Abstract
Zif268 is a transcriptional regulator that plays a crucial role in maintenance of the late phases of hippocampal long-term potentiation (LTP) and consolidation of spatial memories. Because the hippocampal place cell system is essential for long-term spatial memory, we tested the hypothesis that zif268 is required for long-term stability of hippocampal place cell representations by recording CA1 place cells in mice lacking zif268. We found that zif268 gene deletion destabilized the representation of a familiar environment after exposure to a novel environment and impaired the long-term (24 h), but not short-term (1 h), stability of newly formed representations. These impairments could be rescued by repeated exposure to the novel environment, however. These results indicate that zif268 contributes to the long-term stability of spatial representations in CA1 and support the notion that the long-term stability of place cell representations requires transcription-dependent mechanisms similar to those observed in LTP.
Keywords: hippocampus, spatial memory
Current hypotheses on the molecular mechanisms of learning and memory suggest that rapid regulation of gene programs and the synthesis of new proteins leading to persistent synaptic modification constitute a key mechanism for the stabilization of long-term memory. One critical step in this process is the activation of nuclear, inducible transcription factors that interact with promoter regulatory elements on downstream late-response genes. Among the activity-regulated transcription factors, zif268, a member of the Egr family, is best characterized by its role in synaptic plasticity and memory consolidation. Zif268 is rapidly induced in association with long-term potentiation (LTP) and in defined brain structures and circuits after specific learning experiences (1–6). Furthermore, mutant mice with targeted inactivation of the zif268 gene cannot maintain late-phase LTP in the dentate gyrus of the hippocampus and fail to form long-lasting memories (7), with particular sensitivity in hippocampal-dependent spatial memory tasks (8). Our objective in this study was to investigate the possibility that impaired spatial memory caused by zif268 deficiency might be due to the inability of the mutant mice to form or stabilize neural representation of space.
It has long been known that hippocampal CA1 pyramidal cells—the place cells—fire in response to an animal's location within a particular environment (9), with each place cell discharging in a cell-specific, stable region known as a “place field.” The role of place cells as critical elements of a long-term spatial memory system is based on at least 2 properties. First, a given cell reinstates the same place field on multiple exposures to the same environment (10). Second, although the spatial firing pattern of ensemble of cells is environment-specific, remapping—the formation of a new spatial firing map—occurs on exposure to a new environment (11, 12), suggesting that the place cell system elaborates and holds distinct memories for distinct contexts, with each firing pattern reactivated on exposure to the corresponding environment.
Previous data have raised the possibility that molecular mechanisms underlying LTP and memory consolidation are important in enabling place cells to maintain long-term spatial memory representation (13–16). Thus, as a key element in these mechanisms, zif268 may be necessary for the expression of learning and memory properties of place cells. In the present study, we examined the involvement of zif268 in the place cell system's ability to encode and maintain new representations over time. Because zif268 KO mice exhibit deficits in late-phase LTP and long-term memory (7), we hypothesized that formation and short-term maintenance of the representation of a new environment would not be affected, but long-term maintenance would be disrupted.
Results
The data set comprised a total of 274 pyramidal cells with complex-spike firing recorded in CA1, including 94 cells recorded from 7 WT mice, and 180 cells recorded from 7 zif268 KO mice. Six of the 7 KO mice were homozygous mutants (zif268−/−), and 1 KO mouse was a heterozygous mutant (zif268+/−), in which 30 place cells were recorded. There was no evidence of different responses of place cells in the zif268+/− mouse compared with the zif268−/− mice, and so the cells of all 7 zif268 KO mice were pooled for analysis. Because some cells could stop or start firing in the course of the successive recording sessions (possibly due to environmental manipulation), only cells recorded during the 2 sessions for each pair of interest were considered for data analysis based on comparisons between pairs of sessions.
WT and zif268 KO Mice Exhibit No Behavioral Differences.
