Abstract
Human cells tolerate UV-induced cyclobutane pyrimidine dimers (CPD) by translesion DNA synthesis (TLS), carried out by DNA polymerase η, the POLH gene product. A deficiency in DNA polymerase η due to germ-line mutations in POLH causes the hereditary disease xeroderma pigmentosum variant (XPV), which is characterized by sunlight sensitivity and extreme predisposition to sunlight-induced skin cancer. XPV cells are UV hypermutable due to the activity of mutagenic TLS across CPD, which explains the cancer predisposition of the patients. However, the identity of the backup polymerase that carries out this mutagenic TLS was unclear. Here, we show that DNA polymerase ζ cooperates with DNA polymerases κ and ι to carry out error-prone TLS across a TT CPD. Moreover, DNA polymerases ζ and κ, but not ι, protect XPV cells against UV cytotoxicity, independently of nucleotide excision repair. This presents an extreme example of benefit-risk balance in the activity of TLS polymerases, which provide protection against UV cytotoxicity at the cost of increased mutagenic load.
Keywords: carcinogenesis, DNA repair, lesion bypass, replication, ultraviolet
TLS is a fundamental mechanism for tolerating DNA damage that has escaped repair, carried out by specialized low-fidelity DNA polymerases, which synthesize across a wide variety of DNA lesions (1). At least 5 TLS DNA polymerases are present in mammals, four of which, DNA polymerases η, ι, κ, and REV1, belong to the Y superfamily. The fifth TLS polymerase is polζ, which belongs to the B family, and is the only TLS polymerase known to be essential in mammals (2–5). TLS polymerases exhibit a certain degree of specificity for their substrate DNA lesions, and their activity is tightly regulated (6–9). The most well characterized TLS polymerase is polη, which is specialized to bypass cyclobutane pyrimidine dimers (CPD) in a relatively error-free manner. The biological significance of polη is illustrated by the hereditary disease xeroderma pigmentosum variant (XPV), in which germ-line mutations in the POLH gene, encoding polη, cause an extreme 1000-fold increased predisposition to sunlight-induced skin cancer (10, 11). Cells from XPV patients exhibit a slightly increased UV sensitivity, and a dramatic UV hypermutability (12), which is responsible for their extreme cancer predisposition. The UV hypermutability is explained by the activity of a back-up DNA polymerase that performs TLS across CPD with lower efficiency and higher error-frequency. Although there is evidence that polι is involved in TLS across CPD in XPV cells (13–15), additional polymerases may be involved, and the picture is far from being complete. This is an important issue because these polymerases are likely to be driving sunlight-induced skin carcinogenesis in XPV patients.
Here, we show that 3 TLS polymerases, polζ, polκ, and polι, are involved in TLS across CPD in XPV cells. Moreover, polζ and polκ, but not polι, also provide protection against UV cytotoxicity, independently of nucleotide excision repair (NER).
Results
Polζ, polκ, and polι Are Involved in TLS Across a Site-Specific TT CPD in Human XPV Cells.
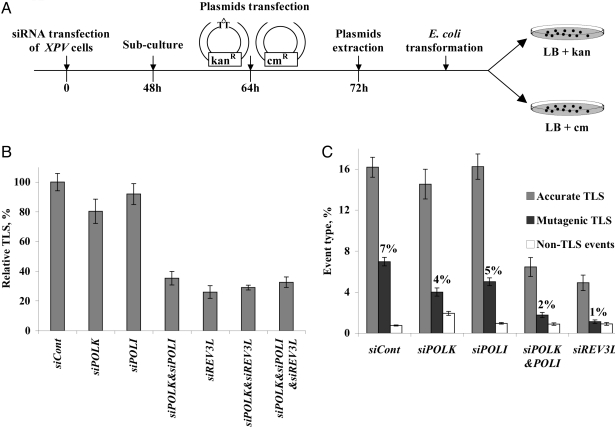
To identify the polymerase responsible for TLS across CPD in XPV cells we used a shuttle gapped-plasmid TLS assay with XPV cells in which the expression of defined DNA polymerases was knocked-down using siRNA. The assay involves transfection of cultured cells with a plasmid carrying a gap opposite a site-specific TT CPD (GP-TT-CPD), along with a control, gapped plasmid, without the CPD (Fig. 1A). After allowing for TLS to occur in the cells, the plasmids are extracted under alkaline conditions, introduced into a TLS-defective E. coli recA strain, and plated in parallel on LB-kanamycin (for repaired GP-TT-CPD) and LB-chloramphenicol (for repaired control plasmid). The ratio of kanR/cmR E. coli colonies is a measure of the efficiency of TLS in the mammalian cells, and sequence analysis of plasmids extracted from individual colonies provides data on DNA sequence changes during TLS. The assay was found to be very useful to study TLS in mammalian cells (8, 16, 17), perhaps because it is a good model system of postreplication gaps (18). Using this system we have shown that TLS across a TT CPD is an order of magnitude more mutagenic in XPV cells compared with normal cells (19), consistent with the hypermutability of XPV cells (12), and the relatively accurate TLS across CPD by purified polη (10, 20).
