hepaten transfer of splenocytes (Fig. 6b). The administration of Padma Hepaten-treated splenocytes, however, decreased hepatic fibrosis (Fig. 6c). The relative fibrosis area of the sirius red staining showed a similar pattern (Fig. 6d). Hepatic fibrosis was then determined in all animals by western blotting of α-SMA in protein liver extracts, and representative samples are shown (Fig. 6e, lower panel). It also shows decreased α-SMA bands in the fibrotic recipients that were reconstituted by i.p. administration of splenocyte from Padma Hepaten-treated donors compared with the non-Padma Hepaten-treated splenocytes. β-actin expression, on the other hand, was almost similar in all groups.
Discussion
Padma Hepaten is a traditional Tibetan medicine herbal preparation, comprised of three myrobalan fruits: chebulic myrobalan (Terminalia chebula Retz.), amla fruit (Phyllanthus emblica L.) and belleric myrobalan (Terminalia bellerica Roxb.) [20–22]. Padma Hepaten and Triphala have a variety of beneficial effects on inflammatory and immune processes. Aqueous extracts derived from this herbal preparation were found to inhibit chemotaxis markedly [39], to possess anti-oxidant and anti-proteinase activities [40,41] and to inhibit inducible nitric oxide synthesis in a macrophage cell line [42]. Also, aqueous and ethanolic extracts of those multi-component formulas exhibited anti-microbial properties for skin infections [43]. Furthermore, it was shown to inhibit oxidative burst in human neutrophils, neutrophil elastase and the peroxidation of lipids as well as killing epithelial and endothelial cells in cultures treated by oxidants [44]. Padma Hepaten was also found to decrease the oxidative burst responses of monocytes and to improve fibrinolysis in patients with intermittent claudication [23], and the potentials of the electroretinogram in lipid metabolism and vascular changes [45]. Aqueous extract of Padma Hepaten decreased production of inflammatory cytokines IL-1β, IL-6, IL-8 and tumour necrosis factor-α strongly and, more moderately, also decreased the anti-inflammatory cytokine IL-10 induced by lipopolysaccharide [46].
Hepatic fibrosis, on the other hand, is also mediated by the immune system [14–19] and the relevance of Padma Hepaten effects could be beneficial. Interestingly, Triphala blocks cellular responses to basic fibroblast growth factor (FGF) and insulin-like growth factor 1 (IGF-1) [47]. These results are encouraging in the presence of the protective effect of IGF-1 on experimental liver cirrhosis [48], and the presence of an agonist role for FGF in specifically insult-induced liver matrix deposition and hepatic fibrogenesis [49]. The aim of the present study was to determine the modulating capacity of Padma Hepaten on an in vivo model of hepatic fibrosis.
Our results suggest strongly that Padma Hepaten administration decreased significantly the severity of hepatic fibrogenesis and ALT serum levels (Figs 1–3) generated by CCl4. Based on our earlier experience [18,15], the use of Padma Hepaten administration during the last two of six CCl4 induction weeks in our model represents an anti-fibrotic effect, rather than prevention of fibrogenesis.
Many mechanistic pathways could explain the Padma Hepaten anti-fibrotic outcome, including anti-oxidation, the involvement of FGF or IGF, cytokine alterations and lymphocyte interactions. In our animal model, the Padma Hepaten anti-fibrotic property was not due to an anti-oxidative mechanism, as no differences were found between Padma Hepaten- and non-Padma Hepaten-treated fibrotic mice (Fig. 4a and b). The data presented show that both liver and serum samples collected from all CCl4-treated animals had higher anti-oxidant capacities as measured by the luminescence cocktail. However, unlike the beneficial effect on the development of hepatic fibrosis, Padma Hepaten failed to change the anti-oxidant capacities induced by CCl4 treatment in both plasma and liver extracts. This raises the question of whether, in this system, Padma Hepaten acts as an anti-oxidant. This might be explained by the accepted assumption that there is a regulatory mechanism in mammalian cells which does not permit drastic alteration in total anti-oxidant capacity. This assumption is supported by recent studies showing that, although supplementation of anti-oxidants to various cell lines in culture showed increased uptake of the selected anti-oxidants, the total anti-oxidant capacity of the cell interior remained unaltered. Therefore, the inability of Padma Hepaten to modify the anti-oxidant capacity of liver extract and plasma is in full agreement with our previous findings [50].