WT and zif268 KO mice exhibited similar object-directed exploratory activity measured during the first sequence of the recording sessions (effect of group, F1,12 = 0.19, P > .05; effect of session, F6,72 = 1.40, P > .05; group × session, F6,72 = 1.38, P > .05) and distance run (effect of group, F 1,12 = 0.35, P > .05; effect of session, F6,72 = 3.04, P < .05; group × session, F6,72 = 1.19, P > .05) throughout the recording sessions.
Place Field Stability in a Familiar Environment and Remapping in a Novel Environment Are Not Affected in zif268 KO Mice.
Place cells recorded in zif268 KO mice exhibited nondegraded basic firing parameters and an even higher information content [see supporting information (SI) Table S1]. In the familiar environment, place fields were stable in both WT and zif268 KO mice, as demonstrated by the similarity score distributions (Fig. S1). Exposure to the novel environment (circle) induced the formation of new spatial firing patterns (remapping) in both WT and zif268 KO mice. There was no difference in the distribution of S2/S3 similarity scores between WT and zif268 KO mice (Fig. S2). In the first recording sequence, mean similarity scores were much lower in S2/S3 than in S1/S2, indicating strong remapping in the 2 groups (effect of group, F1,96 = 0.35, P = .55; effect of novelty, F1,96 = 51.02, P < .0001; group × novelty, F1,96 = 0.93, P = .34) (Fig. S2).
Exposure to the Novel Environment Interferes With Reactivation of the Representation of the Familiar Environment in zif268 KO Mice.
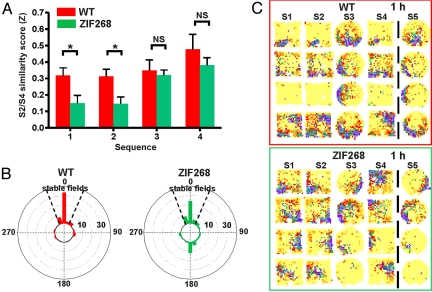
Surprisingly, reexposing zif268 KO mice to the familiar square did not result in reactivation of the familiar representation in many cells, as was the case in WT mice. The S2/S4 similarity scores in zif268 KO mice were lower than both the S1/S2 similarity scores (t152 = 4.18, P < .001) and the S2/S4 similarity scores (t239 = 2.45, P < .05) in WT mice, suggesting a remapping. This was confirmed by examining the rate maps (Fig. S3). Such remapping in the familiar environment was observed only in the first 2 recording sequences (first sequence: WT > zif268, t52 = 2.54, P < .05; second sequence: WT > zif268, t52 = 2.59, P < .05; third and fourth sequences: P > .05) (Fig. 1A). Rotation angles of many place fields in the first 2 sequences clearly departed from the 0° angle (Fig. 1B), and the angle distribution differed from that of WT mice (Watson U2 test for circular data, U2 = 0.655, P < .001).
Fig. 1.
Rotational remapping of place fields in the familiar environment after exposure to the novel environment in zif268 KO mice. (A) S2/S4 similarity scores in the first 4 recording sequences. *P < .05, NS (not significant), P > .05. (B) Distribution in number of cells of the rotation angles (20° bins) corresponding to S2/S4 ZMax pooled for the first 2 sequences in WT and zif268 KO mice (WT: n = 50, mean angle = 0.925°, SD = 4.11°, r = 0.879; zif268: n = 63, mean angle = 5.9°, SD = 16.34°, r = 0.305). In zif268 KO mice, 42.9% (26/63) of cells had fields that shifted (rotation angle exceeding 0° ± 30°). A V test performed on these cells (with cells with stable fields excluded from the analysis) indicated that the rotation angles were not homogeneously distributed but were clustered around 180°, indicating a rotational bias (V test, u = 4.791; P < .001). (C) Rate maps illustrating remapping in S3 (new environment) and rotational remapping in S4 (familiar environment) in zif268 KO mice. The dashed line indicates the 1-h delay.