Fig. 1.
TLS across a TT CPD in XPV cells pretreated with siRNA against specific TLS polymerases. (A) Outline of the gapped plasmid TLS assay. See text for details. (B) Relative TLS extent across TT CPD lesions in XPV cells pretreated with siRNA against TLS polymerases. Each column represents the average of 6–10 measurements. See details in Table S1 and in Materials and Methods. (C) Extent of accurate and mutagenic TLS in XPV cells pretreated with siRNA against TLS polymerases. Sequences were classified as accurate TLS (insertion of AA opposite the TT CPD), mutagenic TLS (nucleotides other than AA inserted opposite the TT CPD, or mutations at the nucleotides flanking the TT CPD), and non-TLS events (large insertions and deletions). The extent of each event type was obtained by multiplying the extent of plasmid repair (Table S1) by the fraction of that event obtained from the DNA sequence analysis (Table S2), as presented in Table S3.
Plasmid GP-TT-CPD was used to transfect a cell line from an XPV patient, in which the expression of POLI, encoding polι, POLK, encoding polκ, and/or REV3L, encoding the catalytic subunit of polζ were knocked-down using siRNA. As shown in Fig. S1, each siRNA, effectively and specifically, knocked-down the expression of its target polymerase. When the efficiency of TLS was assayed, knocking-down POLI or POLK expression had a marginal effect compared with the treatment of cells with control siRNA (Fig. 1B and Table S1). In contrast, knocking down the expression of REV3L caused a significant 74% decrease in TLS. Considering the possibility that polι and polκ may back-up each other, we examined TLS under conditions in which the expression of both POLI and POLK was knocked-down. Under these conditions TLS across the TT CPD decreased by 65%, similar to the effect of REV3L alone (Fig. 1B and Table S1). Knocking-down REV3L expression in combination with POLK, or POLI and POLK, had an effect similar to knocking down REV3L alone (Fig. 1B). Thus, on one hand most TLS events across TT CPD require polκ or polι, but on the other hand most TLS events require polζ. This suggests that in XPV cells polζ cooperates with polκ and/or with polι in 2-polymerase TLS reactions across a TT CPD.
Polζ, Polκ, and Polι Are Responsible for Mutagenic TLS Across TT CPD in XPV Cells.
To determine the extent of mutagenic TLS under conditions in which the expression of certain DNA polymerases was knocked-down, one needs to multiply the efficiency of gap repair by the fraction of mutagenic TLS obtained from DNA sequence analysis of plasmid isolates. To that end we used the extents of TLS from Fig. 1B and Table S1, and the DNA sequence information presented in Table S2. In cells treated with control siRNA, the extent of TLS across the TT CPD was 23%, composed of 7% mutagenic TLS, and 16% accurate TLS (Fig. 1C and Tables S2 and S3). In XPV cells in which the expression of REV3L was knocked down, the extent of mutagenic TLS across TT CPD dramatically decreased by 84% (Fig. 1C and Tables S2 and S3). Similarly, knocking-down the expression of both POLK and POLI decreased the extent of mutagenic TLS by 76%. Knocking-down the expression of POLK or POLI alone had only a marginal effect on the extent of mutagenic TLS (Fig. 1C and Tables S2 and S3). Generally similar effects were observed also for accurate TLS across TT CPD (Fig. 1C and Table S2 and S3). Thus, polκ, polι, and polζ are involved in both mutagenic and accurate TLS across TT CPD in XPV cells.
The mutational signature in XPV cells treated with control siRNA showed that the majority of mutations were targeted to the TT CPD (71%, 20/28 events; Table S2), mostly to the 3′ T of the CPD (80%, 16/20 events) (Table S2), and a significant fraction was semitargeted to the most proximal nucleotides flanking the TT CPD (29%, 8/28 events) (Table S2). One tandem double mutation opposite the CPD was also observed. In contrast to the mutational load (extent of mutagenic TLS), the mutational signature, which is consistent with previous results (19), did not significantly change when the expression of POLK, POLI, REV3L or both POLI and POLK was knocked-down (Table S2; see Discussion).
