Abstract
Acute graft-versus-host disease (GVHD) is an uncommon but often fatal complication following liver transplant. We describe a GVHD case in which a female patient with primary biliary cirrhosis underwent a living-related liver transplant from her son. The human leukocyte antigen typing of the donor was homozygous at all loci. The recipient's human leukocyte antigen type was haplo-identical to that of the donor. A bone marrow aspirate performed for pancytopenia revealed a severely hypoplastic marrow. Fluorescent in situ hybridization (FISH) using X- and Y-chromosome probes demonstrated that 80% of marrow cells were of donor origin. Comparison of Giemsa-stained cell morphology and FISH showed that the erythroid precursor cells were predominantly of male pattern (XY). This report is one of only a few studies that prove the migration of a donor's hematopoietic stem cells to a recipient's bone marrow. We demonstrated that FISH analysis using sex chromosome probes is useful to confirm a diagnosis of GVHD following organ transplantation from a donor of the opposite sex. We also showed that donor hematopoietic stem cells in a liver graft can migrate to the recipient's bone marrow. We suggest that FISH is a rapid and reliable test for confirming the diagnosis of GVHD in a peripheral blood or skin biopsy sample.
Acute graft-versus-host disease (GVHD) following liver transplantation is a rare complication with a high mortality rate.1,2 GVHD occurs when immunocompetent donor lymphocytes originating from the transplanted organ undergo activation and clonal expansion, reacting against the recipient antigens. The clinical course begins with fever or skin rash as an early sign, followed by pancytopenia, overwhelming sepsis, and death.3
The diagnosis of GVHD is a major challenge and thus the condition often goes unrecognized. Detection of donor lymphocytes in peripheral blood or bone marrow in high-level (>1%) is a key to early detection of GVHD.1,3
Case Report
A 49-year-old white female with hilar cholangiocarcinoma associated with primary biliary cirrhosis received a livingrelated liver transplant from her son. She received two units of leukocyte-reduced packed red blood cells for operative blood loss. Post-transplant immunosuppression was with mycophenolate mofetil, tacrolimus, and prednisone. Her post-transplant course was complicated by multiple embolic strokes 6 days after transplantation. Four weeks after transplantation, she developed pancytopenia with nadir blood counts of hemoglobin 8.0 g/dL, platelets 4 x 109/L, and leukocytes 0.2 x 109 cells per L. Treatment with granulocyte-colony stimulating factor was begun but there was no improvement in blood counts.
Six weeks after transplant, she developed diarrhea and an erythematous maculopapular skin rash on her back. The rash was biopsied and pathological findings were consistent with GVHD. Computed tomography of the abdomen showed thickened loops of small intestine. The patient was diagnosed with GVHD and was treated with methylprednisolone. She developed acute respiratory failure, worsening gastrointestinal symptoms, and died 46 days after transplantation.
Materials and Methods
HLA Typing
HLA typing was performed by serological techniques for HLA-A, HLA-B, HLA-C, HLA-DR, and HLA-DQ. The donor (son) is homozygous for all loci (A1; B8; C7; DR17; DQ7). The recipient (mother) shared the haplotype (A1, 3; B8, 18; C7; DR 12, 17, DQ2, 7).
Histology and Cytology
Bone marrow biopsy and aspirate specimens were obtained on day 28 and submitted for routine morphological analysis. Fluorescent in situ hybridization (FISH) was performed on the bone marrow smear specimen using directly labeled centromeric X- and Y-chromosome probes (Vysis/Abbott Molecular, Des Plaines, IL). The probes were hybridized on the smear using standard procedures, followed by counterstaining with 4,6-diamidino-2-phenylindole. Sequential FISH analysis of Giemsa-stained smears was performed to investigate the genotype of the hematopoietic cells. Peripheral blood was obtained on the same day and submitted for FISH analysis. In addition, a skin biopsy obtained from an erythematous lesion was stained with H&E.