Interestingly, visual examination of the rate maps of the cells that remapped in zif268 KO mice suggests that numerous fields underwent rotational remapping (Fig. 1C). The S2/S4 ZMax value was similar to the S1/S2 similarity scores (t25 = 0.822, P > .05), indicating fairly good superimposition of the fields after a simple rotation of the maps. Note that the firing rate in S4 also was affected by the change [high - low/high + low score (17): 0.26 ± 0.04; 1-sample t test, t25 = 6.51; P < .01]. Three out of 7 ensembles of simultaneously recorded neurons (corresponding to 3 mice) displayed rotational remapping. Thus, although cells in zif268 KO mice were able to activate the representation of the familiar square, this representation was oriented incorrectly in many cases.
Although this effect disappeared after the second recording sequence, we examined whether it would reappear if the animals were exposed for 1 recording sequence to a novel, triangular environment. The delay between the first exposure to the circle and the first exposure to the triangle varied across mice, ranging from 1 month to 4 months. A total of 45 place cells (19 cells in 2 WT mice and 26 cells in 3 zif268 KO mice) were recorded. First exposure to the triangle induced remapping in both WT and zif268 KO mice. When reexposed to the square, all groups reactivated the correct representation of the square (Fig. S3). Thus, the remapping seen during reexposure to the familiar environment was not a systematic response to any novel environment, but instead suggests an experience-dependent effect warranting more specific testing.
Short-Term Stability of Place Fields in the Novel Environment Is Not Affected in zif268 KO Mice.
After a 1-h delay, the circle representation was reestablished in both WT and zif268 KO mice (Fig. S4B). The distribution profiles of S3/S5 similarity scores were similar in WT and zif268 KO mice for all recording sequences (Kolmogorov-Smirnov test, P > .1) (Fig. S4A). The mean similarity scores did not differ when considering all (t211 = 0.54, P > .05) or only the first 2 recording sequences (t101 = 1.72, P > .05). Thus, place fields were stable between S3 and S5, indicating that the representation of the novel environment was not degraded over a short delay in either group.
Long-Term Stability of Place Fields in the Novel Environment Is Impaired in zif268 KO Mice.
When reexposed to the circle after a 24-h delay, place cells in zif268 KO mice did not reactivate the corresponding representation (Fig. 2C; see additional examples in Fig. S5). Similarity scores in the novel environment (S5/S7) were much lower in zif268 KO mice than in WT mice in the first 2 recording sequences (unpaired t test, t74 = 3.06, P < .01) (Fig. 2A). This difference was not seen in the next 2 recording sequences (unpaired t test, t74 = 0.53, P > .05), suggesting a temporary impairment. In zif268 KO mice, the S5/S7 (24-h delay) similarity scores also were lower than the S3/S5 (1-h delay) similarity scores (paired t test, t32 = 3.51, P < .01), whereas in WT mice there was no difference between the 2 scores (paired t test, t31 = 1.13, P > .05). Because the instability in the familiar environment in S4 in mutant mice may have some long-lasting effect affecting field stability in S7, we analyzed S5/S7 similarity scores separately for cells found to be stable or unstable in S4. We found low S5/S7 similarity scores regardless of whether cells previously demonstrated unstable or stable place fields in S4 (t33 = 0.48; P > .05), thereby ruling out the possibility of a causal relationship between instability in S4 and instability in S7. As shown in the distribution of rotation angles corresponding to S5/S7 ZMax, unstable fields (departing from an angle of 0° ± 30°; Fig. 2B) were distributed homogeneously over 360° (n = 21; Watson U2 test, U2 = 0.05, P > .05), indicating no angular bias. Taken together, our results demonstrate that place cells in zif268 KO mice were not able to correctly reactivate the representation of the circle 24 h after being exposed to it, suggesting a deleterious effect of zif268 deficiency in long-term maintenance of the novel representation.
Fig. 2.