Polζ and polκ, but Not polι, Protect XPV Cells Against UV Cytotoxicity.
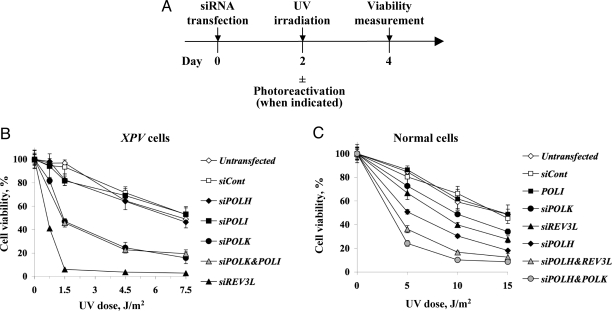
The in vivo involvement of polκ in TLS across a TT CPD was somewhat unexpected, because polκ was reported to be blocked by this lesion (21). To examine whether polκ, and the other polymerases have a similar function in human chromosomes we analyzed the UV sensitivity of XPV cells in which the expression of these polymerases was knocked-down. This was done by assaying ATP level, which rapidly decreases in cells undergoing apoptosis or necrosis (Fig. 2A). As shown in Fig. 2B, knocking-down the expression of POLH had no effect on UV sensitivity of the XPV cells, as expected, because these cells are polη-deficient anyway (Fig. 2B). Similarly, knocking-down the expression of POLI had no effect on UV sensitivity, which was different from several (but not all) previous reports (13–15, 22). In contrast, knocking-down the expression of POLK increased UV sensitivity up to 3.5–5-fold, and REV3L by up to 33- to 39-fold (Fig. 2B). Knocking-down the expression of both POLK and POLI had an effect similar to knocking down POLK alone (Fig. 2B). Measuring UV sensitivity by 5-bromo-2′-deoxyuracil (BrdU) incorporation gave similar results (Fig. S2A). Thus, polζ and polκ, but not polι, protect XPV cells against UV cytotoxicity. Generally consistent, although milder, effects were observed in MRC5 polη-proficient human cells (Fig. 2C). Notably, knocking-down the expression of POLK on top of knocking-down the expression of POLH caused significantly increased UV sensitivity compared with knocking down POLH alone, and a similar effect was observed by knocking down the expression of REV3L along with POLH (Fig. 2C).
Fig. 2.
UV sensitivity of XPV and normal cells pretreated with siRNA against TLS polymerases. (A) Outline of the experimental scheme. (B and C) XPV cells (B) or normal MRC5 human cells (C) were transfected with siRNA, and UV irradiated after 48 h. For MRC5 cells, 1 mM caffeine was added immediately following UV irradiation. Viability was determined 48 h after UV irradiation by measuring cellular ATP as described in Material and Methods.
Protection Against UV Cytotoxicity Provided by polζ and polκ Occurs in the Absence of Nucleotide Excision Repair.
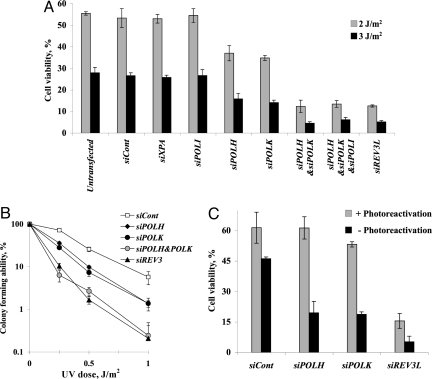
XPV cells are proficient in nucleotide excision repair (NER), and therefore the effects on UV sensitivity observed when the expression of TLS polymerases was knocked-down could be mediated via NER. This possibility is supported by the report that polκ is involved in NER of UV lesions in human cells (23). To examine this possibility we repeated the UV sensitivity experiments with a cell line from an XPA patient, totally defective in NER. As shown in Fig. 3A, a control siRNA and siRNA against XPA had no effect on UV sensitivity of the XPA cell line, as expected. Like in the case of XPV cells, siRNA against POLI had no effect on UV sensitivity. However, knocking-down the expression of POLH or POLK caused an up to 2-fold decrease in UV sensitivity (Fig. 3A). Remarkably, knocking-down the expression of REV3L caused a strong 4- to 5-fold increase in UV sensitivity compared with cells treated with a control siRNA (Fig. 3A). A similarly strong 5- to 6-fold increase in UV sensitivity was observed upon knocking-down the expression of both POLK and POLH (Fig. 3A). Knocking-down the expression of POLI, in addition to POLH and POLK, had no additional effect. Similar results were obtained using 3 different siRNA sequences for each POLK, POLH and REV3L, minimizing the possibility of off-target effects (Fig. S3B). In addition, similar results were obtained using incorporation of BrdU to assay UV sensitivity (Fig. S2B). Surprised by the importance of polκ in protecting XPA cells against UV cytotoxicity, we repeated the experiments using the colony forming ability assay. UV sensitivity of XPA cells in this assay was higher, because cells had been assayed 2 weeks after irradiation, when many more cells complete the UV-induced death process (24). However, also in this assay knocking-down the expression of either POLH or POLK yielded similar UV sensitization, with a bigger effect when both together were knocked-down (Fig. 3B). Thus, the function of polκ and polζ in protection against UV cytotoxicity is due to their involvement in DNA damage tolerance, not NER, consistent with their involvement in TLS across the TT CPD in the gapped plasmid assay.
