Abstract
SURVEYOR is a new mismatch-specific plant DNA endonuclease that is very efficient for mutation scanning in heteroduplex DNA. It is much faster, cheaper, more sensitive, and easier to perform than other “traditional” mutation detection methods such as single-strand conformation polymorphism analysis, denaturing high-performance liquid chromatography, heteroduplex analysis, and phage resolvases. This is the first comprehensive report on the use of SURVEYOR for screening genes implicated in a spectrum of inherited renal diseases. Of the 48.2 kb screened, 44 variations were identified, accounting for one variation per 1.1 kb. The re-sequencing of multiple samples did not reveal any variation that had not been identified by SURVEYOR, attesting to its high fidelity. Additionally, we tested this enzyme against 15 known variants, 14 of which it identified, thus showing a sensitivity of 93%. We showed that the genetic heterogeneity of renal diseases can be easily overcome using this enzyme with a high degree of confidence and no bias for any specific variations. We also showed for the first time that SURVEYOR does not demonstrate any preference regarding mismatch cleavage at specific positions. Disadvantages of using SURVEYOR include enhanced exonucleolytic activity for some polymerase chain reaction products and less than 100% sensitivity. We report that SURVEYOR can be used as a mutation detection method with a high degree of confidence, offering an excellent alternative for low-budget laboratories and for the rapid manipulation of multiple genes.
SURVEYOR is a new mismatch-specific plant DNA endonuclease that is very efficient in scanning for known or unknown mutations and other variants in heteroduplex DNA. It is a member of the CEL family of plant endonucleases, classified as CELII, that cleave DNA with high specificity at sites of mismatches as a result of base substitutions or other distortions.1,2 These DNA endonucleases cut both strands of a DNA heteroduplex on the 3′ side of the mismatch.3,4
CELI nuclease has been used more widely than SURVEYOR (CELII), probably because the latter became commercially available (Transgenomics, Crewe, UK) during the last few years. Despite this, many papers refer to SURVEYOR as a very efficient method for mutation detection in human (see below) as well as non-human5,6 genes for screening of induced point mutations (TILLING) in several organisms,7,8,9 for detecting heteroplasmy,10,11 and for clone sequence validation.12 Its application to human genetic disorders resulted in the discovery and description of many novel mutations in genes such as BRCA1, 1,2,13 EGFR,14 JAK2,15 hCDC4,16 ATRX,17 mitochondrial genes,10,11 ABCC6,18 p53,19 NPHS2,20 TP53,21 COL4A3, and COL4A4.22
There are advantages of SURVEYOR compared with other traditional mutation detection methods like single-strand conformation polymorphism analysis, denaturing high-performance liquid chromatography, and heteroduplex analysis. There is detection of all types of base substitution and insertion/deletion mismatches; cleavage fragments provide information about the location of the mutation; and multiple cleavage products indicate the presence of more than one variant. There is mutation detection in very long DNA fragments when combined with Southern blotting (600 kb has been tested successfully).3 There is the possibility of pooling polymerase chain reaction (PCR) products, thereby improving throughput. Limited experience is required, and analysis can be performed on different platforms. According to our and others' experience, SURVEYOR is a much better mismatch cleavage enzyme than phage resolvases because the latter produce many nonspecific bands, are size-limited, and additional experience is required for experiment assessment.20,22,23,24,25,26
Our laboratory specializes in the genetics of inherited kidney diseases. Due to the fact that the number of responsible genes for inherited renal conditions is very large and is still increasing, a fast, cheap, and easy method for mutation detection like SURVEYOR can solve a lot of problems, especially within the environment of a clinical diagnostic setup. This is a means to accelerate both diagnostic and research procedures. In this study, we summarize our results of the last 5 years using SURVEYOR nuclease for mutation detection in eight kidney-specific genes. These genes are responsible or have been selected as candidates for the genetic defect in renal diseases (Table 1): ACTN4,27 TRPC6,28,29 COL4A3, COL4A4,22 NEPH3 (ENSG00000126259), WTIP (ENSG00000142279) for focal segmental glomerulosclerosis; COL4A3/COL4A430,31 for thin basement membrane nephropathy; NCUG1 (ENSG00000198715) for medullary cystic kidney disease 132; NPHS233,34 for steroid-resistant nephrotic syndrome in children. We found a sum of 44 genetic variants in the above genes. We classified these variations according to the type of nucleotide change, the nucleotide mismatch pairs, the efficiency of enzymatic cleavage, and their position in the PCR product to reveal the properties of SURVEYOR and its efficiency for mutation screening in these genes.
Table 1.
Genes Studied, Encoded Proteins, and Related Diseases
| Gene | Chromosome | Protein | Function | Related disease |
|---|---|---|---|---|
| ACTN4 (NM_004924) | 19q13.1 | α-actinin-4 | Actin-linked protein | Focal segmental glomerulosclerosis (AD) |
| TRPC6 (NM_004621) | 11q22.1 | Transient receptor potential channel 6 | Ca2+ channel | Focal segmental glomerulosclerosis (AD) |
| NPHS2 (AJ279254) | 1q25.2 | Podocin | Part of the slit diaphragm of podocytes | Focal segmental glomerulosclerosis, steroid resistant nephrotic syndrome in children (AR) |
| NEPH3 (NM_032123) | 19q13.1 | Nephrin-like protein 3 (kirrel-2) | Part of the slit diaphragm of podocytes and also found in β-islets of pancreas | No pathology has been associated |
| WTIP (XM_059037) | 19q13.1 | Wilm's tumor 1 interacting protein | Interacts with WT1 and possibly acts also as a transcription factor | No pathology has been associated |
| NCUG1 (NT_079484) | 1q23.1 | Kidney predominant protein | Unknown | No pathology has been associated |
| COL4A3 (NM_000091) | 2q36.3 | Collagen IV chain α3 | Basement membranes network (glomerulus, eyes, cochlea) | Alport syndrome (AR), thin basement membrane nephropathy (AD), focal segmental glomerulosclerosis (AD) |
| COL4A4 (NM_000092) | 2q36.3 | Collagen IV chain α4 | Basement membranes network (glomerulus, eyes, cochlea) | Alport syndrome (AR), thin basement membrane nephropathy (AD), focal segmental Glomerulosclerosis (AD) |
AD, autosomal dominant; AR, autosomal recessive.
