Abstract
The negative-strand RNA viruses (NSRVs) are unique because their nucleocapsid, not the naked RNA, is the active template for transcription and replication. The viral polymerase of nonsegmented NSRVs contains a large polymerase catalytic subunit (L) and a nonenzymatic cofactor, the phosphoprotein (P). Insight into how P delivers the polymerase complex to the nucleocapsid has long been pursued by reverse genetics and biochemical approaches. Here, we present the X-ray crystal structure of the C-terminal domain of P of vesicular stomatitis virus, a prototypic nonsegmented NSRV, bound to nucleocapsid-like particles. P binds primarily to the C-terminal lobe of 2 adjacent N proteins within the nucleocapsid. This binding mode is exclusive to the nucleocapsid, not the nucleocapsid (N) protein in other existing forms. Localization of phosphorylation sites within P and their proximity to the RNA cavity give insight into how the L protein might be oriented to access the RNA template.
Keywords: negative-strand RNA virus, replication, template, transcription, phosphorylation
Vesicular stomatitis virus (VSV) belongs to the family Rhabdoviridae, which also includes rabies virus. The rhabdoviruses are part of the broad group of negative-strand RNA viruses (NSRVs), which contain many medically relevant viruses, including avian influenza, measles, and Ebola. VSV has long served as a prototypic nonsegmented negative-strand RNA virus (NNSRV), partly because of the small number of genes that are encoded by its 11-kb genome (1). These genes include the nucleocapsid protein (N), a phosphoprotein (P), a matrix protein (M), a glycoprotein (G), and a large polymerase protein (L). Each of these proteins has multiple functions and, as a result, has multiple binding partners, including but not limited to each other.
The NNSRVs are characterized by the unique fact that in the entire replication cycle, their genomes do not exist as naked RNA, but rather are encapsidated by their nucleocapsid proteins. The nucleocapsid is the active template for transcription and replication (2, 3). Structures of the nucleocapsid-like particles (NLPs) of VSV and rabies virus have recently been solved (4, 5), showing that the N protein has 2 lobes angled together to form a cavity for encapsidation of the genomic RNA. Each N monomer accommodates 9 bases of RNA. The structures also revealed that each monomer of N interacts with 3 neighboring N molecules across the nucleocapsid. The contacts involve the elongated N terminus and an extended loop (C loop) within the C-terminal lobe, and they are required for RNA encapsidation (6). Residues within this loop have also been implicated in binding to the P (7, 8). Recently, more insight into capsid formation was gained through crystallographic studies of an N protein with a serine-to-tryptophan mutation at residue 290 (called N290; ref. 6). N290 has lost the ability to encapsidate RNA because of the bulky side chain of tryptophan in the RNA cavity, yet the capsid assembly functions of the protein remain intact. Thus, the N protein alone contains all of the information for the assembly of a capsid structure.
The multidomain P protein (Indiana strain) of VSV is the 265-aa nonenzymatic cofactor of the viral polymerase. The N-terminal domain comprises the first 106 residues of P and contains 3 residues (Ser-60, Thr-62, and Ser-64) that may be phosphorylated by host casein kinase II (9–11). These residues are indispensable because they are required for transcription (12–14). The central domain, residues 107–177, is the site of P dimerization and subsequent tetramer formation (15, 16). Previous experiments have shown that multimerization beyond the dimeric state is essential to P's role in replication (11, 17). The majority of the remaining 89 residues form the C-terminal domain (PCTD). PCTD contains 2 additional phosphorylation sites (Ser-226 and Ser-227). The L protein has been shown previously to interact with these residues (13), and although phosphorylation is not required for L binding, phosphorylation of either residue is responsible for regulating levels of replication. This domain of P is also the major site of N protein association (18–23). The structure of the PCTD was determined recently by NMR and shown to form a single compact unit comprising an antiparallel β-turn and 5 α-helices (24).
Upon translation and before polymerization of N onto the genome, N forms an initial complex with P, known as No-P (25–27). This RNA-free, encapsidation-competent complex is delivered to the active replication site, possibly via the secondary interaction of P with the viral polymerase. Here, N comes into contact with the newly synthesized genomic RNA, and the process of encapsidation occurs. In addition to binding N and forming the encapsidation precursor, P binds to the nucleocapsid during the viral replication cycle that involves polynucleotide synthesis. The L polymerase subunit cannot recognize the nucleocapsid alone. The processes of transcription and replication require the association of the P as a component of the viral polymerase complex (2, 28). Thus, P is the determining factor for template recognition for the viral polymerase.
