Abstract
Non-coding (CGG-repeat) expansions in the fragile X mental retardation 1 (FMR1) gene result in a spectrum of disorders involving altered neurodevelopment (fragile X syndrome), neurodegeneration (late-onset fragile X-associated tremor/ataxia syndrome), or primary ovarian insufficiency. While reliable and quantitative assays for the number of CGG repeats and FMR1 mRNA levels are now available, there has been no scalable, quantitative assay for the FMR1 protein (FMRP) in non-transformed cells. Using a combination of avian and murine antibodies to FMRP, we developed a sensitive and highly specific sandwich enzyme-linked immunosorbent assay (ELISA) for FMRP in peripheral blood lymphocytes. This ELISA method is capable of quantifying FMRP levels throughout the biologically relevant range of protein concentrations and is specific for the intact FMRP protein. Moreover, the ELISA is well-suited for replicate protein determinations across serial dilutions in non-transformed cells and is readily scalable for large sample numbers. The FMRP ELISA is potentially a powerful tool in expanding our understanding of the relationship between FMRP levels and the various FMR1-associated clinical phenotypes.
Fragile X syndrome (OMIM #300624), the most common heritable form of intellectual impairment and the leading known single-gene form of autism,1,2 is nearly always caused by lowered (or absence of) expression of the fragile X mental retardation 1 (FMR1) protein (FMRP) in individuals who harbor FMR1 alleles in the full mutation range (>200 CGG repeats) or high premutation range (premutation, 55 to 200 CGG repeats).3,4,5,6,7 Most individuals with a premutation allele have IQs that fall within the normal range, although some children experience attention deficit hyperactivity disorder and autism spectrum disorders.8,9 Moreover, in adults, there is an increased risk of primary ovarian insufficiency,10,11 emotional problems including depression and anxiety,12 and the late-onset neurodegenerative disorder, fragile X-associated tremor/ataxia syndrome.13,14 Although reduction or loss of FMRP is generally believed to be the basis for fragile X syndrome, as well as many of the neurodevelopmental problems in the upper premutation range, quantitative comparisons of molecular (FMRP) and clinical phenotypes are generally lacking due to the absence of a quantitative measure of the protein.
Thus far, the main approaches for measuring protein levels have been indirect, involving immunohistochemical staining of peripheral blood lymphocytes or hair roots. A rapid immunohistochemical test of blood smears, using a mouse monoclonal antibody, was developed by Willemsen et al15,16 In that approach, measurement of FMRP was assessed by counting positively stained lymphocytes, with the fraction of positively staining lymphocytes representing a measure of protein level. Microscopic evaluation of smears is necessary to distinguish positively stained lymphocytes from non-specifically stained monocytes. Moreover, as there is no weighting for the degree or intensity of staining, a weakly stained cell is counted the same as a cell that is intensely stained. Thus, the method is best suited for establishing the absence of FMRP in full mutation males, or the fraction of FMRP positive lymphocytes (a reflection of X activation ratio) in full mutation females. Expression of FMRP has also been studied by immunohistochemical analysis of hair roots.17,18 One potential advantage of this method over the use of blood smears is that skin and neural cells both arise from the ectodermal germ layer, reducing the potential for discordance between cell types, particularly in the case of size- or methylation-mosaicism. However, this method also is not capable of quantifying protein levels.
FMRP levels have been quantified by immunoblot analysis, either in Epstein Barr Virus-transformed lymphoblastoid cells5 or in non-transformed cells.19 The major caveat with the use of transformed cells, other than the effort required for the transformation process per se, is the uncertainty in comparing FMRP expression (or even allele size and methylation status) with the corresponding molecular measures in non-transformed lymphocytes. By contrast, Kaufmann et al19 did quantify FMRP levels in untransformed peripheral blood leukocytes using a Western blot analysis. The protein measures were well-controlled and it is therefore somewhat surprising that additional studies did not follow this methodologic line. One concern may have related to the potential cross-reactivity of the single anti-FMRP antibody with the paralogous proteins, FXR1P and FXR2P.
Given the central importance of FMRP to the presence and severity of the clinical phenotype in fragile X syndrome, a method for accurately and rapidly quantifying FMRP levels is necessary. To this end, we have developed a sandwich enzyme-linked immunosorbent assay (ELISA) for FMRP that precisely determines levels of the protein in circulating lymphocytes in humans. The assay is sensitive to small changes in protein levels, targets intact FMRP specifically, and is a reliable method for the measurement of FMRP in blood. Of course, the caveat of measuring a peripheral protein level for a central nervous system disorder remains incompletely resolved. Notwithstanding this concern, a truly quantitative measure of FMRP will allow a better assessment of its importance in various clinical settings.
Materials and Methods
Lymphocytes
Six to eight ml of whole blood from subjects was collected into BD Vacutainer CPT tubes (Becton-Dickinson, Franklin Lakes, NJ) containing heparin according to University of California, Davis, Institutional Review Board-approved human subject protocols. Lymphocytes were separated, aliquotted with approximately 2.5 × 106 cells per cryovial, and stored in liquid nitrogen within 24 hours of collection according to manufacturer's directions.