Materials and Methods
Blood Samples – DNA Extraction
All blood samples were collected in tubes with EDTA as anticoagulant and sent to our laboratory for research purposes accompanied by signed consent forms approved by the Cyprus National Bioethics Committee. Most patients presented with symptoms suggestive of inherited glomerulopathies such as steroid-resistant nephrotic syndrome (resistance proved on treatment), focal segmental glomerulosclerosis, and thin basement membrane nephropathy on biopsy. Patients with medullary cystic kidney disease belonged to families that were genetically linked to the MCKD1 locus (1q.21) and analyzed specifically for mutations in the NCUG1 as a candidate. The DNA was isolated by one of two methods, either using a salting out procedure35 or using the QiaAmp DNA blood Mini Kit (Qiagen, Hilden, Germany).
PCR Amplification
In appropriate patients, exons of TRPC6 (one patient, one control), NEPH3 (three patients, one control), WTIP (three patients, one control), COL4A3 (10 patients, two controls), COL4A4 (five patients, one control), NCUG1 (two patients, one control), and NPHS2 (24 patients, two controls) were amplified using exon flanking primers (for primer sequences and conditions see Supplementary Tables S1–S7 at http://jmd.amjpathol.org.). The oligonucleotide primers were designed to encompass single or multiple exons as well as at least 60 bp of splice junctions and intronic sequences (Primer 3 software, http://frodo.wi.mit.edu/, last accessed March 24, 2008). ACTN4 was amplified (three patients, one control) through a cDNA approach.27 As expected, some patients were screened for mutations in multiple genes. A 50-μl PCR reaction was set up with 10 ng of genomic DNA, 10 to 15 pmol each of forward and reverse primer, 1 unit of AmpliTaq DNA polymerase (Roche, Mannheim, Germany), 0.2 mmol/L deoxyribonucleotide triphosphates and 10X appropriate PCR buffer (including MgCl2 for final reaction concentration, 1.5 mmol/L). PCR products that required dimethyl sulfoxide were amplified by TaqExpress polymerase, 2 units per reaction (Genpak, Falmer, Brighton, UK). SURVEYOR efficiency is not altered by a dimethyl sulfoxide concentration of up to 5%. PCR amplification was performed in an Eppendorf (Eppendorf, Hamburg, Germany) or Biometra (Biometra, Gottingen, Germany) thermal cycler by cycling for 30 to 40 cycles depending on the amplicon.
SURVEYOR Nuclease Digestion and Analysis by Agarose Gel Electrophoresis – DNA Sequencing
After the final PCR extension, we added the following extra step of melting and reannealing to enhance the formation of DNA heteroduplexes: 95°C for 5 minutes, renature by cooling to 65°C for 30 minutes and 25°C for 30 minutes. For NPHS2 gene for which recessive mutations could exist, PCR products were mixed with an approximately equal amount of PCR product from wild-type DNA (before the denaturation-reannealing step) before forming cross-hybridized sequences to facilitate heteroduplex formation. Quality and quantity of PCR products were assessed by running the products in 1.5% routine agarose gels that contained ethidium bromide and visualizing under ultraviolet light illumination. Three to 16 μl of the PCR product was mixed with 0.5 μl of Enhancer and 0.5 μl of SURVEYOR enzyme (Transgenomics, Crewe, UK) and incubated at 42°C for 20 minutes followed by adding 10X stop solution as per the manufacturer's protocol. Positive and negative mismatch controls, provided by the manufacturer, were included in each digestion set. Some PCR products were incubated for less than 20 minutes because exonucleolytic activity of SURVEYOR was evident, which resulted in DNA degradation. The digestion result was examined by electrophoresis (7.5 V per cm of gel length) on high-resolution Eurobio 3:1 agarose gels, 2–3% density depending on the PCR product size. Ethidium bromide was not incorporated in the gels, but they were stained in 1 L of distilled water containing 0.8 mg of ethidium bromide for 45 minutes, followed by 5 minutes of distaining in distilled water after the end of electrophoresis. This proved to be a better staining procedure for visualizing short and faint DNA fragments. For ultraviolet visualization we used G-Box of SynGene (Cambridge, England) and GeneSnap software (version 6.07) of the same company.
If cleavage was evident, DNA sequencing was performed using a kit for dye terminator cycle DNA sequencing (Beckman Coulter) and fractionated on an automatic DNA sequencer (CEQ2000, Beckman Coulter). Sequences were aligned against the reference sequence, according to Ensembl database, using BioEdit software that utilizes the ClustalW algorithm to perform multiple alignments.36 The entire coding regions of ACTN4 and NPHS2 were directly sequenced for three and 12 patients, respectively, to check for any undetected variants.