Fig. 3.
UV sensitivity of XPA cells pretreated with siRNA against TLS polymerases. Cells were transfected with siRNA, and UV irradiated after 48 h at the indicated doses. Viability was determined 48 h after UV irradiation by measuring cellular ATP (A) or 12 days after UV irradiation by measuring colony forming ability (B). (C) XPA cells stably expressing CPD photolyase were UV irradiated at 3 Jm−2 and immediately illuminated with visible light to photoreactivate CPD lesions. Cell viability was determined as in A. See Materials and Methods for details.
Protection Against UV Cytotoxicity Provided by polκ Occurs at CPD.
To examine whether polκ exerts its protective effect against CPD, we used an XPA cell line expressing the Potorous tridactylus CPD photolyase, which specifically repairs CPD by photoreactivation. As shown in Fig. 3C, in the absence of photoreactivation, knocking-down POLH, POLK, or REV3L expression decreased cell viability upon UV irradiation, as in the parental XPA cells (Fig. 3A). We then repeated the experiment, except that the cells were photoreactivated by illumination with visible light immediately after the UV irradiation (Fig. 3C), a treatment that had eliminated 85% of the CPD (Fig. S4). Under such photoreactivation conditions, knocking-down POLH expression had no effect on UV sensitivity, consistent with the action of polη on CPD (Fig. 3C). Similarly, knocking-down POLK expression had no effect on UV sensitivity after photoreactivation, indicating that the protection provided by polκ is indeed against CPD (Fig. 3C). In contrast, knocking-down REV3L expression still caused a strong increase in UV sensitivity under the same photoreactivation conditions, consistent with its role in TLS across 6–4 PP, in addition to CPD.
Discussion
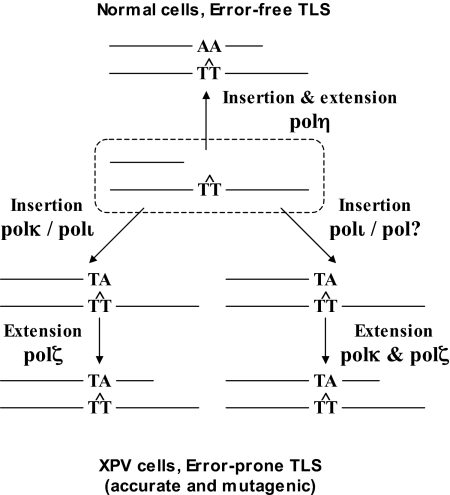
Given the critical role that mutations play in carcinogenesis, an attractive model is that the high error frequency of TLS across CPD in XPV patients is an important cause in their extreme cancer predisposition. The results presented in this study suggest that such a mutagenic bypass involves no less than 3 TLS polymerases, which are needed to back-up the absence of polη. The epistasis siRNA analysis presented above suggests that polζ has a critical role in TLS across a TT CPD, and that it cooperates with polκ and polι, which can back-up each other. This suggests that in XPV cells CPD are bypassed via 2-polymerase mechanisms. Based on the ability of purified polι to inserts nucleotides opposite a TT CPD (25), and the activity of the S. cerevisiae polζ as a general mismatch extender (26), a plausible model is that polι or polκ perform insertion opposite the CPD, and polζ performs the extension (Fig. 4). Although purified human polκ was reported to have extender properties similar to those of the yeast polζ (27), our in vivo results indicate that it was unable to effectively bypass a CPD without the help of polζ. More complex mechanisms are also possible. For example, polι and perhaps another polymerase may each perform the insertion step, whereas extension, which is generally more difficult than misinsertion, may require both polκ and polζ, which is consistent with their extender properties (Fig. 4). Alternatively, insertion opposite the first and the second pyrimidines of a CPD may involve different polymerases, including perhaps polζ. These last 2 possibilities reflect 3-polymerase mechanisms of TLS.