Protein Extraction
Primary lymphocytes were quickly thawed in a 37°C bath and spun at 2,000 × g for 10 minutes. The pelleted cells were resuspended in PBS (137 mmol/L NaCl, 2.7 mmol/L KCl, 4.3 mmol/L Na2KH2PO4, pH 7.4) containing a Complete (Roche Applied Science, Indianapolis, IN) protease inhibitors tablet, and were washed an additional two times with PBS-Complete. The cell pellet following the final wash was resuspended in lysis buffer, MPer (Pierce, Rockford, IL) with additional 150 mmol/L NaCl, Protease Inhibitor Set III (diluted according to manufacturer's instructions) (Calbiochem, San Diego, CA), 10 μg/ml antipain, and 10 μg/ml chymostatin. Cell lysis proceeded overnight at 4°C on a Labquake rotisserie-style rotator, followed by centrifugation at 16,000 × g for 15 minutes at 4°C. The supernatant was carefully removed and aliquotted for storage at −80°C. Quantitation of protein concentration was accomplished with bicinchoninic acid protein assay kit (Pierce, Rockford, IL) according to manufacturer's instructions for the microplate assay.
Recombinant Maltose Binding Protein-FMRP
A technical difficulty with recombinant FMRP, in our hands, was its propensity to form aggregates and precipitate from solution, confounding its use as a reference/control. To circumvent this problem, a maltose binding protein (MBP)-FMRP fusion was used, which was less prone to aggregation. MBP-FMRP constructs were created as follows. Full-length FMRP cDNA was ligated into pET-28c MBP20 using the EcoRI and NotI sites downstream of the tobacco etch virus protease recognition sequence (ENLYFQG). The expression vector was transfected into E. coli BL21 (DE3) cells and grown in 2 L Luria broth medium supplemented with 30 μg/ml kanamycin at 37°C until the absorbance at 600 nm reached 0.5. Protein expression was induced with 1 mmol/L isopropyl thiogalactoside, and the cells were grown an additional 2 hours. Cells were recovered by centrifugation and resuspended in lysis buffer 2 (20 mmol/L HEPES pH 7.4, 200 mmol/L KCl, 1 mmol/L dithiothreitol, 1 mmol/L EDTA, and 1 mmol/L phenylmethylsulfonyl fluoride). Resuspended cells were lysed by French press and then centrifuged at 20,000 × g for 20 minutes at 4°C. The resulting supernatant was applied to 10 ml of amylose resin (New England BioLabs, Ipswich, MA) and then washed in 120 ml lysis buffer 2. The proteins were eluted from the column with 20 ml lysis buffer 2 supplemented with 10 mmol/L maltose. The fractions with the highest protein concentrations were combined and dialyzed overnight in lysis buffer 2. Protein concentrations were determined using a bicinchoninic acid assay kit (Pierce, Rockford, IL) according to manufacturer's instructions.
ELISA Antibodies
A unique peptide sequence, KDRNQKKEKPDSVD, located near the carboxy terminus of all known isoforms of FMRP, was synthesized for the production of chicken antibody (AVES Labs, Inc., Tigard, OR). IgY fractions were collected from immune eggs, and antibody was purified over an affinity matrix. Concentration of IgY was determined by Bradford assay. Other antibodies/specificities include 1C3 mouse monoclonal anti-FMRP (Millipore/Chemicon, Temecula, CA) recognizing an epitope near the amino terminus of FMRP; horseradish peroxidase-conjugated donkey anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA); and horseradish peroxidase conjugated goat anti-chicken (AVES Labs, Inc., Tigard, OR).
ELISA Procedure
Ninety-six-well plates (Lumitrac 600; Greiner Bio-One, Monroe, NC) were coated with 100 μl of 2 μg/ml chicken anti-FMRP antibody diluted in PBS, and rocked for 24 to 48 hours at 4°C to allow complete coverage of the well by the capture antibody. Wells were washed three times with 250 μl of PBS. Aliquots (100 μl) of the cell extracts, or FMRP, were diluted in PBS containing 0.05% polyoxyethylene (20) sorbitan monolaurate (PBS-T), added to prepared wells, and incubated overnight at room temperature. Wells were washed three times with 250 μl of PBS followed by blocking for 2 hours (room temperature) in 250 μl 2% hydrolyzed casein in PBS-T (blocking buffer). Wells were again washed three times with 250 μl of PBS followed by three washes with PBS-T. Detection antibody (100 μl, 1:10,000 v:v mouse anti-FMRP in blocking buffer) was added to each well. Following incubation for 8 to 10 hours at room temperature and six washes with PBS-T, 100 μl of 0.4 μg/ml horseradish peroxidase-conjugated donkey anti-mouse IgG in blocking buffer was added to each well and incubated overnight at room temperature. Wells were again washed six times with PBS-T followed by addition of 100 μl/well PS-Atto (Lumigen, Inc, Southfield, MI). The resulting luminescence was read with a luminometer (Lmax, Molecular Devices, Sunnyvale, CA). Each well was read for 2.5 seconds, approximately 5 minutes after the addition of substrate at room temperature.