Results and Discussion
Identification of mutations in genetically heterogeneous kidney diseases is a difficult task, as the responsible gene can be one among several. Some examples are primary focal segmental glomerulosclerosis (three genes: ACTN4, TRPC6, CD2AP); nephrotic syndromes in children (five genes: NPHS1, NPHS2, WT1, PLCE1, LAMB2); and nephronophthisis (nine genes: NPHP1-9). Here we show that exhaustive screening with SURVEYOR strategy can be routinely used in diagnostic and research laboratories when common or known mutations have already been ruled out. The efficiency of this procedure is attested by the 44 variations we identified (41 single-base substitutions and three small deletions; Table 2). Five of them are novel.22
Table 2.
Mutations and Other Variants Found with SURVEYOR Technique
| No. | Type of variant/gene | Nucleotide change | Exon /intron | PCR size (bp) | Variant position in PCR product |
|---|---|---|---|---|---|
| MUTATION | |||||
| COL4A3 | |||||
| 1 | G871C | c.2611 G>T | Exon 32 | 298 | 179 |
| 2 | G1334E | c.4001 G>A | Exon 45 | 447 | 147 |
| COL4A4 | |||||
| 3 | c.3854delG (frameshift at Ser1217, stop at 1287) | Exon 39 | 500 | 142 | |
| NPHS2 | |||||
| 4 | L305P | c.914 T>C | Exon 8 | 490 | 145 |
| VARIANT | |||||
| NCUG1 | |||||
| 5 | P203S | c.607 C/T | Exon 4 | 419 | 120 |
| 6 | I223V | c.667 A/G | Exon 4 | 419 | 181 |
| NPHS2 | |||||
| 7 | 5′ UTR (−116C/T) | Promoter region | 639 | 512 | |
| 8 | 5′ UTR (−51G/T) | Exon 1 | 639 | 577 | |
| 9 | G34G | c.102 A/G | Exon 1 | 442 | 181 |
| 10 | A318A | c.954 C/T | Exon 8 | 490 | 185 |
| 11 | 3′ UTR (+54G/C) | Exon 8 | 490 | 437 | |
| COL4A3 | |||||
| 12 | IVS5+73C/T | Intron 5 | 374 | 240 | |
| 13 | P141L | c.422 C/T | Exon 7 | 407 | 176 |
| 14 | E162G | c.485 A/G | Exon 9 | 287 | 138 |
| 15 | IVS15+30G/A | Intron 15 | 290 | 164 | |
| 16 | IVS17−80T/C | Intron 17 | 403 | 115 | |
| 17 | K834R | c.2501 A/G | Exon 32 | 298 | 69 |
| 18 | IVS41−110T/G | Intron 41 | 477 | 81 | |
| 19 | IVS42+66C/T | Intron 42 | 477 | 440 | |
| 20 | Q1495R | c.4484 A/G | Exon 49 | 621 | 137 |
| 21 | IVS50−67delA | Intron 50 | 377 | 44 | |
| COL4A4 | |||||
| 22 | P482S | c.1444 C/T | Exon 21 | 300 | 166 |
| 23 | G545A | c.1634 G/C | Exon 23 | 250 | 109 |
| 24 | L1004P | c.3011 T/C | Exon 33 | 271 | 80 |
| 25 | G1198G | c.3594 G/A | Exon 39 | 500 | 90 |
| 26 | K1228K | c.3684 G/A | Exon 39 | 500 | 180 |
| 27 | IVS40+9G/C | Intron 40 | 500 | 414 | |
| 28 | IVS41+34T/C | Intron 41 | 295 | 257 | |
| 29 | M1327V | c.3979 A/G | Exon 42 | 395 | 144 |
| 30 | P1360P | c.4080 A/G | Exon 42 | 395 | 245 |
| 31 | IVS43−36G/A | Intron 43 | 313 | 38 | |
| 32 | P1403S | c.4207 C/T | Exon 44 | 313 | 190 |
| 33 | IVS44−24C/T | Intron 44 | 279 | 55 | |
| 34 | V1516V | c.4548 A/G | Exon 47 | 492 | 136 |
| ACTN4 | |||||
| 35 | P179P | c.537 G/A | Exon 5 | 589 | 290 |
| TRPC6 | |||||
| 36 | IVS3−100G/A | Intron 3 | 427 | 60 | |
| 37 | N561N | c.1683 T/C | Exon 6 | 407 | 263 |
| 38 | IVS10−138C/T | Intron 10 | 457 | 81 | |
| 39 | IVS12−(20_22delCTT) | Intron 12 | 316 | 56 | |
| NEPH3 | |||||
| 40 | A170T | c.508 G/A | Exon 4 | 577 | 80 |
| 41 | A351A | c.1053 G/T | Exon 8 | 590 | 218 |
| 42 | V353M | c.1057 G/A | Exon 9 | 590 | 307 |
| WTIP | |||||
| 43 | Promoter region | G/C (79692)* | 573 | 133 | |
| 44 | IVS4−8G/C | Intron 4 | 794 | 556 |
Nucleotide number 1 is the A of the first ATG codon of translation.
Nucleotide coordinate according to AC008747 locus (NCBI).