Results
Bone Marrow/Peripheral Blood
The bone marrow revealed extreme hypocellularity with occasional clusters of erythroid precursors (Figure 1A). There were also small foci of lymphocytes, mostly T-cells (Figure 1B). Of 500 bone marrow cells analyzed, 79.4% showed one X and one Y signal, and 20.6% showed two X signals. Of eight cells with erythroid precursor morphology, four showed an XY signal pattern while one showed an XX signal pattern (Figure 2, A and B). Another three cells showed morphology of lymphocyte with an XX signal pattern (Figure 2, C and D).
Figure 1.
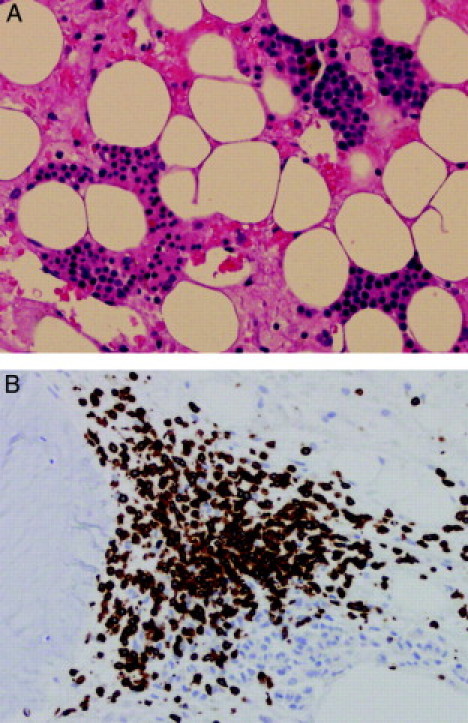
A: Bone marrow biopsy reveals hypocellular marrow with serous degeneration. A few aggregates of erythroid precursor are seen (H&E, magnification = original ×200). B: Immunohistochemical staining for CD3 showing a cluster of T lymphocytes at peritrabecular space (magnification = original ×200).
Figure 2.
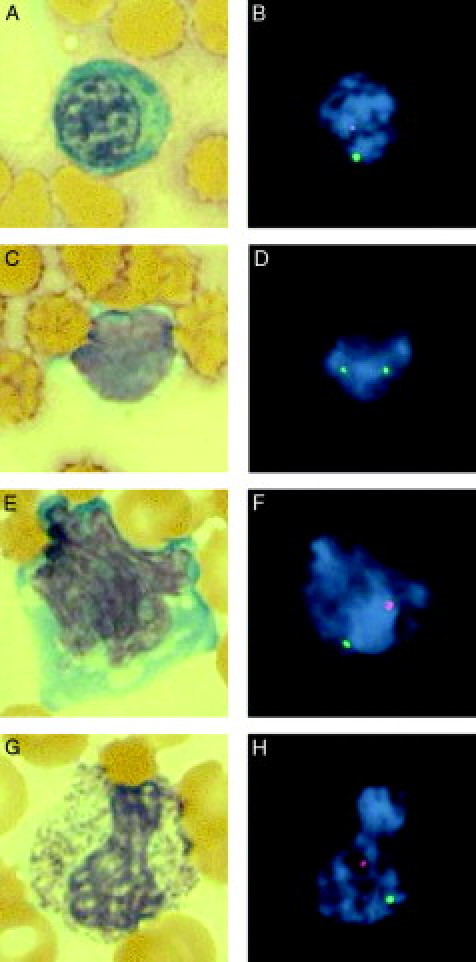
Comparison of Giemsa-stained morphology and FISH with X-chromosome (green) and Y-chromosome (red) probes (magnification = original ×1000). A: Polychromic normoblast in bone marrow (Giemsa). B: FISH analysis of the same cell reveals XY male (donor) pattern. C: Lymphocyte in bone marrow (Giemsa). D: FISH analysis of the same cell reveals XX female (recipient) pattern. E: Activated lymphocyte in peripheral blood (Giemsa). F: FISH analysis of the same cell reveals XY male (donor) pattern. G: Band neutrophil in peripheral blood (Giemsa). H: FISH analysis of the same cell reveals XY male (donor) pattern.