The N, P, and L proteins are unique in that they form distinct complexes at different stages of the replication cycle. As described above, L and P form the RNA polymerase that associates with the N-enwrapped template. Additional experimentation has shown that some P mutants maintain the ability to bind N and form No-P but are inactive in transcription. These complexes were, however, capable of supporting replication (22). Therefore, it was surmised that the replicase and transcriptase were 2 distinct complexes. Subsequently, an active replicase complex was isolated, and in fact it was shown to contain the N, P, and L proteins (29). This tripartite complex was distinct from the transcriptase, a complex that does not contain the N protein. These experiments confer the complexity of the interactions of N, P, and L, and although structures of N and 2 individual domains of P have been published, the precise nature of the direct interaction of N and P together is unknown. Likewise, the nature of the tripartite complex between N, P, and L is not well understood. Ultimately, because these complexes play key roles in the synthetic processes of transcription, replication, and encapsidation of progeny genomes, atomic-level snapshots of their interactions would be invaluable in aiding interpretation of previously published works.
To date, a large body of work has been performed to aide in understanding how the P interacts with N. These studies have relied heavily on mutagenesis and biochemical analysis. However, a concrete model of the direct interaction of N and P was still elusive. The work presented here gives the atomic look at the complex formed between the N protein in NLP and the C-terminal nucleocapsid-binding domain of P. Two adjacent monomers within the nucleocapsid form a unique binding site for P, which is a binding site that can only be found in the nucleocapsid. Binding occurs solely to the C lobes of the 2 N proteins. This explains how the P delivers the viral polymerase specifically to the nucleocapsid for RNA synthesis. New insights are found in the mechanism by which the P allows the polymerase complex to recognize the nucleocapsid as the template for viral RNA synthesis.
Results
N/PCTD Structure.
The N protein is present in the nucleocapsid as a linear polymer. The P protein must recognize the polymeric N protein template and, subsequently, P must remain associated with the nucleocapsid as the polymerase complex moves down the template during RNA synthetic events. These 2 events are essential to the function of the viral polymerases of the NNSRV. To date, it has been unclear exactly how the N protein and P associate. The structure of the middle multimerization domain of P (residues 107–177) was reported previously as a dimer (15). It was also reported recently that the structure of the uncomplexed C-terminal 71 aa of P (residues 195–265) contains a single compact domain comprising an antiparallel β-turn and 5 α-helices (24). Here, we present the crystal structure of PCTD in complex with NLP in the presence and absence of RNA. There are no significant differences between the 2 structures. The overall rmsd between the RNA-encapsidated PCTD complex and the RNA-deficient N290 PCTD complex is 1.41 Å. When superimposed, the main noticeable difference is the orientation of the N lobe with respect to the center of the 10-member ring. These differences were also observed in NLP structures in the absence of PCTD (4, 6). In the NLP–PCTD complex, N exists as a decameric ring with all intramolecular interactions intact, as in the uncomplexed NLP (4, 6), whereas each monomeric PCTD interacts with 2 adjacent N monomers (Fig. 1). This is an important feature because this unique binding site for P could be present only in the nucleocapsid. A total of 18 residues from PCTD contribute to the interaction with the dimeric N-binding site. N donates 17 aa to the interaction: 11 and 6 residues from each N of the binding site, respectively. The residues from N are from a contiguous stretch, between 354 and 386, that encompasses helix α13 and the C loop, which rises above the upper surface of the C-terminal lobe. Twenty-four hydrogen bonds are formed between P and the 2 N monomers. The complete list of bonding partners is provided in Table 1. Two residues of P, Arg-260 and Lys-262, which have been implicated previously in N protein association and mutation of these residues, have also been shown to affect transcription (13). These basic residues are bonded to 2 acidic residues, Asp-358 and Glu-377, which are found on different molecules of the N protein-binding site. Interestingly, Tyr-256 and Asn-257 of P are unique, because each interacts with both molecules of N. The total surface area buried by the interactions between the PCTD and the 2 adjacent N molecules is 956 Å2, whereas the total surface area of the PCTD is 5,100 Å2. Thus, ≈19% of the available surface area of PCTD is in complex with the N protein(s).
Fig. 1.
Ribbon representations of the VSV nucleocapsid in complex with the C-terminal N protein-binding domain of the P protein (PCTD). (A) Three assembled N molecules are represented in red, green, and blue. PCTD, shown in yellow, binds to the extended loop (C loop) within the C lobe of 2 adjacent N molecules (red and green). (B) Scaled-up image of the interaction. Residues of the dual N-binding site that are in contact with PCTD are shown. The illustrations found in this and the subsequent figures were generated with PyMOL (41).
Table 1.