Only nine of the 41 single-base substitutions were transversions, representing 22%, transitions representing the rest, thereby giving a ratio of 1:3.6 (Table 3); according to the literature, random mutations can be divided into transversions and transitions in a 1:2 ratio. Biological data sets tend to have a strong bias toward transitions due to DNA methylation, chemical differences between bases, and differences in DNA repair efficiency for different types of nucleotide mismatches. In the mouse roughly 66.7%37) and in the rat 78.4% are transitions,5 whereas in other reports for humans 64% are transitions.38 Our data here show a similar bias toward transitions (78%). It is not likely that this bias is a result of a decreased sensitivity of SURVEYOR for certain mismatches, since results obtained for CELI1,9 and SURVEYOR (CELII)4 show that all possible heteroduplex mismatches are recognized equally efficiently by these enzymes. Also, it should be noted that 10 of 41 (24.4%) single-base substitutions identified here can putatively be explained as transitions of C to T, at CpG dinucleotides, that are known to be mutation hot spots through deamination and methylation of cytosine residues (Table 3). These variations are equally distributed in collagenous (which contain the characteristic Gly-X-Y repeating motif) and non-collagenous genes of our study, despite the fact that collagen genes are rich in G and C nucleotides (GC content in coding regions: 59% for COL4A4 and 56% for COL4A3) owing to their collagenous sequence of Gly-X-Y, with glycine (GGN) as every third residue while X and Y are frequently prolines (CCN).
Table 3.
Type of Nucleotide Changes Detected by SURVEYOR Endonuclease
| Genotype | Mismatches | Number | % | Type 1* | Type 2* | Variant at CpG† | Transitions |
|---|---|---|---|---|---|---|---|
| C/T | G-T | 32 | 72.7 | 11 | 21 | 10 (24.4%) | 32 (78%) |
| G/A | A-C | ||||||
| A/C | G-A | 4 | 9.1 | 1 | 3 | N/A | N/A |
| T/G | C-T | ||||||
| A/T | A-A | 0 | 0.0 | 0 | 0 | N/A | N/A |
| T-T | |||||||
| G/C | G-G | 5 | 11.4 | 1 | 4 | N/A | N/A |
| C-C | |||||||
| Small deletions | 3 | 6.8 | 1 | 2 | N/A | N/A | |
| Sum | 44 | 100 | 14 | 30 |
Arbitrary classification of SURVEYOR digest grade. Type 1, “strong” digestion; type 2, “weak” digestion.
Variants that can putatively be explained as transitions of C to T.
Our results show that SURVEYOR can detect all kinds of potential single-nucleotide substitutions. The efficiency of cleavage varies but does not seem to be related to the kind of mismatch. “Strong” cleavages and “weak” cleavages can be found in all categories of heteroduplexes (Table 3 and Figure 1A). Some researchers report that this enzyme can detect mutations that were difficult to see by denaturing high-performance liquid chromatography,17 and others report that it can detect heteroduplexes representing as little as 3% of the substrate.39 Due to the fact that SURVEYOR does not cleave completely the PCR substrate,4 the number and intensity of the cleavage fragments can vary when more than one mismatches exist within the same PCR product. As very short cleavage fragments are difficult to detect in agarose gels, resulting assessment is through the detection of the larger of the resulting DNA fragments. In Figure 1B we show the digestion of a PCR product with three variations, with all of the cleavage and fragment combinations predicted, beside the viewable ones in the agarose gel. Hence, investigators using SURVEYOR must be prepared for these cleavage patterns in DNA fragments with multiple variations. Notwithstanding the rather complex pattern expected, the location of the variations can be predicted quite accurately (Figure 1).
Figure 1.
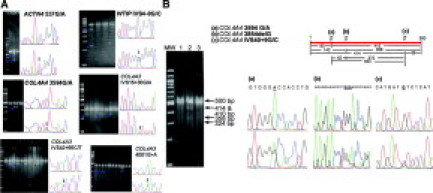
A: Examples of SURVEYOR nuclease single heteroduplex cleavages. Appearance of extra bands in the electrophoresis picture is a sign for a variant. Usually one additional band appears below the original intact PCR product for single nucleotide substitutions or single indels (arrowheads). False positives are rare. The “cleavage strength” is not the same for different variants. The sequencing chromatograms placed next to the agarose gel images correspond to a homozygous sample (top) and to a heterozygous sample (bottom) for the sequence variant. Arrows point to the heterozygous sequence. B: Detection of multiple mismatches within the same DNA fragment of 500 bp. Shown is an example of a DNA fragment representing exon 39 and flanking sequences of COL4A4 where three mismatches result in multiple cleavages and a rather complex electrophoretic pattern. Adding to the complexity is the inherent weakness of the enzyme to proceed to complete cleavage of each mismatch. Careful inspection of the gel allows deduction of number of cleavages and position of mismatches. Very small bands are not visible, either because they are too faint or they run off.
Unfortunately, SURVEYOR and related enzymes like CELI cannot identify 100% of the genetic variants. Otto et al40 found a sensitivity of 92% for CELI (automatic fluorescent detection by WAVE system), checking for 79 known mutations in NPHP genes (the catalogue of detected mutations is not given), and Scaffino et al41 found a sensitivity of 90% (3/3 deletions, 2/2 insertions, and 12/14 single-base substitutions; detection through Eurobio 3:1 agarose gels). The effectiveness of the enzyme may depend on the sequence, but the exact factors are not known.