Fig. 4.
Model describing TLS across CPD in human cells. In human normal cells polη carries out efficient and relatively accurate TLS across CPD. In human XPV cells, TLS across CPD is performed by a back-up system in 2-polymerase reactions, in which polκ or polι perform insertion opposite the CPD, whereas polζ performs the extension step. This pathway is less efficient and more mutagenic than the polη-dependent pathway. A possible 3-polymerase mechanism is also presented. See text for details.
Recently, we showed that TLS across 2 additional DNA lesions in human cells involves 2-polymerase mechanisms: cisplatin-GG, a major intrastrand adduct formed in DNA by the chemotherapeutic drug cisplatin, and benzo[a]pyrene-G, a major adduct formed by tobacco smoke. TLS across these adducts involves polη or polκ cooperating with polζ in combinations that determine error-prone or error-free bypass (17). Taken together with the results presented here, it appears that 2-polymerase mechanisms comprise an important TLS strategy, supporting the original model proposed by the Prakash group (26). The important role of polζ in this process is consistent with the situation in S. cerevisiae (28), and with its essential role during mouse embryonic development (5, 17). It is also consistent with the increased UV sensitivity of normal, XPV and XPA cells in which the expression of REV3L was knocked-down, as shown in this study, and in other cell types in previous studies (15, 29–31). There are also earlier studies, which reported no effect of REV3L on UV sensitivity; this discrepancy might be due to differences in cell lines and methodologies (e.g., antisense RNA versus siRNA) (32–34).
Knocking-down the expression of POLI (confirmed at the mRNA and protein levels; Fig. S1) had no effect on UV sensitivity, which is different from several (but not all) previous reports (13–15, 22). As far as mutagenesis is concerned, we saw essentially no effect on mutagenic TLS across a TT CPD when POLI was knocked-down. Previous studies reported conflicting results of either decreased, altered or unchanged UV mutagenesis in cells deficient in polι (13–15, 35). Like in the case of polζ, this may reflect differences in methodologies or cell types.
The involvement of polκ in TLS across CPD was somewhat surprising, because purified polκ was reported to be blocked by TT CPD (21). Most likely accessory factors present in the cell assist polκ in carrying out insertion opposite CPD. Such situations are not uncommon, e.g., the inability of the purified E. coli polV to carry out TLS in vitro in the absence of RecA (36, 37). Importantly, our data on the involvement of polκ in TLS may explain, at least in part, the puzzling UV sensitivity exhibited by mouse embryonic fibroblasts lacking polκ (38).
The study in ref. 23 reports that polκ functions in NER of UV lesions in human cells. Clearly the protective effects of polκ against UV cytotoxicity described above do not occur via NER, because it was observed also in NER-deficient human XPA cells. Thus, although our results do not exclude the possibility that polκ may function in NER under certain conditions, it is clear that its major protective effect against UV cytotoxicity is mediated via tolerance of DNA damage rather than NER, consistent with the role of polκ in TLS across TT CPD as described above.
The extents of mutagenic TLS decreased 4-fold when expression of POLK and POLI combined was knocked-down, and 7-fold when REV3L was knocked-down, indicating their involvement in mutagenic TLS (Fig. 1C; Table S3). Interestingly, the mutagenic signature under the various conditions was similar (Table S2). This may be dictated by the structure of the CPD in DNA, which maintains the base pairing region of the pyrimidines, and causes only a minor distortion in DNA (39, 40). Alternatively, because TLS under these conditions is very low (Fig. 2), it may reflect residual TLS by the knocked-down polymerases.
Although in the TLS gapped plasmid assay polκ and polι backed-up each other in TLS across a TT CPD, polκ, but not polι, was important for protecting the cells against UV cytotoxicity. In considering this difference it should be noted that, although the gapped plasmid assay specifically measures TLS, the cell UV sensitivity assay involves both DNA damage tolerance, and cell death. It is thus possible that polκ but not polι can act at sites of potentially lethal CPD, such as in overlapping postreplication daughter gaps, or near double-strand breaks (41, 42), where a deficiency in tolerance may cause cell death. In addition, polκ may be involved in DNA damage tolerance via homology-dependent repair (homologous recombination or template switch recombination), mechanisms known to operate in E. coli and S. cerevisiae, but poorly understood in mammalian cells (1). In this context, it is noteworthy that polη was reported to be involved also in homologous recombination (43, 44), and that polκ from T. cruzi can perform DNA synthesis in a recombination intermediate (42).