A possible explanation for the anti-fibrotic effect of the Tibetan formula is the significant alteration of lymphocyte subsets (Fig. 5). Prominent attenuation of the CD8 and NK T cells can explain the Padma Hepaten-induced anti-fibrotic effect, as they were suggested to be profibrotic lymphocytes [15,19]. NK cells, nevertheless, were suggested as an anti-fibrotic subset and their increase in the fibrosis model of CCl4 was explained as a failed anti-fibrotic attempt that does not cope with the high fibrogenic stress of CCl4. Therefore, from the current data, NK attenuation by Padma Hepaten is not expected with the anti-fibrotic result achieved, suggesting that a balanced effect of both NK and NKT subsets is in favour of NK T predominance. Results from the adoptive transfer model of lymphocytes of treated and untreated fibrotic animals further support the hypothesis of a lymphocyte-related anti-fibrotic effect in this model (Fig. 6).
Padma Hepaten has been certified for over-the-counter distribution, or as a food additive, in several European countries as well as in the United States (Padma Basic Inc.). Extracts from Terminalia bellirica Roxb. (one of the plants which constitute Padma Hepaten) and its component gallic acid (a known polyphenol anti-oxidant and a major constitute of this plant) were shown to ameliorate acute liver toxicity of CCl4 in rats [51], and it was proposed that gallic acid stabilizes membranes and may combine directly with free radicals, which leads to their inactivation by suppressing the intracellular concentration of free radicals.
Because Padma Hepaten is a herbal preparation composed of a large variety of biologically active substances, it is assumed that exerting its anti-fibrotic effect in our current study acts as a multi-drug agent [52]. Our data extend our understanding of the homeostasis effect of lymphocyte alterations in hepatic fibrosis, opening a new therapeutic opportunity with safe natural compounds.
Acknowledgments
This work was supported by grants from ‘Ezvonot’ and ‘Bi National Scientific American Israeli Foundation’.
References
- 1.Friedman SL. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 3.Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–72. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 4.Van Waes L, Lieber CS. Early perivenular sclerosis in alcoholic fatty liver: an index of progressive liver injury. Gastroenterology. 1977;73:646–50. [PubMed] [Google Scholar]
- 5.Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84:1780–5. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang SC, Ohata M, Schrum L, Rippe RA, Tsukamoto H. Expression of interleukin-10 by in vitro and in vivo activated hepatic stellate cells. J Biol Chem. 1998;273:302–8. doi: 10.1074/jbc.273.1.302. [DOI] [PubMed] [Google Scholar]
- 7.Winwood PJ, Schuppan D, Iredale JP, Kawser CA, Docherty AJ, Arthur MJ. Kupffer cell-derived 95-kd type IV collagenase/gelatinase B: characterization and expression in cultured cells. Hepatology. 1995;22:304–15. [PubMed] [Google Scholar]
- 8.Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–71. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T-helper cytokine responses. Proc Natl Acad Sci USA. 1997;94:10663–8. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka M, Pham NT, Tsukamoto H. Differential effects of interleukin-1 alpha, tumor necrosis factor alpha, and transforming growth factor beta 1 on cell proliferation and collagen formation by cultured fat-storing cells. Liver. 1989;9:71–8. doi: 10.1111/j.1600-0676.1989.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990;85:1833–43. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czaja MJ, Weiner FR, Flanders KC, et al. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989;108:2477–82. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czaja MJ, Weiner FR, Takahashi S, et al. Gamma-interferon treatment inhibits collagen deposition in murine schistosomiasis. Hepatology. 1989;10:795–800. doi: 10.1002/hep.1840100508. [DOI] [PubMed] [Google Scholar]