To assess the sensitivity of this enzyme in the search for unknown variants, we performed a separate experiment where we tested for 15 previously reported DNA variants that included different kinds of heteroduplexes, most of them on kidney-related genes. SURVEYOR detected 14 of the variants, thereby providing a detection rate of 93% (Figure 2A and Table 4). Surprisingly, the undetected variant was a single-nucleotide deletion, the pathogenic mutation c.3533delC – COL4A3 (autosomal Alport syndrome42). This particular mutation was resistant to detection even when adding more enzyme or increasing digestion time or using PCR products with alternative primer sets (for alternative primer sets and conditions see Supplementary Table 6 at http://jmd.amjpathol.org). DNA sequencing of exon 41 allowed us to detect the above deletion in a heterozygous sample, thus confirming that there was no dropout of the mutant allele during amplification (Figure 2B).
Figure 2.
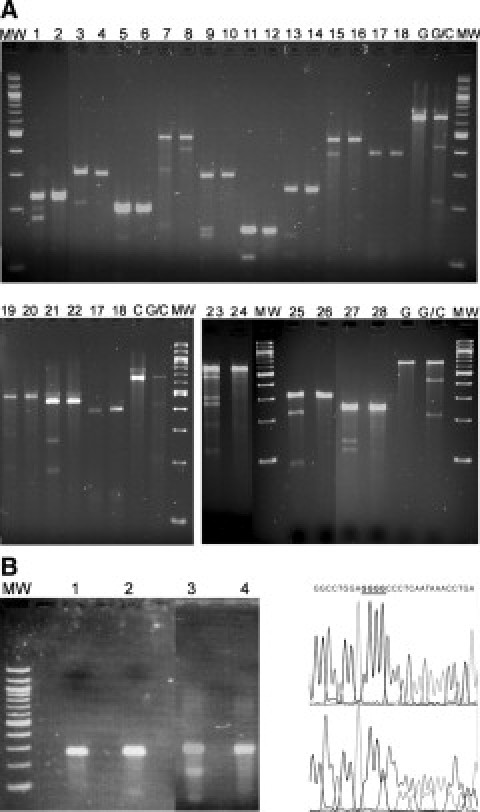
A: Fifteen known genetic variants (Table 4), most of them related with renal diseases, were tested with SURVEYOR to evaluate its sensitivity. Fourteen of them were successfully detected. The undetected one was c.3533delC – COL4A3 mismatch that failed to be cleaved by SURVEYOR (lanes 17 and 18; see text). Based on these results, sensitivity of SURVEYOR can be estimated at 93% (14 of 15). Samples were electrophoresed on 3% Eurobio 3:1 agarose gels. Zygosity status of each sample was known from previous investigations in our laboratory. Apparently sample 23 was heterozygous at more than one location (not sequenced). G & C, homozygous controls (provided by supplier of SURVEYOR); G/C, heterozygous control (provided by supplier of SURVEYOR); MW, 100-bp molecular weight ladder. B: Another demonstration of the failure of SURVEYOR to detect c.3533delC – COL4A3 mutation in COL4A3 exon 41, this time with more enzyme and more PCR product (chromatograms show the reverse sequence; deleted is one of the underlined bases). Lane 1, normal exon 41; lane 2, mutated exon 41; lane 3, SURVEYOR positive control; lane 4, SURVEYOR negative control. MW, 100-bp molecular weight ladder.
Table 4.
Information about the 15 Known Genetic Variants Tested for Detection with SURVEYOR
| Lane no. | Gene | Variant | Zygosity status and variant type |
|---|---|---|---|
| 1 | ADAMTS1 | A227P | Heterozygous G/C |
| 2 | ADAMTS1 | Homozygous G/G | |
| 3 | ATP6V1B1 | R157C | Heterozygous C/T |
| 4 | ATP6V1B1 | Homozygous C/C | |
| 5 | MTHFR | A222V | Heterozygous C/T |
| 6 | MTHFR | Homozygous T/T | |
| 7 | HFE | C282Y | Heterozygous G/A variant; homozygous for IVS3-48G; see sample 8 below |
| 8 | HFE | IVS3-48G/A | Heterozygous G/A variant; homozygous for C282; see sample 7 above |
| 9 | HFE | H63D | Heterozygous C/G |
| 10 | HFE | Homozygous C/C | |
| 11 | SLC3A1 | T216 mol/L | Heterozygous C/T |
| 12 | SLC3A1 | Homozygous C/C | |
| 13 | SLC3A1 | M467K | Heterozygous T/A |
| 14 | SLC3A1 | Homozygous T/T | |
| 15 | SERPINE2 | IVS8+ 111A/G | Heterozygous A/G |
| 16 | SERPINE2 | Homozygous A/A | |
| 17 | COL4A3 | c.3533delC | Heterozygous delC |
| 18 | COL4A3 | Homozygous wild type | |
| 19 | COL4A5 | IVS32-11G/A | Heterozygous G/A |
| 20 | COL4A5 | Homozygous G/G | |
| 21 | COL4A5 | c.3075delT | Heterozygous delT |
| 22 | COL4A5 | Homozygous wild type | |
| 23 | MYH9 | rs4821480 | Heterozygous T/G |
| 24 | MYH9 | Homozygous T/T | |
| 25 | ATP6V1B1 | IVS7+ 1G>T | Heterozygous G/T |
| 26 | ATP6V1B1 | Homozygous G/G | |
| 27 | MYH9 | rs4821481 | Heterozygous T/C |
| 28 | MYH9 | Homozygous T/T |
Lane number in column 1 refers to the results shown in Figure 2A.