Long-term stability of place fields in the novel environment is impaired in zif268 KO mice. (A) Similarity scores for S1/S6 correlations for recording sequences 1 and 2 and S5/S7 correlations for recording sequences 1 and 2 and recording sequences 3 and 4 in WT and zif268 KO mice. **P < .01; NS (non significant), P > .05. (B) Distribution in number of cells of the rotation angles (20° bins) corresponding to S5/S7 ZMax pooled for the first 2 recording sequences (WT: n = 39, mean angle = 359.7°, SD = 31.2°, r = 0.862; zif268: n = 36, mean angle = 354.9°, SD = 82.72°, r = 0.353). In S7, 92% of the fields (36/39) in WT mice and in 42% of the fields (15/36) in zif268 KO mice were found to be stable (χ2 = 22.16, P < .001). (C) Rate maps illustrating remapping in S7 in zif268 KO mice. The dashed line indicates the 1-h delay; the continuous line, the 24-h delay.
Discussion
The basic firing properties of place cells or the characteristics of place fields, such as coherence and information content, measured in a familiar environment were not degraded in zif268 KO mice. When mice were exposed to a novel environment, place cells in both zif268 KO and WT mice readily demonstrated remapping. Thus, consistent with previous reports on place cell activity in conditions of inactivation of molecules implicated in LTP mechanisms (15, 16), the lack of zif268 did not prevent the rapid formation of a new place cell representation.
Our major finding is that zif268 deficiency impaired the ability to maintain a newly formed neuronal representation after a long delay (24 h), but not after a shorter delay (1 h). When mice were placed in a new environment, place fields formed rapidly and equally well in both mutant and WT mice; however, in zif268 KO mice these newly formed place fields were stable after 1 h but were changed significantly at 24 h, in contrast to the long-lasting stability of the place fields in WT mice. Our analyses indicate that this was not due to destabilization during reexposure to the familiar environment. In addition, it is unlikely that this effect can be attributed to alteration of other behavioral abilities, such as locomotor activity, exploratory behavior, anxiety-related response in an open field, visual discrimination and exploration of objects, or novelty detection, because no overt deficits have been observed in these functions in zif268 KO mice (7, 8, 18). Thus, our results provide evidence that zif268 contributes critically to long-term, but not short-term, maintenance of spatial representations by hippocampal place cells. Given that signaling events upstream of zif268 transcription are not affected in the mutant mice (7), we conclude that the lack of long-term stability of the newly formed memory representation is due to a failure in the synthesis of downstream effector proteins encoded by genes for which zif268 is an obligatory transcription factor.
Hippocampal CA1 place cell representation is an essential component of the hippocampal spatial memory system. The compromised long-term stability of place fields in zif268 KO mice parallels that of late-phase, but not early-phase, LTP and the severe deficits in long-term, but not short-term, memory found in several types of tasks, particularly hippocampal-dependent long-term spatial memory (7, 8). This supports the idea that impaired LTP mechanisms are responsible for the deficient stabilization of hippocampal place cell representations, resulting in spatial memory deficits, and is consistent with studies in which manipulations of specific components of intracellular signaling pathways underlying LTP induction or maintenance were found to affect location-specific place cell firing and spatial memory. Pharmacologic or genetic inactivation of NMDA receptors (15, 17), the GluR2 AMPA receptor subunit (19), or mutations affecting plasticity-related proteins, such as the kinase CaMKII or CREB, in transgenic mice (16, 20, 21), all result in degraded and unstable place fields. In addition, both the reduction of forebrain protein kinase A activity in transgenic R(AB) mice (22) and pharmacologic inhibition of protein synthesis using anisomycin in rats (23) were found to affect place cell maps in a manner resembling the effects seen with zif268 inactivation in the present study. The formation of a cell representation in a novel environment (remapping) was normal, and the new representation was stable after 1 h, but not after 24 h. Our results in zif268 KO mice thus support and extend the idea that long-term stability of place cell representations involves some of the same NMDA-dependent activation of intracellular signaling cascades leading to gene transcription and the synthesis of proteins that underlie LTP and are essential for long-term memory.