The UV hypermutability of cells from XPV patients is believed to play a major role in their extremely high predisposition to sunlight-induced skin cancer (1). The results presented here show that backup TLS DNA polymerases protect XPV cells against UV cytotoxicity, but at the cost of increased mutagenesis due to error-prone TLS across CPD, representing an extreme example of benefit-risk balance in responses to DNA damage in human cells.
Materials and Methods
Cell Cultures.
SV40-transformed normal (MRC5), XPA (XP12RO), and XPV (XP30RO) human fibroblasts were gifts from A. R. Lehmann (University of Sussex, Brighton, U.K.). Cells were cultured in MEM Eagle (Sigma) supplemented with 2 mM l-glutamine (GIBCO/BRL), 100 units/mL of penicillin, 100 μg/mL of streptomycin (Biological Industries), and 15% FBS (HyClone). SV40-transformed XPA human fibroblasts (XP12RO) stably expressing a CPD photolyase gene (CPDphr) derived from the rat kangaroo P. tridactylis are described in ref. 24. Cells were cultured in DMEM (GIBCO/BRL) supplemented with 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 10% FBS. The cells were incubated at 37 °C in a 5% CO2 atmosphere, and periodically examined for Mycoplasma contaminations by EZ-PCR test kit (Biological Industries).
Knocking Down the Expression of TLS DNA Polymerase Genes.
The expression of specific DNA polymerase genes in normal, XPV, or XPA cells was knocked-down by transfection with polymerase-specific siRNAs. The extent of knockdown was estimated by RT-PCR using polymerase-specific probes, and by immunoblot analysis for polη and polι, for which good antibodies are available. The experimental details are described in SI Materials and Methods.
Construction of Gapped DNA Substrates.
DNA oligonucleotides without a lesion were supplied by Sigma-Genosys. The construction of the control gapped plasmid is described in ref. 45. A gapped plasmid with a site-specific TT CPD in the sequence context: 5′-GCAAGTTGGAG-3′ (the CPD is underlined) was constructed as described in ref. 19.
TLS Assay in Cultured Human Cells.
The TLS assay is described in refs. 8, 16, and 19. The details of the experiments performed in the current study are presented in SI Materials and Methods.
UV Sensitivity and Photoreactivation Assays.
Viability of UV-irradiated cells was determined using the CellTiter-Glo luminescent cell viability assay (Promega), measuring the amount of cellular ATP present, which signals the presence of metabolically active cells. Cell viability was determined also by incorporation of 5-bromo-2-deoxyuridine (BrdU), and by colony forming ability. The protocols for these assays, and the photoreactivation of XPA cells stably expressing CPD photolyase, are described in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Alan R. Lehmann for the XP and MRC5 cell lines. Z.L. is the Incumbent of the Maxwell Ellis Professorial Chair in Biomedical Research. This work was supported by grants from the Flight Attendant Medical Research Institute (to Z.L.), Florida; Israel Science Foundation Grants 564/04 and 1136/08 (to Z.L.); and U.S. National Institutes of Health Grant CA099194 (to N.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812548106/DCSupplemental.
