Abstract
Suppression by natural CD4+CD25+ regulatory T cells (Tregs) is one mechanism by which tolerance is maintained. However, the way in which Tregs mediate suppression is not well understood. Here, we show that secreted phospholipase A2 (sPLA2)-IID is selectively produced by Tregs. sPLA2-IID is a potent mediator of Treg function, because it strongly suppressed proliferation of CD4+ and CD8+ T cells in vitro and in vivo in a manner independent of its catalytic activity. Furthermore, sPLA2-IID promoted the differentiation of Tregs, presumably via attenuating signaling through the PI3K/Akt/mammalian target of rapamycin pathway. Importantly, administration of a sPLA2-IID-Fc fusion protein inhibited disease development in murine models of colitis and multiple sclerosis, suggesting that sPLA2-IID's immunosuppressive function might be exploited therapeutically.
Keywords: differential screening, suppression, tolerance
The balance between protective immunity and autoimmune disease and immunopathology is tightly controlled by regulatory T cells (Tregs). Depleting Tregs spontaneously leads to various autoimmune diseases (1, 2), clearly demonstrating their crucial importance in keeping the immune system under control. Natural CD4+CD25+Foxp3+ Tregs are continuously produced in the thymus, but additional populations of antigen-specific Tregs with similar suppressive function can be induced from naive CD4+ T cells in the periphery (3).
The transcription factor forkhead box P3 protein (Foxp3) acts as the master regulator in the development and function of natural Tregs (4). Notably, ectopic expression of Foxp3 or TGF-β-mediated induction of Foxp3 in naive CD4+ T cells phenotypically and functionally converts them to Tregs (5, 6). Foxp3 represses IL-2 production and up-regulates the expression of the IL-2 receptor (CD25) as well as other Treg-associated molecules such as cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR) (4, 5). The lack of IL-2 secretion renders Tregs highly dependent on exogenous IL-2 for their survival in the periphery (7). Accordingly, IL-2- and CD25-deficient mice show reduced numbers of CD4+CD25+Foxp3+ Tregs and develop severe lymphoproliferative disease (8).
Tregs suppress the activation and proliferation of many different cell types; however, the exact mechanism of immune regulation is still incompletely understood. In vitro studies suggest that natural CD4+CD25+Foxp3+ Tregs suppress T cell activation in a cell–cell contact-dependent manner, because suppression is inhibited when Tregs and responder T cells are separated by a semipermeable membrane (9). Notably, the cell surface molecules CTLA-4 and GITR contribute to this contact dependency by interacting with CD80 and CD86 molecules expressed by antigen-presenting cells (10, 11). In contrast, inducible Tregs appear to function independently of cell–cell contact and block immune responses through the secretion of immunosuppressive cytokines such as IL-10 and TGF-β (12, 13). There is accumulating evidence that in vivo suppression by natural Tregs also involves the secretion of soluble factors, such as IL-10 and TGF-β (14).
We reasoned that additional soluble mediators of Treg function might exist and carried out a differential screening to identify such proteins. Here, we show that secreted phospholipase A2 (sPLA2)-IID is selectively produced by natural Tregs and acts as a potent suppressor of T cell activation both in vitro and in vivo.
Results
Identification of sPLA2-IID as a Secreted Protein Selectively Released by Tregs.
Screening for genes specifically expressed in Tregs was carried out by suppression subtractive hybridization (SSH) (15). Double-stranded cDNA (ds-cDNA) synthesized from RNA isolated from CD4+CD25+ Tregs was used as the tester, whereas a mixture containing 50% naive CD4+CD25− T cell ds-cDNA and 50% in vitro-activated CD4+CD25+ T cell ds-cDNAs served as the driver. The use of this subtraction strategy allowed for enrichment of genes that are highly expressed in Tregs but not in naive or effector T cells. A library of cDNA fragments was produced and 240 clones were subjected to DNA sequencing. One cDNA fragment contained a 167-bp sequence segment, which in a BLAST search matched with a mRNA encoding the sPLA2-IID (accession no. NM_011109), which is a particular isoform of the family of sPLA2 proteins. Translation of this cDNA yields a secreted protein of 144 aa containing an N-terminal signal peptide (amino acids 1–19), 7 disulfide bonds, and a putative N-glycosylation site as described in refs. 16–18.
Expression Profile of sPLA2-IID.