An additional result was that when the rats were reexposed to the familiar environment after being exposed to the novel environment, what was a stable set of place fields corresponding to the familiar environment was not properly reactivated in zif268 KO mice compared with WT mice. Instead, a large percentage of place fields underwent remapping. Thus, formation of the new representation in zif268 KO mice interfered with the reactivation of the familiar representation. Destabilization did not result in a complete loss, however. Place fields switched to a different angular position in the arena but maintained their radial position (rotational remapping), indicating an incorrect orientation of the place cell map corresponding to the familiar representation (associated with rate change), rather than creation of a totally new representation. Although interference between novel and familiar representations in place cells has been reported (24, 25), it has not been associated with specific molecular mechanisms.
One possible explanation is based on the hypothesis that rotational remapping reflects a generalization effect due to an impaired ability to properly discriminate the 2 environments (26). Generalization may occur because the representation of the familiar environment is not as fully stabilized in zif268 KO mice compared with WT mice, despite extensive training in this environment. This would suggest that zif268 expression is important for the long-term consolidation/stabilization of spatial representations via its role in synaptic plasticity (4, 7). An alternative, albeit more speculative, possibility is that the formation of a new representation in the novel environment occurring shortly after exposure to the familiar environment may destabilize the previously consolidated representation, rendering it labile and vulnerable to interference. If this were the case, then a mechanism of reconsolidation might be required to make the representation available for further recall. Within this framework, the absence of zif268 may be detrimental to this reconsolidation process, a hypothesis consistent with the finding that zif268 is required for the reconsolidation of forms of recognition memory (18). Finally, it is also possible—but remains to be investigated—that zif268 deletion may impair the ability of the head direction cell system to maintain a directional reference that would be used by the place cell system to anchor place fields in space.
Overall, the lack of long-term stability of a newly formed neural representation and the destabilization of more remotely established cellular representations on transfer to a novel environment both suggest that zif268-dependent synaptic plasticity mechanisms are essential for stabilizing CA1 place cell representations. The deficits were confined mostly to the first few recording sequences, however. An explanation for this intriguing result is that place cell maps will progressively stabilize in zif268 KO mice under conditions of repeated exposure to the environment. This fits in with the observation that the long-term memory deficits displayed by zif268 KO mice can be overcome by distributed and extended training (7, 18) and suggests a compensatory mechanism that can bypass the lack of zif268 in certain behavioral conditions. As suggested elsewhere (18), one possibility is the recruitment of other members of the Egr family in conditions of overtraining, because these genes encode closely related transcription factors with a high homology in the zinc-finger DNA binding domain and thus could control expression of some of the same downstream target genes (27). Whether compensation also would be observed in late LTP in zif268 KO mice in conditions of repeated tetanization remains to be determined.
Flexibility is a major characteristic of the place cell system, involving various synaptic plasticity mechanisms that enable both rapid formation of new representations and long-lasting maintenance of familiar representations. Our results lend further support to the hypothesis that at the molecular level, long-term maintenance of spatial representations in the hippocampus requires transcriptional events in neurons, and provide evidence that the transcription factor zif268 plays an important role in this process. The formation of spatial memory presumably involves widespread cortico-hippocampal connectivity networks within which specific regions and inputs may subserve distinct but interactive processing functions (6, 28, 29). Damage to structures conveying information to CA1 in these networks can affect CA1 place cell stability (30–34). Because zif268 inactivation in our mice is not restricted to any particular brain structure, whether the compromised long-term maintenance of place cell representations is due to the lack of zif268-dependent plasticity in CA1 pyramidal cells and/or to deficient plasticity at other sites within cortico-hippocampal circuits cannot be established at this point. Region-specific inactivation of zif268 may provide a means of identifying the critical site(s) in which zif268-dependent plasticity is required in the neural circuits that encode and store spatial representations.