Western Analysis
Separation of cell-extracted proteins was accomplished by electrophoresis at 20mA on a 10.5% to 14% Criterion Tris-HCl in running buffer (25 mmol/L Tris, 192 mmol/L glycine, 0.1% SDS, pH 8.3). Proteins were transferred overnight at 4°C using Criterion Cell Blotter System (BioRad, Hercules, CA) onto nitrocellulose membranes in 25 mmol/L Tris/192 mmol/L glycine/20% methanol. Efficient transfer of proteins was verified with MemCode Blue (Pierce, Rockford, IL) staining of the membrane. Following blocking with 5% nonfat dry milk in T-TBS (100 mmol/L Tris, 150 mmol/L NaCl, 0.1% polyoxyethylene [20] sorbitan monolaurate) the membranes were incubated with either mouse anti-FMRP (1:10,000 v/v), 0.5 μg/ml chicken anti-FMRP, or 2 μg/ml mouse anti-glyceraldehyde 3-phosphate dehydrogenase (Chemicon, Temecula, CA). Membranes were washed five times with T-TBS, and incubated for 2 hours at room temperature with the appropriate horseradish peroxidase-conjugated secondary antibody. Detection of antibodies was accomplished with Super Signal West Dura substrate (Pierce, Rockford, IL). Analysis of band densities was accomplished with ImageJ gel analysis software.21
Statistical Analysis
A series of dilutions of a fiducial lymphocyte extract were used to generate a standard curve for each plate. Concentrations of FMRP relative to the reference were calculated using SoftMax Pro 4-parameter fit logistics curve. Coefficients of variation (SD/mean × 100) were calculated to assess reliability of ELISA measurements across assays and biological stability of lymphocyte FMRP. For the evaluation of two distinct methods of measuring FMRP, ELISA, and Western, Z-scores, (x-<x>mean/SD) were calculated for each of the methods and compared.
Results
Performance Characteristics of the Sandwich ELISA
Avidity and Specificity
Western blot analysis of primary (ie, untransformed) lymphocyte extracts using the ELISA capture and detecting antibodies revealed complementary sensitivity to FMRP. The chicken (ELISA capture) polyclonal antibody bound FMRP with high avidity but also detected a non-FMRP higher molecular weight band (Figure 1A). The mouse anti-FMRP (ELISA detecting) antibody, bound specifically several isoforms of FMRP, but did not recognize the higher-molecular weight non-FMRP band (Figure 1, B and C). Side-by-side comparison of the two immunoblots established that the combination of the two antibodies used in the sandwich ELISA was specific for several isoforms of FMRP. Dilution series of both antibodies determined the optimal signal-to-noise characteristics were obtained with 2 μg/ml capture and 1:10,000 (v:v) detection antibodies. Higher concentrations, particularly of the capture antibody, resulted in elevated background without an increase in signal. Longer incubations with any of the antibodies did not improve the assay, though shorter incubations attenuated its dynamic range.
Figure 1.
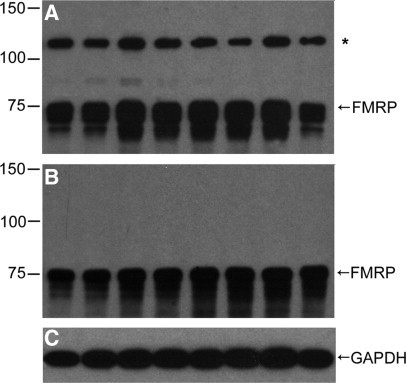
Characterization of protein species in lymphocyte extracts detected by the sandwich ELISA antibodies, mouse monoclonal and chicken polyclonal anti-FMRP. Western blots of lymphocyte extracts, 2 μg/lane from eight individuals with CGG repeats in the normal range. Films were overexposed to demonstrate a minor, cross-reacting protein species (asterisk) larger than 100 kDa to the chicken antibody (A), which is not detected by the mouse antibody (B). Mouse anti-glyceraldehyde-3-phosphate dehydrogenase shows sample loading (C).
Sensitivity
The sandwich ELISA recognized MBP-FMRP. The hybrid protein yielded a sigmoidal dose response (relative light units, RLU) that was proportional to the log of the concentration of the protein assayed. The assay was capable of detecting protein concentrations in the low picomolar range (Figure 2), and had a dynamic range of 0.5 to 16 ng/ml (∼5 to 140 pM) FMRP.
Figure 2.
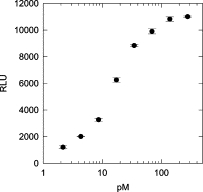
Detection of MBP-FMRP using sandwich ELISA. A dose-response curve of RLUs plotted as a function of log MBP-FMRP concentration. RLU, based on light collected over 2.5 seconds.
Measurability in Biologically Relevant Range
Lymphocyte protein extract from a normal individual was evaluated with the sandwich ELISA. Similar to the results with MBP-FMRP, a sigmoidal dose-response curve was obtained that was proportional to log-protein concentration of the extract (Figure 3A). Based on a working range of protein extracts of up to 6 μg/ml, the ELISA method is capable of quantifying FMRP levels as low as 3% of normal levels, with a variation of 5% of the measured level. In addition, FMRP protein levels were evaluated in extracts obtained from lymphocytes of full mutation patients (one male and one female) to determine whether cross-reactivity with other proteins, including the paralogous FXR1/2 proteins, might limit the sensitivity of the method. As represented in Figure 3B, no FMRP signal was detected by ELISA (<0.1% of normal level) with a protein extract from a male fragile X syndrome patient with no detectable FMR1 RNA expression. FMRP was only detectable at higher protein concentrations in the full mutation female.