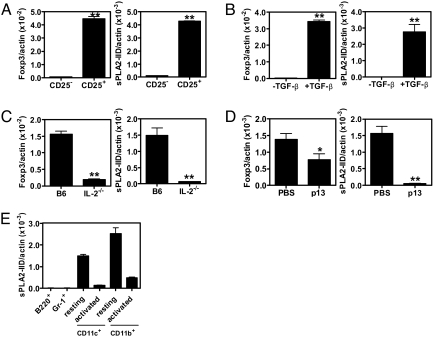
Selective expression of sPLA2-IID by Tregs was validated by analyzing various cell populations for differential gene expression by quantitative real-time PCR. First, the expression level of sPLA2-IID in freshly isolated CD4+CD25+ and CD4+CD25− T cells was compared. In accordance with a possible role of sPLA2-IID in Tregs, a 40-fold higher expression of sPLA2-IID was observed in the regulatory compared with the nonregulatory T cell population, corresponding well with the >80-fold higher expression of Foxp3 (Fig. 1A). Furthermore, resting and activated Tregs stained positive with a sPLA2-IID-specific antibody compared with a control antibody (Fig. S1).
Fig. 1.
Analysis of sPLA2-IID and Foxp3 expression by quantitative real-time PCR. (A) Comparison of expression levels in freshly isolated naive CD4+CD25− T cells (CD25−) and CD4+CD25+ Tregs (CD25+). (B) Expression levels in purified CD4+CD25− T cells stimulated with plate-bound anti-CD3 (coated at 2 μg/mL) and 1 μg/mL soluble anti-CD28 in the presence or absence of 20 ng/mL human TGF-β. (C) CD4+ T cells from B6 mice were compared with CD4+ T cells from IL-2−/− mice. (D) CD4+ T cells from naive and immunized (p13, 50 μg, i.v.) SMARTA-2 mice were compared. (E) sPLA2-IID expression in B220+, Gr-1+, resting and activated (overnight with LPS) CD11c+ and CD11b+cells. (A–E) Representative data are presented as mean expression level normalized to β-actin. Error bars represent SD of 3 reactions. *, P < 0.05; **, P < 0.005. Data are representative of 3 independent experiments.
Next, we sought to induce de novo sPLA2-IID expression in Tregs that had differentiated in vitro. Thus, freshly isolated CD4+CD25− T cells were stimulated with soluble anti-CD28 and plate-bound anti-CD3 in the presence of TGF-β as described in ref. 6. The addition of TGF-β led to the differentiation of Tregs, as shown by massive induction of Foxp3 expression. Significantly, differentiation of Tregs was associated with a >1,200-fold increase in sPLA2-IID expression (Fig. 1B).
To verify the expression of sPLA2-IID by Tregs in vivo, its expression in total CD4+ T cells from C57BL/6 mice was compared with that from IL-2−/− mice. The latter are known to have dramatically reduced numbers of Tregs (8), and we therefore expected them to express lower levels of sPLA2-IID. Indeed, CD4+ T cells isolated from IL-2−/− mice not only showed 8-fold lower Foxp3 expression levels than their wild-type (wt) counterparts but also expressed 23 times less sPLA2-IID (Fig. 1C).
To exclude the possibility that sPLA2-IID is expressed in effector CD4+ T cells, which share the CD25+ phenotype with Tregs, its expression in CD4+ T cells isolated from naive SMARTA-2 mice expressing a T cell antigen receptor (TCR) specific for the H-2 I-Ab-restricted peptide p13 (19) was compared with that in CD4+ T cells from p13-injected SMARTA-2 mice. Importantly, total CD4+ T cells from p13-treated mice expressed >30-fold lower levels of sPLA2-IID than CD4+ T cells from naive SMARTA-2 mice, clearly demonstrating that sPLA2-IID expression is not associated with a classical effector T cell phenotype (Fig. 1D).
In accordance with a possible role as a Treg effector molecule, sPLA2-IID is predominantly expressed in spleen and lymph node (LN) with little or no expression in other tissues (16, 18). We next investigated expression of sPLA2-IID in different cell types. Only trace amounts of sPLA2-IID were detected in nonregulatory T cells (Fig. 1 A–D). To investigate a possible role in non-T cells, sPLA2-IID expression was also analyzed in B220+, Gr-1+, CD11c+, and CD11b+ cells. Although no expression was detectable in B220+ and Gr-1+ cells, significant sPLA2-IID expression levels were found in freshly isolated splenic CD11c+ and CD11b+ cells expressing low levels of CD80 and CD40. However, sPLA2-IID expression by these cells seemed to be limited to the resting state. Indeed, overnight activation in the presence of LPS was associated with a strong down-regulation of sPLA2-IID expression (Fig. 1E).
sPLA2-IID Inhibits T Cell Proliferation in Vitro and in Vivo.