To our knowledge, this is the first time that a single nucleotide deletion proves to be refractory to detection by SURVEYOR. Heteroduplexes formed as a result of indels, compared with single base substitutions, are thought to be more easily detectable by enzyme mismatch cleavage methods due to the fact that any hydrogen bonds between the DNA strands are completely absent at the mismatch locus. Apparently, factors such as specific nucleotide mismatches and neighboring sequence effects can determine the effectiveness of these enzymes. On the other hand, our experience showed that Eurobio 3:1 agarose gels are very effective for the detection of cleavage fragments when using appropriate amount of the digested PCR product; automatic fluorescent analysis probably does not offer significant advantages. As mentioned previously, Otto et al40 and Scaffino et al41 found similar enzyme sensitivity using different techniques. As regards the correct targeting of the SURVEYOR enzyme, we had only two false positive results, one for exon 8 of the WT1 (Wilm's tumor 1) gene and one for the exon 48 of the COL4A4 gene, where our impressions for cleavage by SURVEYOR were not substantiated on DNA re-sequencing (not shown).
Caution should be exercised in regard to the enzyme's occasional strong exonucleolytic activity. The Enhancer reagent provided by the commercial supplier (Transgenomics, UK) is not protective enough for some PCR products. Perhaps the exonucleolytic activity, like the endonucleolytic one, depends on the DNA sequence. In our hands, shorter incubation time and preparing reactions fast on ice can reduce adequately the exonucleolysis. Both activities of SURVEYOR are well preserved during long storage times at −20°C. The enzyme was effectively used in our laboratory even after 3 years of storage time.
The location of the 44 variations in relation to the nearest end of the PCR product is represented in percentage values in Figure 3. It is obvious that there is not any “preference” of SURVEYOR for cleaving mismatches at the ends or in the middle of the PCR products. The positions of the identified variations are equally distributed in a continuous spectrum throughout the entire sequence of the PCR products (Figure 3).
Figure 3.
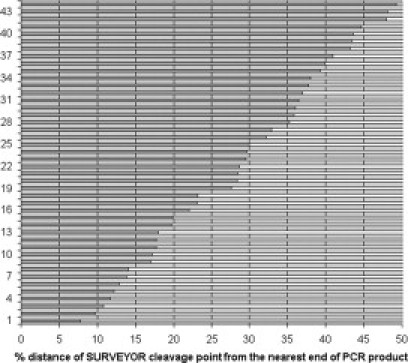
Schematic mapping of the 44 sequence variants identified here, according to their position in the respective PCR product. For any PCR product with a detectable variant sequence shown on the y axis, the x axis presents the distance of the mismatch from the nearest end of the respective PCR DNA fragment, converted to a percentage scale (dark line). For example, the DNA fragment with variant 7 in this figure (corresponding to variant 40 of Table 2) harbored a mismatch very close to one of the ends, hence nearly 14% distance from the nearest end. The nuclease does not seem to have bias for cleaving at specific locations across the DNA fragment. Numbers of variations (y axis) do not correspond with those of Table 2 because they have been sorted solely based on the position of the mismatch within the particular PCR product. See text for further explanation.
In conclusion, we showed that using only conventional equipment belonging to a basic laboratory and commercially available reagents, the SURVEYOR nuclease can detect cheaply and fast a high percentage of DNA variations. This method may be an excellent alternative approach for mutation screening for inherited diseases with increased genetic heterogeneity, like many renal diseases, thus enhancing the diagnostic procedures. Genes with multiple exons, like COL4A3/COL4A4, which are very close to each other (accounting for 100 exons; both genes must be screened), can be handled effectively with this enzyme. In a total of 48.2 kb, mostly coding DNA sequences (328.5 kb when accounting for multiple samples) screened for mutations in this study, 44 variations were found, hence one variation per 1.1 kb. The re-sequencing of multiple PCR products after evidence for cleavage did not detect any variation that had not been identified by the SURVEYOR enzyme, attesting to its high but certainly not absolute fidelity. We validated further the effectiveness of the enzyme by re-sequencing of the entire coding regions of ACTN4 and NPHS2 genes in three and 12 patients, respectively. We did not find any variations not detected by SURVEYOR. The SURVEYOR enzyme does not seem to present any bias for detecting a particular DNA variation; rather it demonstrates unique advantages and robustness. However, despite our confidence in this approach, we should also note that our own experience demonstrates that the efficiency is not 100%, as evidenced by missing mutation c.3533delC in the COL4A3 gene. Finally, to our knowledge our work is the first presentation of data regarding the systematic use of this enzyme for mutation screening and identification in eight genes mutated in inherited kidney diseases.
Footnotes
Supported by grant ENIΣX/0505/02 from the Cyprus Research Promotion Foundation and by the Cyprus Ministry of Health, the Cyprus Kidney Association (scholarship to K.V.), and the University of Cyprus (Research Activities 3/312).
Supplemental material for this article can be found on http://jmd.amjpathol.org.