Figure 3.

ELISA detection of FMRP from lymphocytes. ELISA analysis was performed on protein extracts from controls and fragile X syndrome patients. A: Sigmoidal dose-response curve of RLU (2.5 seconds light collection) as a function of protein concentration for control lymphocytes. Eight concentrations, from 0.2 to 6.0 μg/ml protein, were evaluated in triplicate. B: RLU values for (0.8 to 6.0 μg/ml protein) for two controls (filled circles, male; open circles, female) and two fragile X syndrome patients (triangles), all assayed in triplicate. Lymphocyte extract from the full-mutation male (filled triangles) showed minimal signal at all protein concentrations, whereas extract from a full-mutation female (open triangles; activation ratio, 0.46) yielded a detectable signal at higher concentrations.
Correlation of the ELISA Approach with Western Blot Analysis of Lymphocyte Extracts
Western blot analysis of lymphocyte extracts revealed that levels of FMRP measured in the ELISA were highly concordant with those measured by densitometry of the immunoreactive bands. Lymphocyte extracts showed strong densitometric signals (Figure 4A) from control individuals and progressively weaker signals from full mutation females. No FMRP immunoreactive bands were detected in protein extracts from two full mutation males. The corresponding ELISA analysis revealed the same progression (Figure 4B). Evaluation of Z scores for each of eight samples evaluated by the two methods revealed a strong correlation, with r = 0.99 (Figure 4C).
Figure 4.
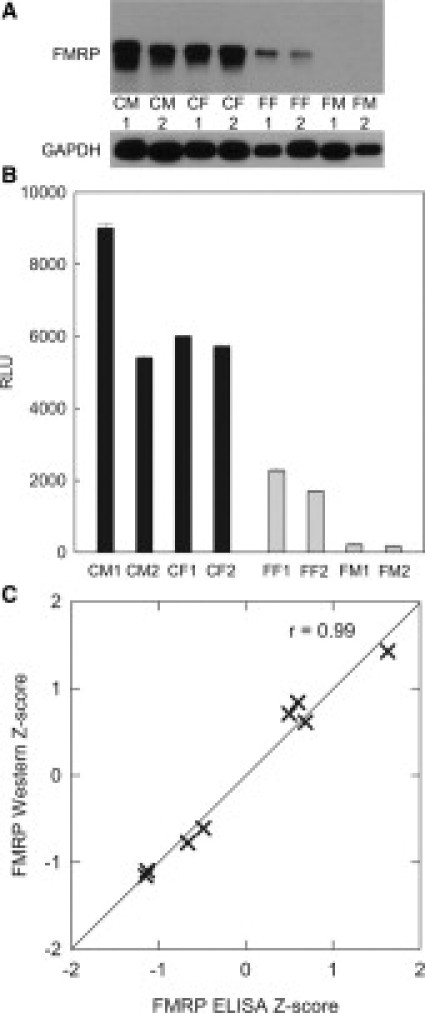
ELISA measurements correlate with Western blot analysis of lymphocyte extracts. A: Immunoblotting of lymphocytes extracts from control and fragile X patients, 2 μg/lane, revealing immunoreactive bands of varying intensities; CM, control male; CF, control female; FF, full-mutation female; FM, full-mutation male (designations 1 and 2 refer to different patient samples.) B: ELISA of FMRP in the same set of samples. Z-scores for the Western blot (integrated density of FMRP:glyceraldehyde 3-phosphate dehydrogenase bands) correspond to the ELISA Z-scores, r = 0.99 (n = 8) (C).
Analytical Recovery
Recovery experiments were performed with full mutation lymphocyte extracts to address the concern that there are substances that may exist within full mutation extracts, which suppress or inhibit the detection of FMRP by the ELISA. As evidenced in Figure 5, exogenous MBP-FMRP was detected without loss of signal when mixed with full mutation lymphocyte extract. Additionally, detection of FMRP from normal lymphocytes was not compromised (no loss of signal) when mixed with full mutation extract.
Figure 5.
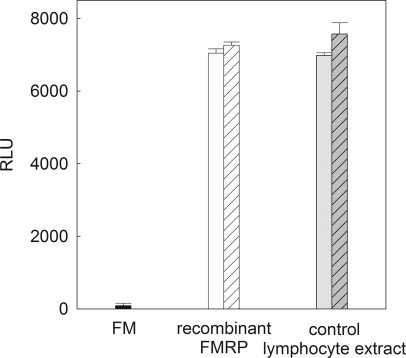
FMRP signal is recovered in mixtures with full-mutation extract. No loss of signal was observed when MBP-FMRP or control lymphocyte extract was mixed with full-mutation extract. Solid black bar indicates RLUs (signal) from lymphocyte extract of a full-mutation male (3 μg/ml), open bar RLUs from FMRP (4 ng/ml), gray bar indicates RLUs from control lymphocyte extract (2 μg/ml), striped bars indicate RLUs from mixture of FMRP or control lymphocyte extract with full-mutation lymphocyte extract.