Tregs are defined as T cells with a suppressive activity on immune responses. We hypothesized that, if sPLA2-IID functions as a Treg effector molecule, it may exhibit a suppressive activity on immune cells. To test whether sPLA2-IID had an effect on T cells, an in vitro proliferation assay was performed. Purified CD4+ and CD8+ T cells were stimulated by plate-bound anti-CD3 antibody in the presence of coated sPLA2-IID-Fc fusion protein or Fc alone. We used a mutated human Fc domain, which is a weak binder of Fc γ receptors and not able to mediate antibody-dependent cellular cytotoxicity (20). Anti-CD3 stimulation led to a strong proliferative response by CD4+ and CD8+ T cells. Significantly, immobilized sPLA2-IID-Fc fusion protein potently inhibited CD4+ and CD8+ T cell proliferation in a dose-dependent manner (Fig. 2A), showing that sPLA2-IID has a suppressive activity. Inhibition of proliferation was not dependent on the presence of an Fc domain, because a variant carrying a FLAG-tag was equally effective (Fig. S2). Of note, cell proliferation of established cell lines was not affected by sPLA2-IID, indicating that sPLA2-IID does not act nonspecifically on cell proliferation (Fig. S3).
Fig. 2.
Inhibition of T cell proliferation by sPLA2-IID. (A) In vitro T cell proliferation assay. Purified CD4+ and CD8+ T cells were stimulated for 3 days in the absence or presence of plate-bound anti-CD3 antibody as indicated and increasing concentrations of plate-bound sPLA2-IID-Fc or Fc (coated as indicated). After 48 h, cultures were pulsed with [3H]thymidine for 12 h and analyzed for the incorporated radioactivity. (B) In vivo T cell proliferation assay. C57BL/6 recipients were injected with 5 × 106 CFSE-labeled SMARTA-2 CD4+ T cells and immunized with p13-Qβ 1 day later. Mice were subjected to daily injections of 50 μg of sPLA2-IID-Fc or Fc starting on the day of transfer. Flow cytometric analysis of adoptively transferred T cells from the draining LN was performed 2 and 5 days after immunization, by gating on CD4+ T cells. One representative mouse is shown. (C) In vitro proliferation of CD4+ T cells in the presence of plate-bound proteins as indicated (coated at 10 μg/mL each). IID, sPLA2-IID-Fc; IID-H66A, sPLA2-IID-H66A-Fc; IIA, sPLA2-IIA-Fc. (D) In vitro proliferation of CD4+ T cells in the absence or presence of 10 μg/mL soluble (s), coated (c), or cross-linked (x) sPLA2-IID-Fc or Fc. (A, C, and D) Representative data are presented as mean cpm ± SD of 2 cultures. *, P < 0.05; **, P < 0.005 compared with corresponding Fc control. Data are representative of 3 independent experiments.
Given the potent inhibitory effect on in vitro proliferation, we tested whether sPLA2-IID was also able to inhibit T cell proliferation in vivo. We assessed this question by using a TCR transgenic mouse model. CD4+ T cells were isolated from naive TCR-transgenic SMARTA-2 mice (19). After labeling with carboxyfluorescein diacetate succinimidyl ester (CFSE), cells were adoptively transferred into C57BL/6 recipients, which were then injected with p13 peptide coupled to the virus-like particle Qβ 1 day later (21). To investigate a possible antiproliferative effect, mice were treated with daily injections of sPLA2-IID-Fc or control Fc starting on the day of the adoptive transfer. Cells from draining LN were isolated 2 and 5 days after immunization, stained for CD4+ expression, and in vivo proliferation was analyzed by CFSE dilution analysis (Fig. 2B). Consistent with the in vitro data (Fig. 2A), sPLA2-IID-Fc treatment reduced the T cell proliferation in vivo.
Whereas many of the biological effects of sPLA2 proteins are critically dependent on their catalytic activity, there is accumulating evidence that certain effects are independent of it (22). To investigate whether the catalytic activity of sPLA2-IID is required to mediate inhibition of T cell proliferation, we prepared a mutated variant of sPLA2-IID in which the catalytic histidine is exchanged by an alanine (H66A). When tested in an in vitro proliferation assay, the mutated protein was as effective as the wt protein in inhibiting proliferation, demonstrating that catalytic activity is not required (Fig. 2C). To verify that inhibition of T cell proliferation is not a property of all sPLA2 isoforms but specific to sPLA2-IID, we tested an additional member of this protein family. Thus, sPLA2-IIA was cloned (23), recombinantly produced as an Fc fusion protein, and tested for its ability to suppress CD4+ T cell proliferation. Notably, sPLA2-IIA had little effect on T cell proliferation compared with sPLA2-IID (30% vs. 93% inhibition), demonstrating that the potent inhibitory activity is indeed a property specific to sPLA2-IID (Fig. 2C).