References
- 1.Friedberg EC, et al. DNA Repair and Mutagenesis. 2nd edition. Washington DC: ASM Press; 2006. [Google Scholar]
- 2.Livneh Z. DNA damage control by novel DNA polymerases: Translesion replication and mutagenesis. J Biol Chem. 2001;276:25639–25642. doi: 10.1074/jbc.R100019200. [DOI] [PubMed] [Google Scholar]
- 3.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: Specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 4.Yang W, Woodgate R. What a difference a decade makes: Insights into translesion synthesis. Proc Natl Acad Sci USA. 2007;104:15591–15598. doi: 10.1073/pnas.0704219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gan GN, Wittschieben JP, Wittschieben BO, Wood RD. DNA polymerase ζ (pol ζ) in higher eukaryotes. Cell Research. 2008;18:174–183. doi: 10.1038/cr.2007.117. [DOI] [PubMed] [Google Scholar]
- 6.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 7.Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase η with monoubiquitinated PCNA: A possible mechanism for the polymerase switch in response to DNA damage. Mol Cell. 2004;14:491–500. doi: 10.1016/s1097-2765(04)00259-x. [DOI] [PubMed] [Google Scholar]
- 8.Avkin S, et al. p53 and p21 regulate error-prone DNA repair to yield a lower mutation load. Mol Cell. 2006;22:407–413. doi: 10.1016/j.molcel.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Soria G, Podhajcer O, Gottifredi V. P21Cip1/WAF1 downregulation is required for efficient PCNA ubiquitination after UV irradiation. Oncogene. 2006;25:2829–2838. doi: 10.1038/sj.onc.1209315. [DOI] [PubMed] [Google Scholar]
- 10.Masutani C, et al. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RE, Kondratick CM, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science. 1999;285:263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- 12.Maher VM, Ouellette LM, Curren RD, McCormick JJ. Frequency of ultraviolet light-induced mutations is higher in xeroderma pigmentosum variant cells than in normal human cells. Nature. 1976;261:593–595. doi: 10.1038/261593a0. [DOI] [PubMed] [Google Scholar]
- 13.Dumstorf CA, et al. Participation of mouse DNA polymerase ι in strand-biased mutagenic bypass of UV photoproducts and suppression of skin cancer. Proc Natl Acad Sci USA. 2006;103:18083–18088. doi: 10.1073/pnas.0605247103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, et al. Evidence that in xeroderma pigmentosum variant cells, which lack DNA polymerase η, DNA polymerase iota causes the very high frequency and unique spectrum of UV-induced mutations. Cancer Res. 2007;67:3018–3026. doi: 10.1158/0008-5472.CAN-06-3073. [DOI] [PubMed] [Google Scholar]
- 15.Gueranger Q, et al. Role of human DNA polymerases η, ι, and ζ in UV resistance and UV-induced mutagenesis. DNA Repair. 2008;7:1551–1562. doi: 10.1016/j.dnarep.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Avkin S, et al. Quantitative analysis of translesion DNA synthesis across a benzo[a]pyrene-guanine adduct in mammalian cells. The Role of DNA polymerase k. J Biol Chem. 2004;279:53298–53305. doi: 10.1074/jbc.M409155200. [DOI] [PubMed] [Google Scholar]
- 17.Shachar S, et al. Two-polymerase mechanisms dictate error-free and error-prone translesion DNA synthesis in mammals. EMBO J. 2009;28:383–393. doi: 10.1038/emboj.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann AR, Fuchs RP. Gaps and forks in DNA Replication: Rediscovering old models. DNA Repair. 2006;5:1595–1598. doi: 10.1016/j.dnarep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Hendel A, Ziv O, Gueranger Q, Geacintov N, Livneh Z. Reduced fidelity and increased efficiency of translesion DNA synthesis across a TT cyclobutane pyrimidine dimer, but not a TT 6–4 photoproduct, in human cells lacking DNA polymerase η. DNA Repair. 2008;7:1636–1646. doi: 10.1016/j.dnarep.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RE, Prakash S, Prakash L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Pol η. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi E, et al. Error-prone bypass of certain DNA lesions by the human DNA polymerase κ. Genes Dev. 2000;14:1589–1594. [PMC free article] [PubMed] [Google Scholar]
- 22.Petta TB, et al. Human DNA polymerase ι protects cells against oxidative stress. EMBO J. 2008;27:2883–2895. doi: 10.1038/emboj.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogi T, Lehmann AR. The Y-family DNA polymerase κ (pol κ) functions in mammalian nucleotide-excision repair. Nat Cell Biol. 2006;8:640–642. doi: 10.1038/ncb1417. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima S, et al. UV light-induced DNA damage and tolerance for the survival of nucleotide excision repair-deficient human cells. J Biol Chem. 2004;279:46674–46677. doi: 10.1074/jbc.M406070200. [DOI] [PubMed] [Google Scholar]