Reliability
Repeated measurement of FMRP on a set of 15 samples was performed to assess the reliability of the sandwich ELISA. Aliquots of extracts stored at −80°C were analyzed across a 9- to 12-month period in two separate assays. Interassay reliability measured by Pearson's correlation was 0.99 (P < 0.01) (Figure 6A). In a second measure of reliability, three sets of triple-repeated measurements were performed to assess inter-run variation on samples similarly prepared and stored as above. For each subject the measured values showed only small variations across the three runs (Figure 6B), with coefficients of variation ranging from 4.61% to 7.29%. Extracts from a normal and a full mutation cell line were used as positive and negative reference standards on all assay plates. Responses relative to a fiducial lymphocyte extract assayed on every plate was used to gauge the interassay reliability.
Figure 6.
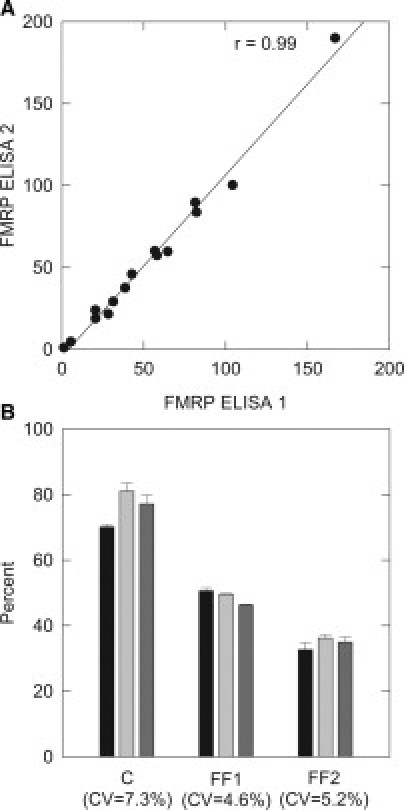
The sandwich ELISA is a reliable tool for the measurement of FMRP. Lymphocyte extracts from individuals were aliquoted and stored at −80°C before assay. A: A set of 15 samples were assayed on two separate occasions, comparison of the measurements shows the reproducibility of the ELISA. B: Another set of three samples was measured in three separate experiments over a 6-month period. Values relative to an internal reference standard (fiducial lymphocyte extract) that was included in each experiment were calculated. Repeated measurement of FMRP in lymphocyte protein extracts yielded reproducible results. CV = coefficient of variation. C indicates control; FF1 and FF2 indicate full-mutation females 1 and 2. Blackened bars indicate FMRP measurement with first ELISA, light gray bars indicate second ELISA, and dark gray bars indicate third ELISA.
Consistency of FMRP Levels in Circulating Lymphocytes
Repeated blood draws were taken from two subjects on three separate occasions over an 11-month period (repeat blood draws at 5 and 11 months) to assess the uniformity of FMRP levels over time. For subject 1, the SD was 1.5% (range = 53.1% to 55.2% of the positive reference standard; canonical value [CV] = 3.30%); for subject 2, the SD was 1.2% (range = 43.1% to 46.0%; CV = 2.12%) (Figure 7). Thus, FMRP levels do not appear to fluctuate significantly over an 11-month period.
Figure 7.
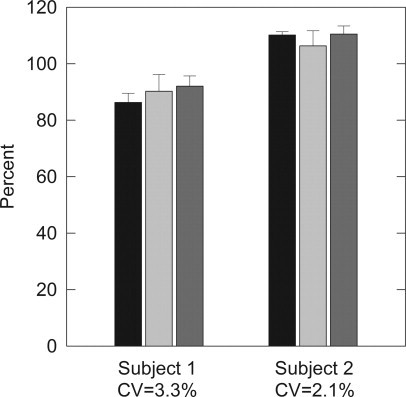
FMRP levels are biologically stable. Two subjects were drawn on three separate occasions over a 5-month period. Lymphocytes extracts were aliquotted and stored at −80°C until evaluation. FMRP levels did not fluctuate significantly for either subject. Black bars represent samples collected on day 1, light gray bars represent samples collected on day 172 (approximately 5 months) and dark gray bars represent samples collected on day 325 (approximately 11 months).
Dynamic Range of FMRP ELISA
FMRP levels were measured in lymphocyte protein extracts derived from full-mutation, mosaic, and control males (Figure 8). The range of FMRP detected in the subjects with hypermethylated, full-mutation alleles was 0.48% to 4.45% (n = 6) of levels observed in control males. Among the lowest values measured (∼0.5% to 1% of the control mean), standard deviations for the measured values were still only approximately one-quarter of the measured mean values. These results indicate that the method is capable of measuring with reasonable precision FMRP levels that are approaching 1% of the mean level in controls. The highest FMRP value (4.45%) corresponded to the only full mutation case with an allele (285 CGG repeats) that was less than 300 repeats. For this case, five distinct alleles were detected, ranging from 285 to 1173 repeats based on Southern gel analysis. We also examined the FMRP levels in a group (n = 8) of size and/or methylation mosaics in which FMRP values ranged from 1.07% to 13.02% of the control (mean), and although a systematic analysis of the range of possible mosaics is beyond the scope of this work, an interesting observation can be made with this small sample. The highest value (13.02%) occurs for a case with two alleles, one (9%) of 170 CGG repeats, and the other (91%) of 229 repeats. This case also has a high mRNA level (3.30 ± 0.15 times normal), reflecting continued transcriptional activity of both alleles. The next highest FMRP level (10.9%) corresponds to a smear of unmethylated alleles (81% of total allele intensity) ranging from 231 to 463 CGG repeats, with a small percentage of methylated alleles at 293 CGG repeats. Thus, for this full-mutation case, also with high mRNA level (3.24 ± 0.17 times normal), the relatively high FMRP level (within the full-mutation range) likely reflects the combination of relatively small alleles and a substantial fraction of unmethylated alleles. The remaining mosaic cases, with lower FMRP levels, generally reflect larger alleles and or lower percentages of either premutation alleles or unmethylated full-mutation alleles.