The binding interactions between the residues of the PCTD and the residues of the dimeric N protein binding site
| Interaction | P residues | Atoms | N residues (subunits 1 and 2) | Atoms |
|---|---|---|---|---|
| Hydrophobic | LEU214 | Side chain | THR361(1) | Side chain |
| H bond | GLN215 | NE2 | ASP359(1) | OD1 |
| H bond | GLN215 | OE1 | SER360(1) | N |
| Hydrophobic | LEU217 | Side chain | LEU364(1) | Side chain |
| Hydrophobic | ILE219 | Side chain | LEU364(1) | Side chain |
| H bond | SER233 | O | THR365(2) | OG1 |
| H bond | VAL234 | O | LEU364(2) | N |
| Hydrophobic | GLY235 | CA | LEU364(2) | Side chain |
| H bond | ARG251 | NH1, NH2 | THR365(2) | OG |
| H bond | LYS253 | NZ | ASN367(1) | ND2 |
| H bond | Lys254 | NZ | ASP384(1) | OD2 |
| H bond | Lys254 | NZ | ASP384(1) | O |
| H bond | Lys254 | O | ASN386(1) | ND2 |
| Hydrophobic | LEU255 | Side chain | THR366(1) | Side chain |
| H bond | TYR256 | OH | ASP358(2) | O |
| H bond | TYR256 | N | ASP384(1) | OD1 |
| H bond | ASN257 | ND2 | ASP359(2) | OD1 |
| H bond | ASN257 | ND2 | ASP359(2) | OD2 |
| H bond | ASN257 | ND2 | LYS354(2) | NZ |
| H bond | ASN257 | ND2 | GLU383(1) | OE2 |
| H bond | ASN257 | OD1 | ASP359(2) | OD1 |
| H bond | ASN257 | OD1 | ASP359(2) | OD2 |
| H bond | ASN257 | OD1 | SER360(2) | OG |
| H bond | ASN257 | OD1 | ASP384(1) | OD1 |
| H bond | ASN257 | N | ASP384(1) | OD1 |
| H bond | GLN258 | N | ASP384(1) | OD1 |
| H bond | ARG260 | NH1 | ASP358(2) | OD1 |
| Hydrophobic | VAL261 | Side chain | VAL367(1) | Side chain |
| H bond | LYS262 | NZ | GLU377(1) | OE2 |
| H bond | TYR263 | OH | GLY362(1) | N |
The numbers 1 and 2 shown in parentheses adjacent to N protein residue names and numbers refer to molecule 1 or 2, respectively, of the N dimer. The list of residues was compiled with the aid of the Protein Interfaces, Surfaces, and Assemblies (PISA) server (42) and visual inspection with COOT (36).
The conserved hydrophobic core of PCTD was described previously (24). Two adjacent surfaces of the PCTD present interesting conserved hydrophobic cavities. The first hydrophobic cavity is positioned between the β-turn and 2 helices (α3 and α4), and the second is situated between helix α1 and the loop connecting helices α2 and α3. The relevance of each cavity is unknown and has been postulated as a potential binding site for the other VSV proteins. The complex structure between N and PCTD showed that the first cavity is capped by the C loop of the N protein. The loop does not penetrate the cavity, but it does lie over the entrance. The second cavity is distal to the N-binding surface of PCTD and is left exposed. This suggests that these hydrophobic cavities most likely form a hydrophobic core that renders stability to this small domain. However, the second cavity is solvent-accessible and could be available to interact with the domains of P that are missing from this structure, or an alternate viral or host protein.
PCTD Structure Changes.
The topology of the PCTD structure was described in a recent report by Ribeiro et al. (24). Although this domain forms a compact unit, the individual secondary structure elements appear to have some flexibility. It is very likely that PCTD undergoes some conformational changes, which may not be global, upon binding to the nucleocapsid. PCTD in the NLP–PCTD complex is topologically identical to that described in the previous study. The size of the recombinant PCTD used in this study was slightly longer. To show structural changes in PCTD upon N binding, the complexed and uncomplexed PCTD structures were superimposed. A single composite structure was created from the 20 lowest-energy deposited NMR structures with CNS (30). Superposition of the composite structure with the N-bound structure resulted in an overall rmsd of 2.16 Å for all atoms. By contrast, the rmsd between the best representative conformer in the ensemble PCTD structure (as defined in ref. 24) and PCTD structure from this study was 2.06 Å. In either case, each of the secondary structure elements and the overall topology of the protein are maintained upon binding the N protein. There are, however, localized shifts of the secondary structure elements, as illustrated in Fig. 2. These displacements correspond to N-binding regions of P, with the most notable shifts occurring at helix α2 and the loop connecting with helix α3 and the β-turn (Fig. 2).
Fig. 2.
Structural overlay of the VSV PCTD in the N protein-bound state (yellow) and the unbound state (cyan; PDB accession ID: 2k47). Residues of PCTD that make contact with the N protein have been shaded magenta on the N-bound representation. (A) Shown is the structure of the PCTD, with secondary structure elements labeled. (B) The overlay of the 2 structures is shown.