Materials and Methods
Animals.
Seven zif268 KO mice and 7 WT mice (30 g) were used (see SI Materials and Methods). Mice were housed in individual cages (36 × 20 × 14 cm) located in a temperature- and light-controlled room with a 12-h light/dark cycle and were provided with ad libitum food and water. All procedures complied with both US and French institutional guidelines.
Surgery.
Under xylasine-ketamine anesthesia, mice were implanted with 4 tetrodes (each comprising 4 twisted 25-μm nichrome wires inserted into a single 30-gauge guide cannula). The tips of the tetrodes were positioned above the right hippocampus (AP, -2 mm; L, -2 mm relative to the bregma). At the completion of the experiment, the mice were perfused, and their brains were sectioned and stained with cresyl violet to identify electrode tracks (see SI Materials and Methods).
Unit Recording and Tracking.
Signals from the electrodes were amplified 10,000 times, bandpass-filtered between 0.3 and 10 kHz using Neuralynx amplifiers, and processed with the animals' position signals using Datawave SciWorks acquisition software. Waveforms of identified units were sampled at 32 kHz and stored in a computer. A single LED positioned on the headstage allowed position tracking (at 50 Hz) and was detected in a grid of 32 × 32 pixels, 25 mm on a side.
Apparatus.
Before implantation, mice were exposed daily (15-min sessions) for 3 weeks to the “familiar environment,” a square enclosure in a dimly lit curtained environment (see SI Materials and Methods. Electrode screening was performed as the animals were running in the square. After 2 initial recording sessions in the familiar environment, the mice were exposed to a “novel environment.” Two novel environments were used, a circular enclosure and a triangular enclosure. All 3 environments had similar floor area (≈1,600 cm2).
Recording Protocol.
Mice were submitted to 7 successive 15-min recording sessions (S1–S7; Fig. 1A). Sessions 1–5 were carried out on day 1, whereas sessions 6 and 7 were performed 24 h later (day 2). Each set of 7 recording sessions is designated a “recording sequence.” During the first recording sequence, mice were exposed to the novel environment for the first time (S3; circle) and then reexposed to it 24 h later (S7); thus the circle became relatively less novel. The same behavioral protocol was repeated while recording different cells in the same mice to collect as much data as possible, reexposing the mice to the circle. Thus, because the circle became progressively more familiar in the subsequent sequences, each recording sequence was analyzed separately to investigate the experience-dependent effects of zif268 deletion. The role of zif268 was examined in different functional aspects of the place cell system: (i) whether place fields are stable in constant conditions (mice were exposed to the familiar, square environment in sessions 1 and 2), (ii) whether place cells are able to form a new representation (mice were exposed to a new environment, circle or triangle, in session 3), (iii) whether place cells are able to maintain short-term stability of the new representation (1 h after reexposure to the square in session 4, mice were placed in the circle in session 5), and (iv) whether place cells are able to maintain long-term stability of the new representation (after a 24-h delay, mice were exposed to the square in S6 and exposed to the circle in S7). With the exception of the S4–S5 and S5–S6 intervals, 5-min intersession intervals were maintained, during which mice were placed back in their home cages. The apparatus was cleaned before each session. As described in Results, an additional experiment involved exposing the animal to a second novel environment, a triangular arena. The recording protocol for the second novel environment was identical to that of the circle-to-square substitution protocol. After the recording sessions, the electrodes were moved to isolate new sets of cells. On the next recording day, a new full behavioral and recording sequence was initiated.
Place Cell Analysis.