Figure 8.
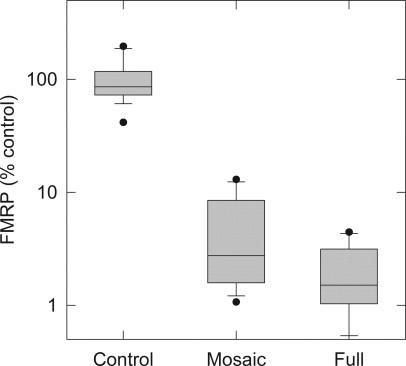
FMRP ELISA is a sensitive measure of protein levels across a range of FMR1 genotypes. Comparison using Mann-Whitney test of FMRP levels from full-mutation lymphocyte protein extracts to controls showed the two groups differed significantly (Mann-Whitney U = 90, control n = 15, full-mutation male n = 6, P < 0.001 two-tailed). Mosaic mutation levels of FMRP also differed significantly from controls (Mann-Whitney U = 120, control n = 15, mosaic n = 8, P < 0.001 two tailed). Lower boundary indicates 25th percentile, line within box indicates median, upper boundary indicates 75th percentile. Whiskers below and above boxes indicate 10th and 90th percentiles; dots indicate outliers.
The difference between FMRP levels in full-mutation versus mosaic males was not statistically significant. However, evaluation of full-mutation to control and mosaic to control FMRP levels using Mann-Whitney test revealed a significant difference with P < 0.001 for both comparisons (Figure 8). The ELISA reliably detected and measured FMRP in lymphocyte protein extracts derived from normal individuals, as well as the very low levels of FMRP associated with mutations of FMR1 gene.
Discussion
The development of the sandwich ELISA for the quantification of FMRP levels in peripheral blood lymphocytes fills a critical need for a quantitative measure of protein expression, since reduced or absent FMRP levels in fragile X syndrome are currently believed to be directly related to the extent of impaired neurodevelopment.22,23,24,25 The current effort was advantaged greatly by the production of a capture antibody with high affinity for human FMRP. Chicken FMRP has a predicted molecular weight of 63.9 kDa and is found to be 92% similar to the human polypeptide.26 While having diverged from mammals approximately 290 million years ago, much of the sequence, as with many X-linked genes, is conserved. However, a peptide sequence near the carboxy terminus of human FMRP, which does not coincide with any sequence within the chicken homolog, proved to be useful as an immunogen for human FMRP. The resultant polyclonal antibody recognized human FMRP in various cell extracts, as well as the synthetic recombinant protein. The high avidity of the chicken antibody made it ideal as a capture antibody of FMRP in very complex mixtures (lymphocytes extracts) for the sandwich ELISA. Detection of the captured FMRP with a highly specific antibody, the mouse anti-FMRP, yielded an extremely sensitive antibody pair that fulfilled the necessary requirements for an ELISA.
An important advantage of the current pair of antibodies is their epitope specificity toward intact FMRP. The capture antibody was made to a sequence not found in either FXR1 or FXR2 near the more variable carboxy terminus, whereas the detection antibody recognizes a region on the opposite end of FMRP near the amino terminus. Thus, the combination of the chicken capture and mouse detection antibodies is not only specific for FMRP but the combination also assures that only intact FMRP is measured with the ELISA. Furthermore, with epitope specificity at opposite ends of FMRP, the likelihood of the antibodies interfering with one another is reduced. While Western blotting with either of these antibodies individually is possible, the specificity and sensitivity of the two-antibody combination cannot be replicated with single-antibody strategies. Under optimized Western blotting conditions, a single measurement of FMRP could be detected in approximately 2 μg of lymphocyte extract protein. Thus, limited mainly by the transfer efficiency and non-linearity of film exposure, Western blotting is at best a semiquantitative tool for the measurement of FMRP, particularly for low levels associated with full-mutation alleles. By contrast, up to 12 replicates can be made conveniently with the ELISA in a 96-well format from the same amount of protein, allowing replicates and concentration profiles for each sample; the use of such replicates dramatically improves the precision of the method. Therefore, quantification with the FMRP sandwich ELISA is capable of providing a reliable estimate of FMRP levels in a complex mixture and permits the evaluation of more replicates and samples.