Changes in the N Protein upon P Binding.
Upon P binding, the NLP maintains the intermolecular N interactions described previously (4, 6), with very little discernible difference in their overall structures. The main dissimilarity is in the C loop, as shown in Fig. 3. Because there seem to be structural changes of N side chains in the NLP–PCTD complex, we used the higher-resolution structure of N290 NLP in complex with PCTD to identify any changes in N. N290 is an N protein with a mutation of serine to tryptophan at residue 290 (6). This mutated N has lost the ability to bind RNA. Superposition of the N290 structure with the PCTD-bound structure determined here revealed that the C loop is shifted, with many of the side chains rearranged to accommodate binding of the PCTD (Fig. 3). The remainder of the N290 structure superimposed quite well with the PCTD-bound structure. The 5 monomers overlaid as a single rigid body had an rmsd of 1.117 Å. If the loops (residues 256–369) were removed from the calculation, the rmsd was lowered to a value of 1.075 Å. By comparison, the rmsd for the N RNA structure to its PCTD-bound counterpart was 1.223 Å, or 1.066 Å with the loops removed. Interestingly, in the absence of PCTD, the extended loops were disordered in 3 of the 5 N monomers in the previously determined N RNA structure. In the PCTD-bound structure presented here, all 5 of the loops were observed in the electron density maps and were easily traced. In this case, PCTD promoted ordering of the loop. The inherent flexibility of the loop could aid in snaring P because P delivers the L protein to the nucleocapsid for RNA synthesis.
Fig. 3.
Structural similarity of the N protein in the PCTD-bound and unbound states. Two molecules of the PCTD-bound N290 molecules are colored red and green. Two additional molecules (colored gray) of the previously determined structure N290 (PDB accession ID: 2qvj) were superimposed upon the current complex structure. The main dissimilarity between the structures is observed in the conformation of the C loops (acknowledged with arrows). This corresponds to the site of PCTD binding. The models are rotated 180° with respect to Fig. 1; in this case, looking from the exterior of the protein rather than the RNA cavity face of N.
Discussion
Polynucleotide synthesis is an essential part of the viral replication cycle. The NSRVs have evolved to perform this reaction with their own specialized polymerase proteins. For the nonsegmented NSRVs, including VSV, this requires a concerted set of events involving the N protein-enwrapped genome, the L protein, and the nonenzymatic P. One of the roles of P is to deliver L to the active template. The L protein is unable to bind the template directly but, rather, binds to the P and is then delivered to the active template as P binds to N. The structure presented here of an NLP bound to the PCTD shows that the N protein forms a unique binding site for P that involves 2 adjacent monomers within the nucleocapsid. This binding site is formed by a contiguous stretch of residues (354–386) that includes helix α13 and the extended loop of the C lobe, the C loop. The loop from each N protein snares P by clamping onto it from opposite sides. Such a binding site is exclusive to the assembled nucleocapsid and is in accordance with the fact that the active template for transcription and replication is the N-enwrapped genome. Comparison with the unbound NLP structure shows that N is largely unchanged upon binding P, with the most significant variation occurring in the C loops that bind to PCTD (Fig. 3). Likewise, topologically, P is unchanged upon binding to the nucleocapsid. There is, however, a shift of the secondary structure elements in relation to one another—the 2 most notable movements are helix α2 and the loop connecting to helix α3 and the β-turn (Fig. 2). Each of these elements is involved in N-binding. It is possible that the induced conformational changes in N and P allow the two to be associated more tightly when P binds to the nucleocapsid.