- 14.Muhanna N, Horani A, Doron S, Safadi R. Lymphocyte–hepatic stellate cell proximity suggests a direct interaction. Clin Exp Immunol. 2007;148:338–47. doi: 10.1111/j.1365-2249.2007.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safadi R, Ohta M, Alvarez CE, et al. Immune stimulation of hepatic fibrogenesis by CD8 lymphocytes and its attenuation by transgenic interleukin 10 from hepatocytes. Gastroenterology. 2004;127:870–82. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 16.Melhem A, Muhanna N, Bishara A, et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–52. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 18.Horani A, Muhanna N, Pappo O, et al. The beneficial effect of glatiramer acetate (Copaxone®) on immune modulation of experimental hepatic fibrosis. Am J Physiol Gastr Liver Physiol. 292:G628–38. doi: 10.1152/ajpgi.00137.2006. [DOI] [PubMed] [Google Scholar]
- 19.Safadi R, Zigmond E, Pappo O, Shalev Z, Ilan Y. Amelioration of hepatic fibrosis via beta-glucoslyceramide mediated immune modulation is associated with altered CD8 and NKT lymphocyte distribution. Int Immunol. 2007;19:1021–9. doi: 10.1093/intimm/dxm069. [DOI] [PubMed] [Google Scholar]
- 20.Tasduq SA, Singh K, Satti NK, Gupta DK, Suri KA, Johri RK. Terminalia chebula (fruit) prevents liver toxicity caused by sub-chronic administration of rifampicin, isoniazid and pyrazinamide in combination. Hum Exp Toxicol. 2006;25:111–18. doi: 10.1191/0960327106ht601oa. [DOI] [PubMed] [Google Scholar]
- 21.Tasduq SA, Mondhe DM, Gupta DK, Baleshwar M, Johri RK. Reversal of fibrogenic events in liver by Emblica officinalis (fruit), an Indian natural drug. Biol Pharm Bull. 2005;28:1304–6. doi: 10.1248/bpb.28.1304. [DOI] [PubMed] [Google Scholar]
- 22.Naik GH, Priyadarsini KI, Bhagirathi RG, et al. In vitro antioxidant studies and free radical reactions of Triphala, an ayurvedic formulation and its constituents. Phytother Res. 2005;19:582–6. doi: 10.1002/ptr.1515. [DOI] [PubMed] [Google Scholar]
- 23.Brunner-La Rocca HP, Schindler R, Schlumpf M, Saller R, Suter M. Effects of the Tibetan herbal preparation PADMA 28 on blood lipids and lipid oxidisability in subjects with mild hypercholesterolaemia. Vasa. 2005;34:11–17. doi: 10.1024/0301-1526.34.1.11. [DOI] [PubMed] [Google Scholar]
- 24.Drabaek H, Mehlsen J, Himmelstrup H, Winther K. A botanical compound, PADMA-28, increases walking distance in stable intermittent claudication. Angiology. 1993;44:863–7. doi: 10.1177/000331979304401103. [DOI] [PubMed] [Google Scholar]
- 25.Gieldanowski J, Dutkiewicz T, Samochowiec L, Wojcicki J. PADMA-28 modifies immunological functions in experimental atherosclerosis in rabbits. Arch Immunol Ther Exp (Warsz) 1992;40:291–5. [PubMed] [Google Scholar]
- 26.Gladysz A, Juszczyk J, Brzosko WJ. Influence of PADMA 28 on patients with chronic active hepatitis B. Phytother Res. 1993;7:244–7. [Google Scholar]
- 27.Sabina EP, Rasool M. An in vivo and in vitro potential of Indian ayurvedic herbal formulation Triphala on experimental gouty arthritis in mice. Cascul Pharmacol. 2008;48:14–20. doi: 10.1016/j.vph.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Sandhya T, Lathika KM, Pandey BN, Mishra KP. Potential of traditional ayurvedic formulation, Triphala, as a novel anticancer drug. Cancer Lett. 2006;231:206–14. doi: 10.1016/j.canlet.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 29.Srikumar R, Jeya Parthasarathy N, Sheela Devi R. Immunomodulatory activity of Triphala on neutrophil functions. Biol Pharm Bull. 2005;28:1398–403. doi: 10.1248/bpb.28.1398. [DOI] [PubMed] [Google Scholar]
- 30.Melzer J, Brignoli R, Diehm C, Reichling J, Do DD, Saller R. Treating intermittent claudication with Tibetan medicine Padma 28: does it work? Atherosclerosis. 2006;189:39–46. doi: 10.1016/j.atherosclerosis.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 31.Saller R, Kristof O, Reichling J. Padma 28 – ein traditionelles und modernes Phytotherapeutikum [Padma 28 – a traditional and modern phytotherapeutic] Schweiz Zeitschr Ganzheitsmed. 1999;11:28–37. [Google Scholar]
- 32.Suter M, Richter C. Anti- and pro-oxidative properties of PADMA 28, a Tibetan herbal formulation. Redox Rep. 2000;5:17–22. doi: 10.1179/rer.2000.5.1.17. [DOI] [PubMed] [Google Scholar]
- 33.Ginsburg I, Sadovnik M, Sallon S, Milo-Goldzweig I, Mechoulam R, Breuer A. PADMA-28, a traditional Tibetan herbal preparation inhibits the respiratory burst in human neutrophils, the killing of epithelial cells by mixtures of oxidants and pro-inflammatory agonists and peroxidation of lipids. Inflammapharmacology. 1999;7:47–62. doi: 10.1007/s10787-999-0025-9. [DOI] [PubMed] [Google Scholar]