During the development of the ELISA assay, several features of the method were found to be critical for accurate, reproducible detection of FMRP levels in peripheral blood lymphocytes. First, the traditional method of isolating lymphocytes from heparinized blood by Ficoll gradient fractionation did not yield reliable results, particularly if the heparinized blood was allowed to remain more than 24 hours before processing. This lack of reliability appeared to be due to a variable degree of protein degradation, as evidenced by Western blot analysis (data not shown), in part as a consequence of the release of proteases from other cell types during subsequent steps of protein isolation. To address this problem, CPT Vacutainers were used to afford better cell fractionation, thus reducing the cross-contamination by proteases from other cell types. Extraction of intact FMRP was improved by isolating lymphocytes within 24 hours of blood collection, beyond that period the recovery was less reliable. Second, the presence of an appropriate combination of protease inhibitors is essential during protein extraction; ineffective protease inhibition appears to result in some degradation even on storage at −80°C, or during freezing and thawing of the samples. In this regard, with few exceptions (eg,19), previous studies of FMRP levels by direct methods (eg, Western blot analysis) have been performed on Epstein Barr Virus-transformed lymphocytes (lymphoblastoid cells).5,27 This predilection for the use of transformed cells likely reflects, at least in part, the near absence of protease-induced degradation of FMRP during isolation.
Also of critical importance to the success of the ELISA method is the order of antibody use. Reversal of the roles of the antibodies, with the mouse antibody for capture and the chicken for detection, resulted in signal barely detectable above background. This observation provides a possible explanation as to why previous attempts to develop FMRP ELISAs using mouse antibodies have not been successful; namely, that mouse antibodies generated to-date lack the affinity required for efficient capture. A range of concentrations of antibodies was tested, the combination yielding the greatest signal-to-noise ratio was selected. Other neutral salt buffers were considered, but the phosphate-based buffer functioned best for all incubations and washes.
Several blocking agents were tested, including milk and bovine serum albumin. None of the agents tested, other than hydrolyzed casein, were able to suppress the background sufficiently to allow efficient detection of the FMRP signal. With respect to detection, a variety of peroxidase substrates was tested; again, most failed to give sufficient signal above background for lymphocyte extracts. Blocking following, rather than before, addition of antigen expanded significantly the dynamic range of the assay. We speculate that the ability of the capture antibody to detect and bind FMRP from protein extract was impeded by the presence of the blocking buffer. Blocking before the application of the detecting or secondary antibodies was effective as background RLUs were minimal (800 to 1100 RLUs).
Finally, MBP-FMRP was evaluated as a protein standard for the ELISA. Unfortunately, the recombinant protein proved to be insufficiently stable in solution (or on storage) over a several month period, perhaps due to its propensity to precipitate from solution. On further investigation, it was found that the solubility of FMRP is poor, with a CV of 3.02 and CV − CV′ equal to 1.31, based on the protein solubility model of Davis et al28 This same model asserts FMRP has a 78.8% chance of being insoluble. However, lymphocyte protein extracts are very stable when collected, prepared, and stored under proper conditions. While insolubility might still be a problem with lymphocyte extracts, the significantly lower FMRP concentrations in mammalian cells favor its solubility during extraction and analysis. Thus, the most efficient and reliable interplate FMRP standard was protein extract derived from lymphocytes.
Concluding Remarks
An FMRP sandwich ELISA has been developed that is capable of accurately and reliably determining protein levels over the entire practical range of expression levels for expanded CGG repeat alleles of the FMR1 gene. This method is directly applicable to peripheral blood lymphocytes without the need for transformation, and should provide a highly quantitative alternative to indirect methods such as immunocytochemical staining or hair-root analysis, long the mainstay of fragile X research. Furthermore, the ELISA method is readily adaptable for large-scale use in various multiwell automated formats. The method should prove to be a powerful tool for further investigation of the relationships between FMRP and the diverse clinical phenotypic domains. Large-scale studies are necessary to recognize the value of the measurement and how FMRP influences the multitude of phenotypes associated with the FMR1 gene.
Acknowledgements
We thank the families who have supported and participated in our fragile X research program.
Footnotes
Supported by the National Institutes of Health Interdisciplinary Research Consortium (Roadmap) grant (UL1DE19583, P.J.H.; RL1AG032119, P.J.H.; RL1AG032115, R.J.H.), NICHDR01HD036071 (R.J.H.), grant UL1 RR024146 from the National Center for Research Resources (D.V.N.), R01HD055510 (F.T.), and by the UC Davis M.I.N.D. Institute for general infrastructure.
Current address of D.Y.: Department of Microbiology and Immunology, University of California, Davis, School of Medicine, Davis, CA.