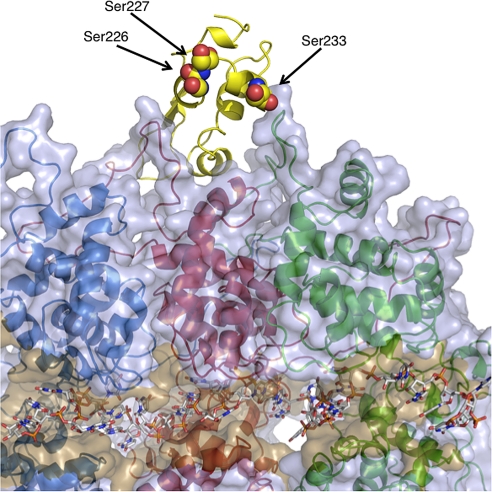
The structure of the NLP shows that the RNA is encapsidated in a cavity located between the 2 lobes of the N protein. During transcription and replication, the L protein must be positioned at the mouth of this cavity to gain access to the genome. The L protein has been shown previously to interact with 2 essential phosphorylation sites (Ser-226 and Ser-227) on P (13). These residues reside in the C-terminal domain of P and are observed in our structure, but they are not phosphorylated. L binding to P is not dependent on their phosphorylation, but phosphorylation of either residue is responsible for regulating levels of replication. Ser-226 and Ser-227 are found on the loop that connects 310-helix 1 and helix α2 (Fig. 4). The loop is positioned such that these residues face the interior of the N protein ring. Neither serine makes contact with the N protein but, rather, each sits exposed ≈50 Å directly above the entrance of the RNA encapsidation cavity and aligned with the interior face of the C lobe of the N protein. The interaction of L with the 2 phosphorylated serine residues would not prevent PCTD in the P–L complex from docking on the nucleocapsid. At the same time, PCTD could bring L in close contact with the RNA, because the 2 serine residues may be viewed as the boundary of contact between L and N. Because of the way that the RNA is sequestered while encapsidated, the N protein must undergo some conformational adjustments in order for L to gain access to the genome. How this occurs is unknown at this point. One likely scenario may involve the N-terminal 22 residues of the N protein. These residues form an arm that extends to the C lobe of a neighboring N within the nucleocapsid and holds the 2 lobes of N in the proper orientation for the formation of the RNA-encapsidation cavity. Previous mutational studies showed that if the N-terminal arm is removed, the ability to bind P remains intact, but the ability to retain RNA is lost (6). Interestingly, the integrity of the assembled nucleocapsid is not completely broken. This feature is important because it dictates that the N-terminal arm can dissociate to expose the RNA locally without affecting the global association of the nucleocapsid. The L protein or the L–P complex could cause this local dissociation, exposing the RNA in the course of polynucleotide synthesis. P binds to the extended loop of the C lobe of N, and the opposite face of the loop is in contact with the N-terminal arm of N. Upon the P–L complex association with the nucleocapsid, it could be possible that the loop–arm interaction is destabilized, causing N to open. Subsequently, after transcription or replication has occurred, the arm is repositioned, and the RNA is again stored in the cavity.
Fig. 4.
Phosphorylation of the P protein. Cartoon models of 3 molecules of the N protein (colored as in Fig. 1) are shown with encapsidated RNA (shown in stick representation). A semitransparent surface covering the 3 molecules of the N protein is shown in a shade of light blue, with the RNA cavity colored a darker shade of orange. The bound PCTD is shown in yellow. Residues that are phosphorylated are shown as space-filling spheres and are labeled accordingly. The perspective is the same as that in Fig. 1.
Here, we propose a model for the process of polynucleotide processivity based on the structure described in this work. For L to encounter the viral genome–nucleocapsid template, the dimeric P is required. This dimer is associated with both L and N. Within the dimer, 1 PCTD associates with the nucleocapsid. To process through the genome, N is forced to open temporarily and expose the RNA as the polymerase passes along. The mechanism for forcing the conformational change resulting in the opening of N is due to a local destabilization event caused by P–L binding. Once the binding occurs, N is opened, exposing the RNA; however, upon exit of the active bubble of polynucleotide synthesis, N returns to the closed state (illustrated in Fig. 5). The PCTD, as shown in the previous figures, binds to the C lobe of 2 adjacent N monomers within the nucleocapsid. The association should be fleeting as the polymerase complex moves along the template. Thus, PCTD is expected to repetitively bind and release the template as the polymerase complex moves down the template. In this scenario, the dimer is always associated with L and intermittently associated with N.
Fig. 5.
Model of the interactions of P and L with the encapsidated template. The N, P, and L proteins are labeled. Polarity of the template and progeny RNA (both shown as a yellow ribbon) are also labeled with 5′ and 3′. The P dimer in the viral polymerase delivers the L subunit to the nucleocapsid template. The nucleocapsid-bound viral polymerase forms an RNA synthesis chamber in which the genomic RNA is temporarily unencapsidated to allow the catalytic region of the polymerase to use it as the template for RNA synthesis. As the viral polymerase moves along the nucleocapsid, the previous unveiled region of the RNA genome is reencapsidated, and the next segment becomes available for RNA synthesis. The P dimer maintains the association of the viral polymerase with the nucleocapsid throughout the process of RNA synthesis. Three successive steps are labeled A, B, and C.
There must be differences between the processes of transcription and replication. For some time, the general thought has been that the difference is the presence of a sufficient quantity of No-P (25–27). As amounts of this encapsidation precursor complex increase, the switch to replication is initiated. However, a different hypothesis was proposed, stating that the presence of No-P promotes the formation of a tripartite replicase that switches to replication (29). Multimerization of P seems to be necessary, because 2 previous studies have shown that both the deletion of residues 191–210 of P or addition of an artificial peptide with this sequence affects multimerization and, subsequently, transcription and replication (16, 31). This region of P is observed in our structure and is not in contact with the N protein. In our model a single C-terminal domain of P is required for recognition of the nucleocapsid. Multimerization of P may be through the central domain region, as shown by our previous structure of the central domain of P, which can form a dimer or tetramer (15). Further structural studies of the complete polymerase complex are required to fully understand the direct interaction of L with the P-bound template.