- 34.Neurauter G, Wirleitner B, Schroecksnadel K, Schennach H, Ueberall F, Fuchs D. PADMA 28 modulates interferon-gamma-induced tryptophan degradation and neopterin production in human PBMC in vitro. Int Immunopharmacol. 2004;4:833–9. doi: 10.1016/j.intimp.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Miranda SR, Erlich S, Visser JW, et al. Bone marrow transplantation in acid sphingomyelinase-deficient mice: engraftment and cell migration into the brain as a function of radiation, age, and phenotype. Blood. 1997;90:444–52. [PubMed] [Google Scholar]
- 36.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 37.Ginsburg I, Sadovnic M, Oron M, Kohen R. Novel chemiluminescence-inducing cocktails, part I: the role in light emission of combinations of luminal with SIN-1, selenite, albumin, glucose oxidase and Co2+ Inflammopharmacology. 2004;12:289–303. doi: 10.1163/1568560043696263. [DOI] [PubMed] [Google Scholar]
- 38.Ginsburg I, Sadovnic M, Oron M, Kohen R. Novel chemiluminescence-inducing cocktails, part II: measurement of the anti-oxidant capacity of vitamins, thiols, body fluids, alcoholic beverages and edible oils. Inflammopharmacology. 2004;12:305–20. doi: 10.1163/1568560043696218. [DOI] [PubMed] [Google Scholar]
- 39.Matzner Y, Sallon S. The effect of PADMA 28, a traditional Tibetan herbal preparation, on human neutrophil function. J Clin Lab Immunol. 1995;46:13–23. [PubMed] [Google Scholar]
- 40.Suter M, Richter C. Anti- and pro- oxidative properties of PADMA 28, a Tibetan herbal formulation. Redox Rep. 2000;5:17–22. doi: 10.1179/rer.2000.5.1.17. [DOI] [PubMed] [Google Scholar]
- 41.Stämpfli S, Schwabli B. The antioxidative and anti-inflammatory properties of PADMA 28. Schweiz Zschr Ganzheits Medizin. 2001;13:242. [Google Scholar]
- 42.Moeslinger T, Friedl R, Volf I, Brunner M, Koller E, Spieckermann PG. Inhibition of inducible nitric oxide synthesis by the herbal preparation PADMA 28 in macrophage cell line. Can J Physiol Pharmacol. 2000;78:861–6. [PubMed] [Google Scholar]
- 43.Ginsburg I, Sadovnik M, Sallon S, et al. PADMA 28, a traditional Tibetan herbal preparation inhibits the respiratory burst in human neutrophils, the killing of epithelial cells by mixtures of oxidants and pro-inflammatory agonists and peroxidation of lipids. Inflammopharmacology. 1999;7:47–62. doi: 10.1007/s10787-999-0025-9. [DOI] [PubMed] [Google Scholar]
- 44.Weseler A, Saller R, Reichling J. Comparative investigation of the antimicrobial activity of PADMA 28 and selected European herbal drugs. Forsch Komplementarmed Klass Naturheilkd. 2002;9:346–51. doi: 10.1159/000069234. [DOI] [PubMed] [Google Scholar]
- 45.Samochowiec J, Palacz A, Bobnis W, Lisiecka B. Oscillating potentials of the electroretinogram in the evaluation of the effects of PADMA 28 on lipid metabolism and vascular changes in humans. Phytother Res. 1992;6:200–4. [Google Scholar]
- 46.Barak V, Kalickman I, Halperin T, Birkenfeld S, Ginsburg I. PADMA-28, a Tibetan herbal preparation is an inhibitor of inflammatory cytokine production. Eur Cytokine Netw. 2004;15:203–9. [PubMed] [Google Scholar]
- 47.Navab R, Aingorn H, Fallavollita L, et al. PADMA-28, a traditional Tibetan herbal preparation, blocks cellular responses to bFGF and IGF-I. Inflammopharmacology. 2004;12:373–89. doi: 10.1163/1568560043696227. [DOI] [PubMed] [Google Scholar]
- 48.Cantürk NZ, Cantürk Z, Ozden M, Dalçik H, Yardimoglu M, Tülübas F. Protective effect of IGF-1 on experimental liver cirrhosis-induced common bile duct ligation. Hepatogastroenterology. 2003;50:2061–6. [PubMed] [Google Scholar]
- 49.Yu C, Wang F, Jin C, et al. Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis. Am J Pathol. 2003;163:1653–62. doi: 10.1016/S0002-9440(10)63522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koren E, Zverev I, Ginsburg I, Kohen R. Supplementation with antioxidants fails to increase the total antioxidant capacity of several cell lines in culture. Biomed Pharmacother. 2008;62:179–88. doi: 10.1016/j.biopha.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Jadon A, Bhadauria M, Shukla S. Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol. 2007;109:214–8. doi: 10.1016/j.jep.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 52.Ginsburg I. Multi-drug strategies are necessary to inhibit the synergistic mechanism causing tissue damage and organ failure in post infectious sequelae. Inflammopharmacology. 1999;7:207–17. doi: 10.1007/s10787-999-0004-1. [DOI] [PubMed] [Google Scholar]