Spikes from single CA1 hippocampal pyramidal cells were identified and isolated using Datawave SciWorks cluster cutting software. Only cells with clear location-specific activity were included in the data set. Color-coded firing rate maps were then constructed for each session to visualize the positional firing distribution (12). A place field was defined as a set of at least 9 contiguous pixels with a firing rate above the mean firing rate. Several measures of spatial firing were used to compare place cells in zif268 KO and WT mice: in-field mean firing rate, in-field peak firing rate, spatial coherence, and information content (35) (see SI Materials and Methods). Place field similarity between sessions was measured by calculating pixel-by-pixel cross-correlations between pairs of firing rate arrays (similarity score; see refs. 15 and 36). The place field angular shift between the 2 sessions was measured by calculating cross-correlation as the firing rate array of the first session was rotated in 6° steps relative to the firing rate array of the second session. The angle associated with the highest correlation (ZMax) was taken as the rotation angle of the place field between the 2 sessions (see SI Materials and Methods).
Supplementary Material
Acknowledgments.
We thank Patrick Charnay and Piotr Topilko for providing the zif268 mutant mice breeders and Nathalie Samson and Pascale Le Blanc for providing animal care and rearing and genotyping the mice. This work was supported by CNRS, by ACI “Neurosciences Intégratives et Computationnelles” Grant NIC0027 from the French Ministry of Research (to S.D. and B.P.), and by a fellowship from the Fondation pour la Recherche Médicale (to S.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900484106/DCSupplemental.
References
- 1.Abraham WC, Dragunow M, Tate WP. The role of immediate early genes in the stabilization of long-term potentiation. Mol Neurobiol. 1991;5:297–314. doi: 10.1007/BF02935553. [DOI] [PubMed] [Google Scholar]
- 2.Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning. Behav Brain Res. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 3.Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: Selective activation of hippocampal CA1 neurons during recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain. Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- 6.Poirier GL, Amin E, Aggleton JP. Qualitatively different hippocampal subfield engagement emerges with mastery of a spatial memory task by rats. J Neurosci. 2008;28:1034–1045. doi: 10.1523/JNEUROSCI.4607-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones MW, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- 8.Bozon B, Davis S, Laroche S. Regulated transcription of the immediate-early gene Zif268: Mechanisms and gene dosage–dependent function in synaptic plasticity and memory formation. Hippocampus. 2002;12:570–577. doi: 10.1002/hipo.10100. [DOI] [PubMed] [Google Scholar]
- 9.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 10.Thomson LT, Best PJ. Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Res. 1990;509:299–308. doi: 10.1016/0006-8993(90)90555-p. [DOI] [PubMed] [Google Scholar]
- 11.Lever L, Wills T, Cacucci F, Burgess N, O'Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416:90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- 12.Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dragoi G, Harris KD, Buzsàki G. Place representation within hippocampal networks is modified by long-term potentiation. Neuron. 2003;39:843–853. doi: 10.1016/s0896-6273(03)00465-3. [DOI] [PubMed] [Google Scholar]
- 14.Ekstrom AD, Meltzer J, McNaughton BL, Barnes CA. NMDA receptor antagonism blocks experience-dependent expansion of hippocampal “place fields.”. Neuron. 2001;31:631–638. doi: 10.1016/s0896-6273(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 15.Kentros C, et al. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science. 1998;280:2121–2126. doi: 10.1126/science.280.5372.2121. [DOI] [PubMed] [Google Scholar]
- 16.Rotenberg A, Mayford M, Hawkins RD, Kandel ER, Muller RU. Mice expressing activated CaMKII lack low-frequency LTP and do not form stable place cells in the CA1 region of the hippocampus. Cell. 1996;87:1351–1361. doi: 10.1016/s0092-8674(00)81829-2. [DOI] [PubMed] [Google Scholar]