Methods
Wild-type VSV N/RNA, N (Ser-290→Trp) mutant (N290), and the PCTD residues 183–265 were expressed in Escherichia coli and purified as described previously (refs. 6, 15, and 18, respectively). Purified protein samples of N/RNA, N290, and PCTD [or selenomethionine-substituted PCTD (Se-PCTD)] were concentrated to 11, 9, and 12 mg/mL. N (or N290) was mixed with PCTD (or Se-PCTD) at a 1:1.2 molar ratio. N290/Se-PCTD crystals were grown by the hanging drop vapor diffusion method in 24-well VDX plates (Hampton Research) at 22 °C (32).
Crystals of the N/RNA–PCTD complex were grown by the hanging drop method at 4 °C. Crystallization drops were formed by mixing equal volumes of protein with reservoir solution containing 7% PEG 4000, 250 mM NaCl, and 100 mM citrate buffer, pH 5.6. This crystal form was cryoprotected with reservoir solution containing a final concentration of 20% PEG-4000 (Hampton Research) and 20% glycerol. This crystal form belonged to the orthorhombic space group P21212. Maximal crystal growth occurred within 2 weeks. Data were collected at the Stanford Synchrotron Radiation Lightsource (SSRL) beamline 11-1 at a wavelength of 1.0 Å, with an oscillation angle of 0.3° and crystal-to-detector distance of 450 mm on a Mar 325 CCD (Marresearch) detector at cryotemperature.
The N290–Se-PCTD protein complex was mixed with a 1:1 volume equivalent of reservoir solution consisting of 0.8–1.0 M K/Na tartrate, 200 mM NaCl, and 100 mM imidazole buffer, pH 8.0. Orthorhombic crystals grew within 1 to 2 weeks. Before data collection, crystals were cryoprotected stepwise to a final solution containing reservoir solution plus 20% glycerol and were flash frozen in liquid nitrogen. Data were collected at the South East Regional Collaborative Access Team (SER-CAT) BL22-ID at Advanced Photon Source (APS) at a wavelength of 0.94 Å, with an oscillation angle of 0.3° and crystal-to-detector distance of 450 mm on a MAR 325 CCD (Marresearch) detector at cryotemperature.
In all cases, raw intensity images were processed with the HKL2000 package (33), and structure factors were calculated with TRUNCATE (34) through the CCP4 program suite. Location of the N protein/RNA decamers in the N/RNA–PCTD complex was achieved by molecular replacement with COMO (35) using the previously determined VSV N/RNA structure [Protein Data Bank (PDB) ID code: 2gic] as the search model. An initial model of the PCTD was built with COOT (36) in 2Fo − Fc maps of the orthorhombic data. The N290/Se-PCTD structure was solved by molecular replacement with the previously determined N290 model (PDB ID code: 2qvj). Crude placement of the PCTD domains was performed with the aid of an intermediate VSV N/RNA–PCTD model using the superpositioning protocols in O (37). The asymmetric unit of each crystal of the N/RNA–PCTD or the N290–PCTD complexes contains one-half of the decameric nucleocapsid-like particle bound to 5 PCTD monomers. The stoichiometry of N:P is such that each available N-binding site (10:10) is occupied when averaged over the entire crystal. However, there is a reduced occupancy of the PCTD in certain positions. The highest substitution occurs in alternating rather than adjacent N-binding positions. The lack of complete P substitution is not surprising, because N and P do not exist in a 1:1 ratio in mature virions (38). Rigid body refinement of the individual domains and extensions was carried out with REFMAC5 (39). Manual model building and real-space refinement were performed with COOT. Selenium sites were used to aid in confirmation of the constructed sequences. Restrained and TLS refinement were performed with REFMAC5. Refinement statistics are shown in Table 2. No outliers were found in the Ramachandran plot. All superimpositions for determining the differences between bound and unbound complexes as discussed in the text were carried out by using the secondary structure mapping procedure in COOT (40).
Table 2.