We investigated the mechanism of action in more detail by testing the effect of soluble and antibody-cross-linked sPLA2-IID-Fc on T cell proliferation. Similar to coated protein, antibody-cross-linked sPLA2-IID-Fc potently inhibited CD4+ T cell proliferation (Fig. 2D). In contrast, soluble sPLA2-IID-Fc had no detectable inhibitory activity. Thus, it appears likely that sPLA2-IID exerts its effect on T cells by binding and cross-linking of a cell surface receptor.
It is noteworthy that, similar to other sPLA2 proteins including sPLA2-IIA, sPLA2-IID is highly cationic and binds strongly to heparan sulfate proteoglycans (HPSGs) (16). Indeed, our recombinantly produced sPLA2-IID-Fc fusion protein bound to numerous cell types, and this binding could be blocked by heparin (Fig. S4). Thus, in vivo, sPLA2-IID is not expected to be in a soluble form but bound to HPSGs on the cell surface. Hence, as observed for coated sPLA2-IID in vitro, sPLA2-IID is also expected to be able to cross-link a putative receptor in vivo.
sPLA2-IID Interferes with T Cell Activation and Induces Foxp3+ T Cells.
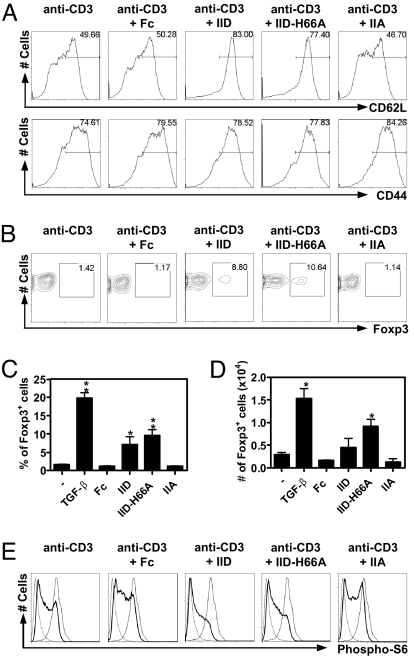
Given its inhibitory effect on T cell proliferation, we next investigated whether sPLA2-IID interferes with T cell activation. Resting T cells are known to constitutively express CD62L, which is essential for LN homing (24). Activation leads to down-regulation of CD62L and the concomitant up-regulation of the activation marker CD44. To assess the effect of sPLA2-IID on T cell activation, we stimulated CD4+ T cells for 3 days with anti-CD3 in the presence of sPLA2-IID-Fc, sPLA2-IID-H66A-Fc, sPLA2-IIA-Fc, or Fc. Anti-CD3 stimulation in the presence of Fc or sPLA2-IIA-Fc led to strong activation of CD4+ T cells, as shown by down-regulation of CD62L and up-regulation of CD44 (Fig. 3A). In marked contrast, cells treated with the wt or mutated sPLA2-IID-Fc protein were unable to down-regulate CD62L on anti-CD3 stimulation (Fig. 3A). However, they were able to up-regulate CD44, indicating that sPLA2-IID allows them to become partially activated (Fig. 3A).
Fig. 3.
Effect of sPLA2-IID treatment on CD4+ T cell activation and Foxp3 expression. (A) Cell surface expression of CD62L and CD44. CD4+ T cells were stimulated with anti-CD3 antibody (coated at 1 μg/mL) alone or together with the indicated proteins (coated at 10 μg/mL) and analyzed by flow cytometry for CD62L and CD44 expression, gating on living CD4+ T cells. (B) Analysis of Foxp3 expression. Sorted CD4+CD25− T cells were stimulated in the presence of 1 μg/mL soluble anti-CD28 antibody, immobilized anti-CD3 antibody (coated at 1 μg/mL), and the indicated proteins (coated at 50 μg/mL). (C and D) Data from B is presented as percentage (C) and absolute numbers (D) of Foxp3+ cells ± SD of 3 cultures. TGF-β, treated with soluble TGF-β at 20 ng/mL. *, P < 0.05; **, P < 0.005 compared with Fc. (E) Phosphorylation status of ribosomal protein S6. Splenocytes were stimulated with anti-CD3 (coated at 10 μg/mL) in the presence of the indicated proteins (—, coated at 100 μg/mL). Cells were analyzed for phospho-S6 status by flow cytometry, gating on T cells (CD90.2+). ···, Rapamycin treated; —, PDBu-treated. (A–E) Anti-CD3, anti-CD3 stimulated cells; IID, sPLA2-IID-Fc; IID-H66A, sPLA2-IID-H66A-Fc; IIA, sPLA2-IIA-Fc. The analysis was repeated twice with a similar result.