Of 500 interphase nuclei analyzed, 89.4% showed an XY signal pattern, and 10.6% showed an XX signal pattern. Morphology and FISH comparison was successful in two cells, one lymphocyte and one band neutrophil, with an XY signal pattern in both cells (Figure 2E–H).
Skin Biopsy
The morphological examination of skin biopsy revealed lymphocytic infiltration in the epidermis, epidermal-dermal junction, and superficial dermis. Vacuolization and spongiosis were seen in the basal layer of the epidermis. Occasional individual cell necrosis was also noted in keratocytes (Figure 3A). X- and Y-chromosome FISH analysis revealed XX and XY patterns in the lymphocytes occupying the perivascular spaces (Figure 3, B and C). Lymphocytes with an XY signal pattern were also noted in the epidermis.
Figure 3.
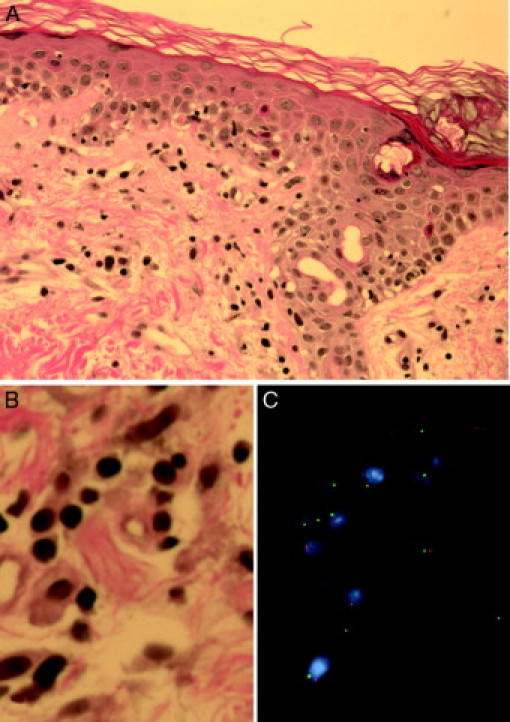
A: Skin biopsy reveals lymphocytic infiltration in epidermis, epidermal-dermal junction and superficial perivascular spaces. Occasional keratocytes with eosinophilic necrosis are also seen in epidermis (H&E, magnification = original ×400). B: High-power view of superficial dermis with perivascular lymphocytic infiltrate (H&E, magnification = original ×1000). C: FISH analysis with X- (green) and Y-chromosome (red) probes showing mixture of XY and XX cells (magnification = original ×1000).
Discussion
HLA match between donor and recipient is a significant risk factor for GVHD in liver transplant.4 Although HLA matching is not considered for organ assignment in cadaveric liver transplant, recipients of complete HLA-matched grafts by chance appear to be at increased risk of GVHD.3 The proposed mechanism for GVHD in these cases is that hepatic lymphocytes in the well-matched graft are not recognized as foreign by the recipient immune system and survive to react against the recipient tissues. Although close HLA matching is unlikely in cadaveric transplant given the random graft assignment, it is much more common in living-related liver transplants. The risk of GVHD seems to be exaggerated when a graft from an HLA-homozygous donor is transplanted to a haplo-identical recipient, ie, donor-dominant one-way HLA match.5,6 The incidence of HLA homozygosity is variable among different ethnic groups. It is reported to be 1.6% among the Caucasian population, and 3.2% among blood donors in Japan.6 Most GVHD cases in living-related liver transplant have been reported from Japan,5,6,7,8 where living-related transplant predominate over cadaveric transplant due to ethical and/or cultural concerns regarding the use of cadaveric transplant.