Supplementary data
References
- 1.Oleykowski CA, Bronson Mullins CR, Godwin AK, Yeung AT. Mutation detection using a novel plant endonuclease. Nucleic Acids Res. 1998;26:4597–4602. doi: 10.1093/nar/26.20.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang B, Wen X, Kodali NS, Oleykowski CA, Miller CG, Kulinski J, Besack D, Yeung JA, Kowalski D, Yeung AT. Purification, cloning, and characterization of CEL I nuclease. Biochemistry. 2000;39:3533–3541. doi: 10.1021/bi992376z. [DOI] [PubMed] [Google Scholar]
- 3.Sokurenko EV, Tchesnokova V, Yeung AT, Oleykowski CA, Trintchina E, Hughes KT, Rashid RA, Brint JM, Moseley SL, Lory S. Detection of simple mutations and polymorphisms in large genomic regions. Nucleic Acids Res. 2001;29:e111. doi: 10.1093/nar/29.22.e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu P, Shandilya H, D'Alessio JM, O'Connor K, Durocher J, Gerard GF. Mutation detection using Surveyor nuclease. BioTechniques. 2004;36:702–707. doi: 10.2144/04364PF01. [DOI] [PubMed] [Google Scholar]
- 5.Smits BMG, van Zutphen BFM, Plasterk RHA, Cuppen E. Genetic variation in coding regions between and within commonly used inbred rat strains. Genome Res. 2004;14:1285–1290. doi: 10.1101/gr.2155004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi R, Otomo K, Yamada H, Tatsumi T, Sugawara I. Temperature-mediated heteroduplex analysis for the detection of drug-resistant gene mutations in clinical isolates of Mycobacterium tuberculosis by denaturing HPLC: SURVEYOR nuclease. Microbes Infect. 2006;8:128–135. doi: 10.1016/j.micinf.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Till BJ, Reynolds SH, Greene EA, Codomo CA, Enns LC, Johnson JE, Burtner C, Odden AR, Young K, Taylor NE, Henikoff JG, Comai L, Henikoff S. Large-scale discovery of induced point mutations with high throughput TILLING. Genome Res. 2003;13:524–530. doi: 10.1101/gr.977903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry JA, Wang TL, Welham TJ, Gardner S, Pike JM, Yoshida S, Parniske M. A TILLING reverse genetics tool and a Web-accessible collection of mutants of the legume Lotus japonicus. Plant Physiol. 2003;131:866–871. doi: 10.1104/pp.102.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wienholds E, van Eeden F, Kosters M, Mudde J, Plasterk RHA, Cuppen E. Efficient target-selected mutagenesis in zebrafish. Genome Res. 2003;13:2700–2707. doi: 10.1101/gr.1725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bannwarth S, Procaccio V, Paquis-Flucklinger V. Rapid identification of mitochondrial DNA (mtDNA) mutations in neuromuscular disorders by using surveyor strategy. Nat Protoc. 2006;1:2037–2047. doi: 10.1038/nprot.2006.318. [DOI] [PubMed] [Google Scholar]
- 11.Bannwarth S, Procaccio V, Rouzier C, Fragaki K, Poole J, Chabrol B, Desnuelle C, Pouget J, Azulay JP, Attarian S, Pellissier JF, Gargus JJ, Abdenur JE, Mozaffar T, Calvas P, Labauge P, Pages M, Wallace DC, Lambert JC, Paquis-Flucklinger V. Rapid identification of unknown heteroplasmic mutations across the entire human mitochondrial genome with mismatch-specific Surveyor nuclease. Mitochondrion. 2008;8:136–145. [Google Scholar]
- 12.Qiu P, Shandilya H, Gerard GF. A method for clone confirmation using a mismatch-specific DNA endonuclease. Mol Biotechnol. 2005;29:11–18. doi: 10.1385/MB:29:1:11. [DOI] [PubMed] [Google Scholar]
- 13.Kulinski J, Besack D, Oleylowski CA, Godwin AK, Yeung AT. CEL I enzymatic mutation detection assay. BioTechniques. 2000;29:44–48. doi: 10.2144/00291bm07. [DOI] [PubMed] [Google Scholar]
- 14.Janne PA, Borras AM, Kuang Y, Rogers AM, Joshi VA, Liyanage H, Lindeman N, Lee JC, Halmos B, Maher EA, Distel RJ, Meyerson M, Johnson BE. A rapid and sensitive enzymatic method for epidermal growth factor receptor mutation screening. Clin Cancer Res. 2006;12:751–758. doi: 10.1158/1078-0432.CCR-05-2047. [DOI] [PubMed] [Google Scholar]
- 15.Sattler M, Walz C, Crowley BJ, Lengfelder E, Janne PA, Rogers AM, Kuang Y, Distel RJ, Reiter A, Griffin JD. A sensitive high-throughput method to detect activating mutations of Jak2 in peripheral-blood samples. Blood. 2006;107:1237–1238. doi: 10.1182/blood-2005-07-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowak D, Mossner M, Baldus CD, Hopfer O, Hofmann WK. Mutation analysis of hCDC4 in AML cells identifies a new intronic polymorphism. Int J Med Sci. 2006;3:148–151. doi: 10.7150/ijms.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada T, Fukushima Y, Saitoh S. A new detection method for ATRX gene mutations using a mismatch-specific endonuclease. Am J Med Genet A. 2006;140:1519–1523. doi: 10.1002/ajmg.a.31310. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Terry SF, Terry PF, Bercovitch LG, Gerard GF. Development of a rapid, reliable genetic test for Pseudoxanthoma elasticum. J Mol Diagn. 2007;9:105–112. doi: 10.2353/jmoldx.2007.060093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitani N, Niwa Y, Okamoto Y. Surveyor nuclease-based detection of p53 gene mutations in haematological malignancy. Ann Clin Biochem. 2007;44:557–559. doi: 10.1258/000456307782268174. [DOI] [PubMed] [Google Scholar]