Low-intensity activation or immunosuppressive cytokines such as TGF-β favor the development of Tregs from naive T cells (8). Thus, we next tested whether sPLA2-IID can promote induction of CD4+CD25+Foxp3+ cells. Therefore, sorted CD4+CD25− cells were stimulated as above and analyzed for Foxp3 expression. Stimulation of cells in the presence of wt or mutated sPLA2-IID-Fc resulted in 5-fold higher frequencies of Foxp3+ cells compared with Fc (Fig. 3 B and C). Furthermore, absolute numbers of CD4+Foxp3+ cells were also increased (Fig. 3D), indicating that sPLA2-IID promotes the differentiation of Foxp3+ cells. We found similar percentages and frequencies of Foxp3+ cells when using CD4+GFP− T cells isolated from Foxp3-GFP mice (Fig. S5), indicating that these cells are indeed induced de novo and not present because of expansion of CD4+CD25−Foxp3+ cells. Hence, sPLA2-IID is a protein secreted by Tregs that specifically favors the Treg phenotype.
Sustained activation of the PI3K/Akt/mammalian target of rapamycin (mTOR) network has been shown to block Treg differentiation and to down-regulate CD62L (25–27). Given the lack of CD62L down-regulation in sPLA2-IID-treated cells, we investigated whether a blockade of the PI3K/Akt/mTOR pathway might be involved. As an indirect readout of the PI3K/Akt/mTOR pathway, the phosphorylation status of the ribosomal protein S6, the target of PI3K- and mTOR-regulated p70S6 kinase, was investigated. Splenocytes were stimulated with anti-CD3 in the presence of sPLA2-IID-Fc, sPLA2-IID-H66A-Fc, sPLA2-IIA-Fc, or Fc, and after 30 min, the phosphorylation status of S6 was analyzed. High levels of S6 phosphorylation were observed in a large fraction of Fc- and sPLA2-IIA-Fc-treated cells, whereas only modest S6 phosphorylation was observed in cells stimulated in the presence of wt or mutated sPLA2-IID-Fc (Fig. 3E). This provides further evidence that sPLA2-IID inhibits signaling pathways downstream of PI3K/Akt/mTOR and relates sPLA2-IID signaling to Treg differentiation.
Therapeutic Efficacy in Preclinical Disease Models.
There is evidence suggesting that IL-10 and TGF-β are involved in Treg-mediated control of chronic colitis and experimental autoimmune encephalomyelitis (EAE) (28) and to suppress CD4+ T cell proliferation (29, 30). Thus, we investigated whether sPLA2-IID might also be able to negatively regulate development of these diseases.
We induced chronic colitis by the transfer of naive CD4+CD25−CD45RBhigh T cells into RAG1−/− mice. Mice were subjected to biweekly injections with sPLA2-IID-H66A-Fc fusion protein or Fc, starting 1 day before the transfer. After 35 days, the colons of Fc-treated mice were enlarged (Fig. 4A) and tissue sections were characterized by epithelial hyperplasia, inflammatory infiltrates, erosion, and goblet cell depletion (Fig. 4 B and C). In striking contrast, sPLA2-IID-H66A-Fc-treated mice displayed no macroscopic or histological evidence of intestinal inflammation (Fig. 4 A–C).
Fig. 4.
sPLA2-IID protects from colitis. RAG1−/− mice were injected with 4 × 105 CD4+CD25−CD45RBhigh T cells to induce colitis and treated biweekly with sPLA2-IID-H66A-Fc (IID-H66A) or Fc. Mice were killed at day 35 postinjection and analyzed. (A) A representative macroscopic image of the colon is shown. (B) A representative histological section (H&E staining) of the colon is displayed. (Magnification, 10× and 20×.) (C) Score of colons (average of proximal, middle, and distal parts) were analyzed for clinical signs as indicated. (D) Mesenteric LN cells were stained for TNF-α, IFN-γ, IL-17, and Foxp3 expression and analyzed by gating on CD4+ T cells. n = 4 mice per group. ***, P < 0.005; **, P < 0.001; *, P < 0.05. Error bars represent SD. Data are representative of 3 independent experiments.
A hallmark of colitis pathogenesis is the production of inflammatory cytokines by the donor lymphocytes (31). Indeed, on day 35 after disease induction, a high percentage of CD4+ T cells from control animals were positive for TNF-α, IFN-γ, and IL-17 on ex vivo stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Fig. 4D). Significantly, the fraction of cells positive for these cytokines was dramatically reduced when CD4+ T cells were isolated from sPLA2-IID-H66A-Fc-treated mice (Fig. 4D). Furthermore, in line with the reduced inflammation in sPLA2-IID-H66A-Fc-treated mice, there was a 3-fold higher percentage of Tregs compared with Fc-treated mice, an observation that is compatible with a role of Tregs in controlling chronic colitis (Fig. 4D Right). This demonstrates that treatment with sPLA2-IID reduces the development of Th17 cells, whereas Treg induction is promoted.