- 25.Vaisman A, et al. Sequence context-dependent replication of DNA templates containing UV-induced lesions by human DNA polymerase ι. DNA Repair. 2003;2:991–1006. doi: 10.1016/s1568-7864(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature. 2000;406:1015–1019. doi: 10.1038/35023030. [DOI] [PubMed] [Google Scholar]
- 27.Washington MT, Johnson RE, Prakash L, Prakash S. Human DINB1-encoded DNA polymerase κ is a promiscuous extender of mispaired primer termini. Proc Natl Acad Sci USA. 2002;99:1910–1914. doi: 10.1073/pnas.032594399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbs PE, McDonald J, Woodgate R, Lawrence CW. The relative roles in vivo of Saccharomyces cerevisiae Pol η, Pol ζ, Rev1 protein and Pol32 in the bypass and mutation induction of an abasic site, T-T (–4) photoadduct and T-T cis-syn cyclobutyl dimer. Genetics. 2005;169:575–582. doi: 10.1534/genetics.104.034611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittschieben JP, Reshmi SC, Gollin SM, Wood RD. Loss of DNA polymerase zeta causes chromosomal instability in mammalian cells. Cancer Res. 2006;66:134–142. doi: 10.1158/0008-5472.CAN-05-2982. [DOI] [PubMed] [Google Scholar]
- 30.Zander L, Bemark M. Immortalized mouse cell lines that lack a functional Rev3 gene are hypersensitive to UV irradiation and cisplatin treatment. DNA Repair (Amsterdam) 2004;3:743–752. doi: 10.1016/j.dnarep.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 31.Sonoda E, et al. Multiple roles of Rev3, the catalytic subunit of pol ζ in maintaining genome stability in vertebrates. EMBO J. 2003;22:3188–3197. doi: 10.1093/emboj/cdg308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibbs PE, McGregor WG, Maher VM, Nisson P, Lawrence CW. A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase zeta. Proc Natl Acad Sci USA. 1998;95:6876–6880. doi: 10.1073/pnas.95.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, et al. hREV3 is essential for eror-prone translesion synthesis past UV or benzopyrene diol expoxide-induced DNA lesions in human fibroblasts. Mutat Res. 2002;510:71–80. doi: 10.1016/s0027-5107(02)00253-1. [DOI] [PubMed] [Google Scholar]
- 34.Diaz M, et al. Decreased Frequency and Highly Aberrant Spectrum of Ultraviolet-Induced Mutations in the hprt Gene of Mouse Fibroblasts Expressing Antisense RNA to DNA Polymerase ζ. Mol Cancer Res. 2003;1:836–847. [PubMed] [Google Scholar]
- 35.Choi JH, Besaratinia A, Lee DH, Leeb CS, Pfeifer GP. The role of DNA polymerase ι in UV mutational spectra. Mutat Res. 2006;599:58–65. doi: 10.1016/j.mrfmmm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Tang M, et al. UmuD'(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc Natl Acad Sci USA. 1999;96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuven NB, Arad G, Maor-Shoshani A, Livneh Z. The mutagenesis protein UmuC is a DNA polymerase activated by UmuD', RecA and SSB, and specialized for translesion replication. J Biol Chem. 1999;274:31763–31766. doi: 10.1074/jbc.274.45.31763. [DOI] [PubMed] [Google Scholar]
- 38.Ogi T, Shinkai Y, Tanaka K, Ohmori H. Pol κ protects mammalian cells against the lethal and mutagenic effects of benzopyrene. Proc Natl Acad Sci USA. 2002;99:15548–15553. doi: 10.1073/pnas.222377899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling H, Boudsocq F, Plosky BS, Woodgate R, Yang W. Replication of a cis-syn thymine dimer at atomic resolution. Nature. 2003;424:1083–1087. doi: 10.1038/nature01919. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, et al. Nucleotide insertion opposite a cis-syn thymine dimer by a replicative DNA polymerase from bacteriophage T7. Nat Struct Mol Biol. 2004;11:784–790. doi: 10.1038/nsmb792. [DOI] [PubMed] [Google Scholar]
- 41.Garinis GA, et al. Transcriptome analysis reveals cyclobutane pyrimidine dimers as a major source of UV-induced DNA breaks. EMBO J. 2005;24:3952–3962. doi: 10.1038/sj.emboj.7600849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajao MA, et al. DNA polymerase κ from Trypanosoma cruzi localizes to the mitochondria, bypasses 8-oxoguanine, and performs DNA synthesis in a recombination intermediate. Mol Microbiol. 2008 doi: 10.1111/j.1365-2958.2008.06521.x. In press. [DOI] [PubMed] [Google Scholar]
- 43.Kawamoto T, et al. Dual roles for DNA polymerase η in homologous DNA recombination and translesion DNA synthesis. Mol Cell. 2005;20:793–799. doi: 10.1016/j.molcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 44.McIlwraith MJ, et al. Human DNA polymerase η promotes DNA synthesis from strand invasion intermediates of homologous recombination. Mol Cell. 2005;20:783–792. doi: 10.1016/j.molcel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Tomer G, Livneh Z. Analysis of unassisted translesion replication by the DNA polymerase III holoenzyme. Biochemistry. 1999;38:5948–5958. doi: 10.1021/bi982599+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.