- 20.Voskarides K, Makariou C, Papagregoriou G, Stergiou N, Printza N, Alexopoulos E, Elia A, Papachristou F, Georgaki E, Pierides A, Deltas C. NPHS2 screening with SURVEYOR nuclease in Hellenic children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2008;23:1373–1375. doi: 10.1007/s00467-008-0804-3. [DOI] [PubMed] [Google Scholar]
- 21.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D, Saunders J, Westra W, Sidransky D, Koch WM. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voskarides K, Damianou L, Neocleous V, Zouvani I, Christodoulidou S, Hadjiconstantinou V, Ioannou K, Athanasiou Y, Patsias C, Alexopoulos E, Pierides A, Kyriakou K, Deltas C. COL4A3/COL4A4 mutations producing focal segmental glomerulosclerosis and renal failure in thin basement membrane nephropathy. J Am Soc Nephrol. 2007;18:3004–3016. doi: 10.1681/ASN.2007040444. [DOI] [PubMed] [Google Scholar]
- 23.Nollau P, Wagener C. Methods for detection of point mutations: performance and quality assessment. IFCC Scientific Division, Committee on Molecular Biology Techniques. Clin Chem. 1997;43:1114–1428. [PubMed] [Google Scholar]
- 24.Mean R, Pierides A, Deltas C, Koptides M. Modification of the enzyme mismatch cleavage method using T7 endonuclease I and silver staining. BioTechniques. 2004;36:758–760. doi: 10.2144/04365BM01. [DOI] [PubMed] [Google Scholar]
- 25.Yeung AT, Hattangadi D, Blakesley L, Nicolas E. Enzymatic mutation detection technologies. BioTechniques. 2005;38:749–758. doi: 10.2144/05385RV01. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji T, Niida Y. Development of a simple and highly sensitive mutation screening system by enzyme mismatch cleavage with optimized conditions for standard laboratories. Electrophoresis. 2008;29:1473–1483. doi: 10.1002/elps.200700729. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 28.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 29.Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemmink HH, Nillesen WN, Mochizuki T, Schroder CH, Brunner HG, van Oost BA, Monnens LA, Smeets HJ. Benign familial. hematuria due to mutation of the type IV collagen α4 gene. J Clin Invest. 1996;98:1114–1118. doi: 10.1172/JCI118893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rana K, Wang YY, Buzza M, Tonna S, Zhang KW, Lin T, Sin L, Padavarat S, Savige J. The genetics of thin basement membrane nephropathy. Semin Nephrol. 2005;25:163–170. doi: 10.1016/j.semnephrol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Christodoulou K, Tsingis M, Stavrou C, Eleftheriou A, Papapavlou P, Patsalis PC, Ioannou P, Pierides A, Constantinou Deltas C. Chromosome 1 localization of a gene for autosomal dominant medullary cystic kidney disease (ADMCKD) Hum Mol Genet. 1998;7:905–911. doi: 10.1093/hmg/7.5.905. [DOI] [PubMed] [Google Scholar]
- 33.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 34.Karle SM, Uetz B, Ronner V, Glaeser L, Hildebrandt F, Fuchshuber A. Novel mutations in NPHS2 detected in both familial and sporadic steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2002;13:388–393. doi: 10.1681/ASN.V132388. [DOI] [PubMed] [Google Scholar]
- 35.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 37.Lindblad-Toh K, Winchester E, Daly MJ, Wang DG, Hirschhorn JN, Laviolette JP, Ardlie K, Reich DE, Robinson E, Sklar P, Shah N, Thomas D, Fan JB, Gingeras T, Warrington J, Patil N, Hudson TJ, Lander ES. Large-scale discovery and genotyping of single-nucleotide polymorphisms in the mouse. Nat Genet. 2000;24:381–386. doi: 10.1038/74215. [DOI] [PubMed] [Google Scholar]
- 38.Halushka MK, Fan JB, Bentley K, Hsie L, Shen N, Weder A, Cooper R, Lipshutz R, Chakravarti A. Patterns of single-nucleotide polymorphisms in candidate genes for blood-pressure homeostasis. Nat Genet. 1999;22:239–247. doi: 10.1038/10297. [DOI] [PubMed] [Google Scholar]
- 39.Bannwarth S, Procaccio V, Paquis-Flucklinger V. Surveyor nuclease: a new strategy for a rapid identification of heteroplasmic mitochondrial DNA mutations in patients with respiratory chain defects. Hum Mutat. 2005;25:575–582. doi: 10.1002/humu.20177. [DOI] [PubMed] [Google Scholar]
- 40.Otto EA, Helou J, Allen SJ, O'Toole JF, Wise EL, Ashraf S, Attanasio M, Zhou W, Wolf MT, Hildebrandt F. Mutation analysis in nephronophthisis using a combined approach of homozygosity mapping: CEL I endonuclease cleavage, and direct sequencing. Hum Mutat. 2008;29:418–426. doi: 10.1002/humu.20669. [DOI] [PubMed] [Google Scholar]
- 41.Scaffino MF, Pilotto A, Papadimitriou S, Sbalzarini M, Ansaldi S, Diegoli M, Porcu E, Grasso M, Brega A, Arbustini E. Heteroduplex detection with a plant DNA endonuclease for standard gel electrophoresis. Transgenics. 2004;4:157–166. [Google Scholar]
- 42.Heidet L, Arrondel C, Forestier L, Cohen-Solal L, Mollet G, Gutierrez B, Stavrou C, Gubler MC, Antignac C. Structure of the human type IV collagen gene COL4A3 and mutations in autosomal Alport syndrome. J Am Soc Nephrol. 2001;12:97–106. doi: 10.1681/ASN.V12197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