We next assessed whether sPLA2-IID is also able to reduce the symptoms of EAE. Notably, sPLA2-IID-H66A-Fc-treated mice developed EAE of milder severity at the peak of disease, consistent with lower clinical scores, reduced weight loss, and little sign of inflammation (Fig. S6).
Taken together, our data demonstrate that sPLA2-IID is able to interfere with the development of autoimmune and inflammatory diseases, pointing to a potential use for the treatment of human disorders.
Discussion
We describe here the identification of sPLA2-IID as an effector protein selectively secreted by CD4+CD25+Foxp3+ Tregs. We show that sPLA2-IID inhibits the in vitro and in vivo proliferation of T cells, through a mechanism independent of its catalytic activity, possibly involving binding of a specific surface receptor. sPLA2-IID also promoted differentiation to Tregs correlating with inhibition of PI3K/Akt/mTOR signaling. A therapeutic potential of sPLA2-IID was revealed by its potent inhibitory activity in 2 preclinical disease models.
We show here expression of sPLA2-IID in Foxp3+ Tregs, which is consistent with previous reports showing prominent expression of the molecule in the spleen and in LN (16–18, 32). However, in addition to its expression in Tregs, we also found sPLA2-IID expression in resting dendritic cells (DCs) and macrophages. This is consistent with a broader immune modulating function of sPLA2-IID, because resting DCs and tissue macrophages are known to express immunosuppressive molecules and to mediate peripheral T cell tolerance rather than activation (33).
The primary function of secretory and nonsecretory PLA2s is the cleavage of glycerophospholipids at the sn-2 position, into free fatty acid and lysophospholipid (34). In mammalian cells, this activity is critical for the release of arachidonic acid from membrane phospholipids, the precursor for the synthesis of prostaglandins, thromboxane, and leukotrienes (35). However, some biological functions of sPLA2s have been shown to be independent of enzymatic activity and the generation of arachidonic acid (22). In accordance with this, we found that an enzymatically inactive variant of sPLA2-IID still potently inhibited T cell activation and proliferation in vitro and in vivo. It seems likely that binding to a cell surface receptor is involved, because only sPLA2-IID immobilized on plastic or cross-linked by an antibody displayed inhibitory activity on in vitro proliferation of T cells. Strikingly, a different member of the sPLA2 family, sPLA2-IIA, did not interfere with T cell activation or proliferation. sPLA2-IIA is highly homologous to sPLA2-IID (48% identity), sharing an identical distribution of 14 cysteine residues and HPSG binding (16), supporting the notion that PLA2 catalytic activity per se is not responsible for the observed effects. A 180-kDa lectin dubbed the M-type receptor is now recognized to be a high-affinity receptor for many sPLA2 isoforms (36). Notably, the M-type receptor binds sPLA2-IIA with high affinity, whereas it does not bind sPLA2-IID (37). Thus, it seems likely that sPLA2-IID exerts its effect on T cells via a yet-to-be identified cell surface receptor. However, our attempts at identifying this receptor, hampered by sPLA2-IID's strong binding to HSPGs (16), have thus far not been successful.
Our data show that sPLA2-IID not only strongly blocks T cell activation in vitro and in vivo but also promotes differentiation of Tregs, possibly via blockage of the PI3K/Akt/mTOR network. This is in line with recent reports that propose that Foxp3 expression is regulated by the PI3K/Akt/mTOR pathway (25–27). Hence sPLA2-IID is a protein secreted by Tregs that specifically promotes the generation of Tregs, leading to a positive-feedback loop that may be important under physiological conditions to effectively counterbalance a disease-promoting proinflammatory milieu. In this respect, sPLA2-IID may be similar to other antiinflammatory molecules, such as IL-10 and TGF-β, which are known to foster Treg generation. Furthermore, in our in vivo model of colitis, we have seen reduced presence of inflammatory Th17 cells. Whether this is a direct effect of sPLA2-IID or is mediated indirectly via the induction of Tregs remains to be determined.
In conclusion, we have identified sPLA2-IID as a potent immunosuppressive protein. sPLA2-IID blocks T cell activation in vitro and in vivo and promotes differentiation of Tregs in vitro, presumably creating a positive-feedback loop. This identifies sPLA2-IID as a potential candidate for therapeutic intervention for the treatment of inflammatory and autoimmune conditions.