- 17.McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell. 1996;87:1339–1349. doi: 10.1016/s0092-8674(00)81828-0. [DOI] [PubMed] [Google Scholar]
- 18.Bozon B, Davis S, Laroche S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron. 2003;40:695–701. doi: 10.1016/s0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
- 19.Yan J, et al. Place-cell impairment in glutamate receptor 2 mutant mice. J Neurosci. 2002;22:RC204:1–5. doi: 10.1523/JNEUROSCI.22-03-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cacucci F, Wills TJ, Lever C, Giese KP, O'Keefe J. Experience-dependent increase in CA1, place cell spatial information, but not spatial reproducibility, is dependent on the autophosphorylation of the alpha-isoform of the calcium/calmodulin-dependent protein kinase II. J Neurosci. 2007;27:7854–7859. doi: 10.1523/JNEUROSCI.1704-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho YH, Giese KP, Tanila H, Silva AJ, Eichenbaum H. Abnormal hippocampal spatial representations in αCaMKIIT286A and CREBαΔ− mice. Science. 1998;279:867–869. doi: 10.1126/science.279.5352.867. [DOI] [PubMed] [Google Scholar]
- 22.Rotenberg A, Abel T, Hawkins RD, Kandel ER, Muller RU. Parallel instabilities of long-term potentiation, place cells, and learning caused by decreased protein kinase A activity. J Neurosci. 2000;20:8096–8102. doi: 10.1523/JNEUROSCI.20-21-08096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agnihotri NT, Hawkins RD, Kandel ER, Kentros C. The long-term stability of new hippocampal place fields requires new protein synthesis. Proc Natl Acad Sci USA. 2004;101:3656–3661. doi: 10.1073/pnas.0400385101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanila H, Sipilä P, Shapiro M, Eichenbaum H. Brain aging: Impaired coding of novel environmental cues. J Neurosci. 1997;17:5167–5174. doi: 10.1523/JNEUROSCI.17-13-05167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Hoz L, Wood ER. Dissociating the past from the present in the activity of place cells. Hippocampus. 2006;16:704–715. doi: 10.1002/hipo.20207. [DOI] [PubMed] [Google Scholar]
- 26.Bostock E, Muller RU, Kubie JL. Experience-dependent modifications of hippocampal place cell firing. Hippocampus. 1991;1:193–205. doi: 10.1002/hipo.450010207. [DOI] [PubMed] [Google Scholar]
- 27.Swirnoff AH, Milbrandt J. DNA-binding specificity of NGFI-A and related zinc finger transcription factors. Mol Cell Biol. 1995;15:2275–2287. doi: 10.1128/mcb.15.4.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 29.Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- 30.Brun VH, et al. Impaired spatial representation in CA1-impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 31.Muir GH, Bilkey DK. Instability in the place field location of hippocampal place cells after lesions centered on the perirhinal cortex. J Neurosci. 2001;21:4016–4025. doi: 10.1523/JNEUROSCI.21-11-04016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paz-Villagràn V, Lenck-Santini PP, Save E, Poucet B. Properties of place cell firing after damage to the visual cortex. Eur J Neurosci. 2002;16:771–776. doi: 10.1046/j.1460-9568.2002.02154.x. [DOI] [PubMed] [Google Scholar]
- 33.Save E, Paz-Villagràn V, Alexinsky T, Poucet B. Functional interaction between the parietal cortex and hippocampal place cell firing in the rat. Eur J Neurosci. 2005;21:522–530. doi: 10.1111/j.1460-9568.2005.03882.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Cauter T, Poucet B, Save E. Unstable CA1 place cell representation in rats with entorhinal cortex lesions. Eur J Neurosci. 2008;27:1933–1946. doi: 10.1111/j.1460-9568.2008.06158.x. [DOI] [PubMed] [Google Scholar]
- 35.Markus EJ, Barnes CA, McNaughton BL, Gladden VL, Skaggs WE. Spatial information content and reliability of hippocampal neurons: Effects of visual input. Hippocampus. 1994;4:410–421. doi: 10.1002/hipo.450040404. [DOI] [PubMed] [Google Scholar]
- 36.Paz-Villagràn V, Save E, Poucet B. Independent coding of connected environments by place cells. Eur J Neurosci. 2004;20:1379–1390. doi: 10.1111/j.1460-9568.2004.03570.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.