Abstract
Monoclonal antibodies reactive with three different viral polypeptides were evaluated singly and in combination as the capture antibody(s) in an enzyme-linked immunosorbent assay system for the detection of bovine enteric coronavirus. Similar levels of sensitivity were found for all combinations tested. A sensitive, highly specific, and reproducible assay for the detection of bovine enteric coronavirus was developed, using a mixture of two of these monoclonal antibodies reactive with antigenic components either external or internal to the virion. These monoclonal antibodies were bound indirectly to 96-well plates via rabbit anti-mouse immunoglobulin. After sample application and incubation, virus was detected by using rabbit anti-coronavirus peroxidase conjugate followed by enzyme substrate and chromagen. Fecal samples from a single herd of cows were screened for the presence of coronavirus by this assay. Five percent of clinically normal cows were found to be shedding coronavirus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar R., Conklin R. H., Vollet J. J., Pickering L. K., DuPont H. L., Walters D. L., Kohl S. Rotavirus in travelers' diarrhea: study of an adult student population in Mexico. J Infect Dis. 1978 Mar;137(3):324–327. doi: 10.1093/infdis/137.3.324. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut P., Debouck P., Pensaert M. Enzyme-linked immunosorbent assay for the detection of the coronavirus-like agent and its antibodies in pigs with porcine epidemic diarrhea. Vet Microbiol. 1982 Sep;7(4):295–306. doi: 10.1016/0378-1135(82)90009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrystie I. L., Totterdell B. M., Banatvala J. E. Asymptomatic endemic rotavirus infections in the newborn. Lancet. 1978 Jun 3;1(8075):1176–1178. doi: 10.1016/S0140-6736(78)90967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch C. F., Raybould T. J. Comparison of different antigen preparations as substrates for use in passive hemagglutination and enzyme-linked immunosorbent assays for detection of antibody against bovine enteric coronavirus. J Clin Microbiol. 1983 Jul;18(1):146–149. doi: 10.1128/jcm.18.1.146-149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukor G., Berry M. K., Blacklow N. R. Simplified radioimmunoassay for detection of human rotavirus in stools. J Infect Dis. 1978 Dec;138(6):906–910. doi: 10.1093/infdis/138.6.906. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Frankel M. E., Gerhard W. The rapid determination of binding constants for antiviral antibodies by a radioimmunoassay. An analysis of the interaction between hybridoma proteins and influenza virus. Mol Immunol. 1979 Feb;16(2):101–106. doi: 10.1016/0161-5890(79)90051-8. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Wyatt R. G., Valdesuso J., Kalica A. R., London W. T., Chanock R. M., Kapikian A. Z. Solid-phase microtiter radioimmunoassay for detection of the Norwalk strain of acute nonbacterial, epidemic gastroenteritis virus and its antibodies. J Med Virol. 1978;2(2):97–108. doi: 10.1002/jmv.1890020204. [DOI] [PubMed] [Google Scholar]
- Kemeny L. J. Isolation of transmissible gastroenteritis virus from pharyngeal swabs obtained from sows at slaughter. Am J Vet Res. 1978 Apr;39(4):703–705. [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Dunsmoor C. L. Principles, problems, and strategies in the use of antigenic mixtures for the enzyme-linked immunosorbent assay. J Clin Microbiol. 1983 Apr;17(4):655–665. doi: 10.1128/jcm.17.4.655-665.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
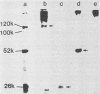
- King B., Brian D. A. Bovine coronavirus structural proteins. J Virol. 1982 May;42(2):700–707. doi: 10.1128/jvi.42.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langpap T. J., Bergeland M. E., Reed D. E. Coronaviral enteritis of young calves: virologic and pathologic findings in naturally occurring infections. Am J Vet Res. 1979 Oct;40(10):1476–1478. [PubMed] [Google Scholar]
- Marsolais G., Assaf R., Montpetit C., Marois P. Diagnosis of viral agents associated with neonatal calf diarrhea. Can J Comp Med. 1978 Apr;42(2):168–171. [PMC free article] [PubMed] [Google Scholar]
- Mebus C. A., Stair E. L., Rhodes M. B., Twiehaus M. J. Pathology of neonatal calf diarrhea induced by a coronavirus-like agent. Vet Pathol. 1973;10(1):45–64. doi: 10.1177/030098587301000105. [DOI] [PubMed] [Google Scholar]
- Moon H. W., McClurkin A. W., Isaacson R. E., Pohlenz J., Skartvedt S. M., Gillette K. G., Baetz A. L. Pathogenic relationships of rotavirus, Escherichia coli, and other agents in mixed infections in calves. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):577–583. [PubMed] [Google Scholar]
- Morin M., Larivière S., Lallier R. Pathological and microbiological observations made on spontaneous cases of acute neonatal calf diarrhea. Can J Comp Med. 1976 Jul;40(3):228–240. [PMC free article] [PubMed] [Google Scholar]
- Petric M., Middleton P. J., Grant C., Tam J. S., Hewitt C. M. Lapine rotavirus: preliminary studies on epizoology and transmission. Can J Comp Med. 1978 Jan;42(1):143–147. [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Wolters G., Kuijpers L., Kacaki J., Schuurs A. Solid-phase enzyme-immunoassay for detection of hepatitis B surface antigen. J Clin Pathol. 1976 Oct;29(10):873–879. doi: 10.1136/jcp.29.10.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H. Enzyme immunoassays for the detection of infectious antigens in body fluids: current limitations and future prospects. Rev Infect Dis. 1982 Jan-Feb;4(1):35–68. doi: 10.1093/clinids/4.1.35. [DOI] [PubMed] [Google Scholar]
- Yolken R., Wyatt R. G., Kapikian A. Z. ELISA for rotavirus. Lancet. 1977 Oct 15;2(8042):819–819. doi: 10.1016/s0140-6736(77)90750-4. [DOI] [PubMed] [Google Scholar]