Materials and Methods
SSH.
For the isolation of CD4+CD25+ Tregs, CD4+ T cells isolated from spleen and LN of C57BL/6 mice were purified by sorting on a FACSVantage (Becton Dickinson). As a control, CD4+CD25− naive T cells were also sorted. Half of these cells were used immediately, the other half were activated overnight in the presence of 500 ng/mL ionomycin and 50 ng/mL PMA (Sigma). Total RNA was isolated by using TRI reagent (Molecular Research Center). ds-cDNA was synthesized from each of the RNAs by using the SMART PCR cDNA synthesis kit (Clontech). Enrichment of genes specifically expressed in Tregs was carried out by SSH with the PCR Select kit (Clontech). The resulting cDNA fragments were cloned into pCR II-TOPO and subjected to DNA sequencing.
Quantitative Real-Time PCR.
Synthesis of single-stranded cDNA (ss-cDNA) from total RNA was done by using random nonamers (Microsynth) and SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's protocol. Template RNA was digested with 2 units of RNase H (New England BioLabs) at 37 °C for 20 min. The ss-cDNA was then used as template for quantitative real-time PCR (iCycler; Bio-Rad) with the gene-specific primers β-actin-F (5′-CCC TGA AGT AC CCC ATT GAA C-3′), β-actin-B (5′-CTT TTC ACG GTT GGC CTT AG-3′), Foxp3-F (5′-AGT GGC CTG GTT GTG AGA AG-3′), Foxp3-B (5′-GGA AAA GGA GAA GCT GGG AG-3′), sPLA2-IID-F (5′-GGA GTC CCC TAG AAC CAA GC-3′), and sPLA2-IID-B (5′-CCG GAG CCT GAG CTA TTA TG-3′), by using the Brilliant SYBR Green QPCR Master Mix (Stratagene) according to the manufacturer's protocol.
Production of Mouse sPLA2-IID-Fc, sPLA2-IID-H66A-Fc, and sPLA2-IIA-Fc Fusion Proteins.
wt and H66A variants of mouse sPLA2-IID (NP_035239), as well as mouse sPLA2-IIA (NP_001076000), were expressed in HEK-293T cells as Fc-fusion proteins. A C-terminal mutated human Fc γ1 domain was generated by exchanging 4 aa in the CH2 domain (L234A, L235E, G238A, and P331S), which are the critical residues for interaction with Fc γ receptors (20). Fc-fusion proteins were then purified by protein G-Sepharose affinity chromatography.
Proliferation Assays.
Magnetic-activated cell sorting (MACS)-purified CD4+ and CD8+ T cells (2 × 105 cells per well) were cultured in complete Iscove's modified Dulbecco's medium (Lonza). Wells were coated with 0.5–1 μg/mL anti-CD3 antibody (2C11; BD Biosciences) and 1–100 μg/mL indicated protein overnight at 4 °C. Cross-linking of sPLA2-IID-Fc was done by adding goat anti-human IgG (Jackson ImmunoResearch Laboratories) in a 4-fold molar excess over soluble sPLA2-IID-Fc. After 48 h of culture, cells were pulsed with 1 μCi of [3H]thymidine (Hartmann Analytic) for 6–12 h and then analyzed on a beta counter (1450 MicroBeta Trilux; PerkinElmer Wallace). Alternatively, cells were analyzed by flow cytometry.
CFSE Labeling and Adoptive Transfer.
MACS-purified SMARTA-2 CD4+ T cells were labeled in a 0.5 μM CFSE solution (Molecular Probes) and incubated for 7 min at 37 °C. A total of 5 × 106 labeled CD4+ T cells were injected i.v. into C57BL/6 recipients. After 16 h, recipients were vaccinated s.c. with 50 μg of p13 peptide (GLNGPDIYKGVYQFKSVE) coupled to Qβ (21). Animals were subjected to daily i.v. injections of 50 μg of sPLA2-IID-Fc or Fc.
T Cell Reconstitution.
Colitis was induced in RAG1−/− mice by the adoptive transfer of 4 × 105 CD4+CD25+CD45RBhigh T cells as described in ref. 28. Mice were injected i.v. with 50 μg of sPLA2-IID-H66A-Fc or Fc. Mesenteric LN and colons were removed, fixed with 10% paraformaldehyde (Sigma), and analyzed by H&E staining.
Flow Cytometry.