Data collection and refinement statistics
| Complex | N290-Pctd | N/RNA-Pctd |
|---|---|---|
| Space group | P21212 | P21212 |
| Unit cell, Å | ||
| a | 170.6 | 166.5 |
| b | 234.5 | 235.2 |
| c | 95.0 | 96.1 |
| Resolution | 30.0–2.70 | 30.0–3.50 |
| High-resolution bin | 2.80–2.70 | 3.63–3.50 |
| Reflections (unique/total) | 77,814/228,906 | 48,185/316,345 |
| Completeness, % | 73.1 (44.9) | 98.3 (88.4) |
| I/σI | 20.79 (2.10) | 21.48 (4.31) |
| Rmerge | 0.073 (0.419) | 0.116 (0.434) |
| Rcryst | 0.263 | 0.258 |
| Rfree | 0.296 | 0.323 |
| Mean B value, Å2 | 74.58 | 28.09 |
| Model | ||
| No. of atoms | 19,495 | 19,455 |
| No. of residues | 2,460 | 2,460 |
| No. of nucleic acid bases | 0 | 45 |
| rmsd | ||
| Bonds, Å | 0.006 | 0.008 |
| Angles, ° | 0.957 | 1.179 |
| Ramachandran | ||
| Favored, % | 98.6 | 96.0 |
| Allowed, % | 100.0 | 100.0 |
Values in parentheses represent values within high-resolution shells.
Acknowledgments.
We thank the staff of the SER-CAT at the APS, Argonne National Laboratory, for assistance in data collection. We thank the generosity of the staff at the SSRL. Portions of this research were carried out at the SSRL, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. Use of the APS was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract W-31-109-Eng-38. SER-CAT supporting institutions may be found at www.ser-cat.org/members.html. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health (NIH), National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. This work was supported in part by NIH Grant AI050066.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
The atomic coordinates for the N/RNA–PCTD and N290–PCTD complex structures have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID codes 3HHZ and 3HHW, respectively).
References
- 1.Huang AS, Wagner RR. Comparative sedimentation coefficients of RNA extracted from plaque-forming and defective particles of vesicular stomatitis virus. J Mol Biol. 1966;22:381–384. doi: 10.1016/0022-2836(66)90143-4. [DOI] [PubMed] [Google Scholar]
- 2.Emerson SU, Wagner RR. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972;10:297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naito S, Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976;251:4307–4314. [PubMed] [Google Scholar]
- 4.Green TJ, Zhang X, Wertz GW, Luo M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science. 2006;313:357–360. doi: 10.1126/science.1126953. [DOI] [PubMed] [Google Scholar]
- 5.Albertini AA, et al. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science. 2006;313:360–363. doi: 10.1126/science.1125280. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Green TJ, Tsao J, Qiu S, Luo M. Role of intermolecular interactions of vesicular stomatitis virus nucleoprotein in RNA encapsidation. J Virol. 2008;82:674–682. doi: 10.1128/JVI.00935-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietzschold B, et al. Localization and immunological characterization of antigenic domains of the rabies virus internal N and NS proteins. Virus Res. 1987;8:103–125. doi: 10.1016/0168-1702(87)90023-2. [DOI] [PubMed] [Google Scholar]
- 8.Toriumi H, Kawai A. Structural difference recognized by a monoclonal antibody #404–11 between the rabies virus nucleocapsid (NC) produced in virus infected cells and the NC-like structures produced in the nucleoprotein (N) cDNA-transfected cells. Microbiol Immunol. 2005;49:757–770. doi: 10.1111/j.1348-0421.2005.tb03666.x. [DOI] [PubMed] [Google Scholar]
- 9.Barik S, Banerjee AK. Phosphorylation by cellular casein kinase II is essential for transcriptional activity of vesicular stomatitis virus phosphoprotein P. Proc Natl Acad Sci USA. 1992;89:6570–6574. doi: 10.1073/pnas.89.14.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das T, et al. Role of cellular casein kinase II in the function of the phosphoprotein (P) subunit of RNA polymerase of vesicular stomatitis virus. J Biol Chem. 1995;270:24100–24107. doi: 10.1074/jbc.270.41.24100. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Lenard J. Multimerization and transcriptional activation of the phosphoprotein (P) of vesicular stomatitis virus by casein kinase-II. EMBO J. 1995;14:1240–1247. doi: 10.1002/j.1460-2075.1995.tb07107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takacs AM, Barik S, Das T, Banerjee AK. Phosphorylation of specific serine residues within the acidic domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. J Virol. 1992;66:5842–5848. doi: 10.1128/jvi.66.10.5842-5848.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JL, Das T, Banerjee AK. Phosphorylated states of vesicular stomatitis virus P protein in vitro and in vivo. Virology. 1997;228:200–212. doi: 10.1006/viro.1996.8401. [DOI] [PubMed] [Google Scholar]