References
- 1.Belmonte MK, Mazziotta JC, Minshew NJ, Evans AC, Courchesne E, Dager SR, Bookheimer SY, Aylward EH, Amaral DG, Cantor RM, Chugani DC, Dale AM, Davatzikos C, Gerig G, Herbert MR, Lainhart JE, Murphy DG, Piven J, Reiss AL, Schultz RT, Zeffiro TA, Levi-Pearl S, Lajonchere C, Colamarino SA. Offering to share: how to put heads together in autism neuroimaging. J Autism Dev Disord. 2008;38:2–13. doi: 10.1007/s10803-006-0352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagerman RJ, Rivera SM, Hagerman PJ. The fragile X family of disorders: a model for autism and targeted treatments. Curr Pediat Rev. 2008;4:40–52. [Google Scholar]
- 3.Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993;4:335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, Lakkis L, Devys D, Warren ST. Quantitative comparison of FMR1 gene expression in normal and premutation alleles. Am J Hum Genet. 1995;56:106–113. [PMC free article] [PubMed] [Google Scholar]
- 5.Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 6.Tassone F, Hagerman RJ, Ikle DN, Dyer PN, Lampe M, Willemsen R, Oostra BA, Taylor AK. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84:250–261. [PubMed] [Google Scholar]
- 7.Tassone F, Hagerman RJ, Taylor AK, Mills JB, Harris SW, Gane LW, Hagerman PJ. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet. 2000;91:144–152. doi: 10.1002/(sici)1096-8628(20000313)91:2<144::aid-ajmg14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27:S137–S144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 9.Goodlin-Jones BL, Tassone F, Gane LW, Hagerman RJ. Autistic spectrum disorder and the fragile X premutation. J Dev Behav Pediatr. 2004;25:392–398. doi: 10.1097/00004703-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 11.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, Simpson JL, Nelson LM. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Barbato I, Rice C, Gould E, Hall DA, Grigsby J, Wegelin JA, Harris S, Lewin F, Weinberg D, Hagerman PJ, Hagerman RJ. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- 13.Amiri K, Hagerman RJ, Hagerman PJ. Fragile X-associated tremor/ataxia syndrome: an aging face of the fragile X gene. Arch Neurol. 2008;65:19–25. doi: 10.1001/archneurol.2007.30. [DOI] [PubMed] [Google Scholar]
- 14.Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, Grigsby J, Bourgeois JA, Finucane B, Jacquemont S, Brunberg JA, Zhang L, Lin J, Tassone F, Hagerman PJ, Hagerman RJ, Leehey MA. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 2007;22:2018–2030. doi: 10.1002/mds.21493. quiz 2140. [DOI] [PubMed] [Google Scholar]
- 15.Willemsen R, Mohkamsing S, de Vries B, Devys D, van den Ouweland A, Mandel JL, Galjaard H, Oostra B. Rapid antibody test for fragile X syndrome. Lancet. 1995;345:1147–1148. doi: 10.1016/s0140-6736(95)90979-6. [DOI] [PubMed] [Google Scholar]
- 16.Willemsen R, Smits A, Mohkamsing S, van Beerendonk H, de Haan A, de Vries B, van den Ouweland A, Sistermans E, Galjaard H, Oostra BA. Rapid antibody test for diagnosing fragile X syndrome: a validation of the technique. Hum Genet. 1997;99:308–311. doi: 10.1007/s004390050363. [DOI] [PubMed] [Google Scholar]
- 17.de Vries BB, Severijnen LA, Jacobs A, Olmer R, Halley DJ, Oostra BA, Willemsen R. FMRP expression studies in blood and hair roots in a fragile X family with methylation mosaics. J Med Genet. 2003;40:535–539. doi: 10.1136/jmg.40.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willemsen R, Anar B, De Diego Otero Y, de Vries BB, Hilhorst-Hofstee Y, Smits A, van Looveren E, Willems PJ, Galjaard H, Oostra BA. Noninvasive test for fragile X syndrome, using hair root analysis. Am J Hum Genet. 1999;65:98–103. doi: 10.1086/302462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann WE, Abrams MT, Chen W, Reiss AL. Genotype, molecular phenotype, and cognitive phenotype: correlations in fragile X syndrome. Am J Med Genet. 1999;83:286–295. [PubMed] [Google Scholar]
- 20.Fraser CS, Berry KE, Hershey JW, Doudna JA. eIF3j is located in the decoding center of the human 40S ribosomal subunit. Mol Cell. 2007;26:811–819. doi: 10.1016/j.molcel.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, MD: 1997–2007. [Google Scholar]
- 22.Hall SS, Burns DD, Lightbody AA, Reiss AL. Longitudinal changes in intellectual development in children with fragile X syndrome. J Abnorm Child Psychol. 2008;36:927–939. doi: 10.1007/s10802-008-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10:31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- 24.Menon V, Leroux J, White CD, Reiss AL. Frontostriatal deficits in fragile X syndrome: relation to FMR1 gene expression. Proc Natl Acad Sci USA. 2004;101:3615–3620. doi: 10.1073/pnas.0304544101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera SM, Menon V, White CD, Glaser B, Reiss AL. Functional brain activation during arithmetic processing in females with fragile X Syndrome is related to FMR1 protein expression. Hum Brain Mapp. 2002;16:206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price DK, Zhang F, Ashley CT, Jr, Warren ST. The chicken FMR1 gene is highly conserved with a CCT 5′-untranslated repeat and encodes an RNA-binding protein. Genomics. 1996;31:3–12. doi: 10.1006/geno.1996.0002. [DOI] [PubMed] [Google Scholar]
- 27.Primerano B, Tassone F, Hagerman RJ, Hagerman P, Amaldi F, Bagni C. Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. RNA. 2002;8:1482–1488. [PMC free article] [PubMed] [Google Scholar]
- 28.Davis GD, Elisee C, Newham DM, Harrison RG. New fusion protein systems designed to give soluble expression in Escherichia coli. Biotech Bioeng. 1999;65:382–388. [PubMed] [Google Scholar]