Cells were then stained with the following antibodies obtained from BD Biosciences: allophycocyanin (APC) CD4 (LT34), PerCP CD4 (RM4-5), FITC CD62L (MEL-14), phycoerythrin (PE) CD44 (IM7), FITC CD25 (3C7), PE IFN-γ (XMG1.2), Alexa 647 IL-17 (TC11-18H10), and FITC TNF-α (MP6-XT22). APC Foxp3 (FJK-16s) was purchased from eBioscience. Analysis of phosphorylation of S6 was done as in ref. 38. As a negative control, cells were treated with 20 nM rapamycin (Sigma) and as a positive control with 4 nM phorbol dibutyrate (PDBu) (Sigma).
Supplementary Material
Acknowledgments.
We thank Prof. M. Kopf and Prof. N. Harris for helpful discussions. This work was supported by funding under the Sixth Research Framework Program of the European Union, Project MUGEN (MUGEN LSHG-CT-2005-005203).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812569106/DCSupplemental.
References
- 1.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 3.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 6.Chen W, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 8.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 9.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 11.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: Suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 14.Miyara M, Sakaguchi S. Natural regulatory T cells: Mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Diatchenko L, et al. Suppression subtractive hybridization: A method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valentin E, et al. Cloning and recombinant expression of a novel mouse-secreted phospholipase A2. J Biol Chem. 1999;274:19152–19160. doi: 10.1074/jbc.274.27.19152. [DOI] [PubMed] [Google Scholar]
- 17.Ishizaki J, et al. Cloning and characterization of novel mouse and human secretory phospholipase A2s. J Biol Chem. 1999;274:24973–24979. doi: 10.1074/jbc.274.35.24973. [DOI] [PubMed] [Google Scholar]
- 18.Shakhov AN, Rubtsov AV, Lyakhov IG, Tumanov AV, Nedospasov SA. SPLASH (PLA2IID), a novel member of phospholipase A2 family, is associated with lymphotoxin deficiency. Genes Immun. 2000;1:191–199. doi: 10.1038/sj.gene.6363659. [DOI] [PubMed] [Google Scholar]
- 19.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: Effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 20.Armour KL, Clark MR, Hadley AG, Williamson LM. Recombinant human IgG molecules lacking Fcgamma receptor I binding and monocyte triggering activities. Eur J Immunol. 1999;29:2613–2624. doi: 10.1002/(SICI)1521-4141(199908)29:08<2613::AID-IMMU2613>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Storni T, et al. Critical role for activation of antigen-presenting cells in priming of cytotoxic T cell responses after vaccination with virus-like particles. J Immunol. 2002;168:2880–2886. doi: 10.4049/jimmunol.168.6.2880. [DOI] [PubMed] [Google Scholar]
- 22.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 23.Kadam S, Deshpande C, Coulier F, Mulherkar R. Sequence analysis of full length cDNA for enhancing factor/phospholipase A2. Indian J Exp Biol. 1998;36:553–558. [PubMed] [Google Scholar]
- 24.Arbones ML, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 25.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 27.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mottet C, Uhlig HH, Powrie F. Cutting edge: Cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 29.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 30.Uhlig HH, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13:1016–1023. doi: 10.1002/ibd.20148. [DOI] [PubMed] [Google Scholar]
- 32.Eerola LI, et al. Analysis of expression of secreted phospholipases A2 in mouse tissues at protein and mRNA levels. Biochim Biophys Acta. 2006;1761:745–756. doi: 10.1016/j.bbalip.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 33.van den Broek M. Dendritic cells break bonds to tolerize. Immunity. 2007;27:544–546. doi: 10.1016/j.immuni.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Granata F, et al. Secretory phospholipases A2 as multivalent mediators of inflammatory and allergic disorders. Int Arch Allergy Immunol. 2003;131:153–163. doi: 10.1159/000071481. [DOI] [PubMed] [Google Scholar]
- 35.Mayer RJ, Marshall LA. New insights on mammalian phospholipase A2(s); comparison of arachidonoyl-selective and -nonselective enzymes. FASEB J. 1993;7:339–348. doi: 10.1096/fasebj.7.2.8440410. [DOI] [PubMed] [Google Scholar]
- 36.Cupillard L, et al. Both group IB and group IIA secreted phospholipases A2 are natural ligands of the mouse 180-kDa M-type receptor. J Biol Chem. 1999;274:7043–7051. doi: 10.1074/jbc.274.11.7043. [DOI] [PubMed] [Google Scholar]
- 37.Rouault M, et al. Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry. 2007;46:1647–1662. doi: 10.1021/bi062119b. [DOI] [PubMed] [Google Scholar]
- 38.Hinton HJ, Alessi DR, Cantrell DA. The serine kinase phosphoinositide-dependent kinase 1 (PDK1) regulates T cell development. Nat Immunol. 2004;5:539–545. doi: 10.1038/ni1062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.