- 14.Pattnaik AK, et al. Phosphorylation within the amino-terminal acidic domain I of the phosphoprotein of vesicular stomatitis virus is required for transcription but not for replication. J Virol. 1997;71:8167–8175. doi: 10.1128/jvi.71.11.8167-8175.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding HT, Green TJ, Lu S, Luo M. Crystal structure of the oligomerization domain of the phosphoprotein of vesicular stomatitis virus. J Virol. 2005;80:2808–2814. doi: 10.1128/JVI.80.6.2808-2814.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das SC, Pattnaik AK. Role of the hypervariable hinge region of phosphoprotein P of vesicular stomatitis virus in viral RNA synthesis and assembly of infectious virus particles. J Virol. 2005;79:8101–8112. doi: 10.1128/JVI.79.13.8101-8112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, Lenard J. Cooperative binding of multimeric phosphoprotein (P) of vesicular stomatitis virus to polymerase (L) and template: Pathways of assembly. J Virol. 1995;69:7718–7723. doi: 10.1128/jvi.69.12.7718-7723.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green TJ, et al. Study of the assembly of vesicular stomatitis virus N protein: Role of the P protein. J Virol. 2000;74:9515–9524. doi: 10.1128/jvi.74.20.9515-9524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill DS, Chattopadhyay D, Banerjee AK. Identification of a domain within the phosphoprotein of vesicular stomatitis virus that is essential for transcription in vitro. Proc Natl Acad Sci USA. 1986;83:8873–8877. doi: 10.1073/pnas.83.23.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takacs AM, Das T, Banerjee AK. Mapping of interacting domains between the nucleocapsid protein and the phosphoprotein of vesicular stomatitis virus by using a two-hybrid system. Proc Natl Acad Sci USA. 1993;90:10375–10379. doi: 10.1073/pnas.90.21.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emerson SU, Schubert M. Location of the binding domains for the RNA polymerase L and the ribonucleocapsid template within different halves of the NS phosphoprotein of vesicular stomatitis virus. Proc Natl Acad Sci USA. 1987;84:5655–5659. doi: 10.1073/pnas.84.16.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das T, et al. Basic amino acid residues at the carboxy-terminal eleven amino acid region of the phosphoprotein (P) are required for transcription but not for replication of vesicular stomatitis virus genome RNA. Virology. 1997;238:103–114. doi: 10.1006/viro.1997.8823. [DOI] [PubMed] [Google Scholar]
- 23.Paul PR, Chattopadhyay D, Banerjee AK. The functional domains of the phosphoprotein (NS) of vesicular stomatitis virus (Indiana serotype) Virology. 1988;166:350–357. doi: 10.1016/0042-6822(88)90505-3. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro EA, Jr, et al. Solution structure of the C-terminal nucleoprotein-RNA binding domain of the vesicular stomatitis virus phosphoprotein. J Mol Biol. 2008;382:525–538. doi: 10.1016/j.jmb.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Masters PS, Banerjee AK. Complex formation with vesicular stomatitis virus phosphoprotein NS prevents binding of nucleocapsid protein N to nonspecific RNA. J Virol. 1988;62:2658–2664. doi: 10.1128/jvi.62.8.2658-2664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peluso RW. Kinetic, quantitative, and functional analysis of multiple forms of the vesicular stomatitis virus nucleocapsid protein in infected cells. J Virol. 1988;62:2799–2807. doi: 10.1128/jvi.62.8.2799-2807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peluso RW, Moyer SA. Viral proteins required for the in vitro replication of vesicular stomatitis virus defective interfering particle genome RNA. Virology. 1988;162:369–376. doi: 10.1016/0042-6822(88)90477-1. [DOI] [PubMed] [Google Scholar]
- 28.Emerson SU, Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975;15:1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta AK, Shaji D, Banerjee AK. Identification of a novel tripartite complex involved in replication of vesicular stomatitis virus genome RNA. J Virol. 2003;77:732–738. doi: 10.1128/JVI.77.1.732-738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 31.Chen M, Ogino T, Banerjee AK. Mapping and functional role of the self-association domain of vesicular stomatitis virus phosphoprotein. J Virol. 2006;80:9511–9518. doi: 10.1128/JVI.01035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mcpherson A. Preparation and Analysis of Protein Crystals. New York: John Wiley; 1982. [Google Scholar]
- 33.Otwinowski Z, Minor W. Processsing of x-ray diffraction data collected in oscillation mode. In: Carter CW, Sweet RM, editors. Methods in Enzymology. Vol 276. New York: Academic; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 34.French S, Wilson K. On the treatment of negative intensity observations. Acta Crystallogr A. 1978;34:517–525. [Google Scholar]
- 35.Jogl G, Tao X, Xu Y, Tong L. COMO: A program for combined molecular replacement. Acta Crystallogr D Biol Crystallogr. 2001;57:1127–1134. doi: 10.1107/s0907444901006783. [DOI] [PubMed] [Google Scholar]
- 36.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 37.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 38.Thomas D, et al. Mass and molecular composition of vesicular stomatitis virus: A scanning transmission electron microscopy analysis. J Virol. 1985;54:598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 40.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 41.DeLano WL. The PyMOL User's Manual. San Carlos, CA: Delano Scientific; 2002. [Google Scholar]
- 42.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]