One of the earliest clinical findings of acute GVHD is an erythematous skin rash.2,3 Routine skin biopsy may reveal typical histological features including lichenoid lymphocytic infiltrate vacuolar changes in the epidermal basal layer, and dyskeratosis. These features are highly suggestive, but not pathognomonic for GVHD.9,10 A firm diagnosis requires demonstration of chimerism from donor lymphocytes in recipient tissue, peripheral blood and/or bone marrow.3 The existence of donor lymphocytes can be demonstrated by HLA markers, PCR-based microsatellite markers, or if the graft is from sex-mismatched donor, FISH analysis using sex chromosome specific probes.10 Transient lymphocytic chimerism without subsequent development of GVHD is common in patients following liver transplant. Schlitt et al studied post-liver transplant donor-derived lymphocyte populations in 16 patients. All recipients demonstrated between 1% and 24% donor lymphocytes 1 week after transplant. In the second week, donor lymphocytes were detected in 8 of the 14 patients and ranged from 2% and 23%. After the second week, donor lymphocytes were still detected in two patients at very low number.11
A few cases of liver transplant with donor-derived hematopoiesis have been reported.12,13 It is not clear if this is a rare event, or relatively common but not diagnosed clinically. In the current case, the donor cell hematopoiesis was proven after the onset of the symptoms of GVHD. Donor cell hematopoiesis may be a part of the progression of GVHD. One patient had a long-term (17 month) survival with donor cell hematopoiesis, but with no evidence of GVHD and graft failure.13
FISH analysis is commonly used to identify chimerism following allogeneic bone marrow transplant. Quantitative analysis of chimerism may help to detect relapse of the disease, and to monitor the disease course. The procedure can detect as low as 1% donor or recipient cells, although simple demonstration of low-level chimerism has little value for diagnosis of GVHD. HLA typing using serology provides quantitative information of donor-derived lymphocytes in the circulation of recipients. However, the method is not informative when the HLA type of the donor is haplo-identical to that of the recipient. The short tandem-repeat assay using PCR is another quantitative method to analyze chimerism in the recipient. The method has been used in bone marrow transplant patients to monitor engraftment, and was recently applied to detect donor lymphocyte in liver transplant recipients.14 In contrast to these methods, FISH analysis can provide morphological information, if necessary, in addition to the origin of each hematopoietic cell. The major limitation of FISH analysis based on sex chromosome probes is that it can be applied only to the transplant cases with opposite sex donors. Recent studies by Wu et al15 showed a novel FISH approach to detect chimerism even in same-sex transplant cases using copy number polymorphisms. This approach, once standardized for routine clinical diagnosis, will help further understand the dynamics of cellular chimerism after transplantation.
As in the present case, demonstration of a large number of donor-derived hematopoietic cells in bone marrow and peripheral blood specimens is strongly suggestive of GVHD. The effect of donor-derived hematopoiesis in recipient bone marrow on GVHD is uncertain. FISH analysis using sex chromosome probes is useful to confirm the diagnosis following organ transplantation from a donor of the opposite sex.
References
- 1.Taylor AL, Gibbs P, Sudhindran S, Key T, Goodman RS, Morgan CH, Watson CJ, Delriviere L, Alexander GJ, Jamieson NV, Bradley JA, Taylor CJ. Monitoring systemic donor lymphocyte macrochimerism to aid the diagnosis of graft-versus-host disease after liver transplantation. Transplantation. 2004;77:441–446. doi: 10.1097/01.TP.0000103721.29729.FE. [DOI] [PubMed] [Google Scholar]
- 2.Kohler S, Pascher A, Junge G, Sauer IM, Nagy M, Schonemann C, Koch M, Neumann U, Pratschke J, Neuhaus P. Graft versus host disease after liver transplantation - a single center experience and review of literature. Transpl Int. 2008;21:441–451. doi: 10.1111/j.1432-2277.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- 3.Taylor AL, Gibbs P, Bradley JA. Acute graft versus host disease following liver transplantation: the enemy within. Am J Transplant. 2004;4:466–474. doi: 10.1111/j.1600-6143.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 4.Chan EY, Larson AM, Gernsheimer TB, Kowdley KV, Carithers RL, Jr, Reyes JD, Perkins JD. Recipient and donor factors influence the incidence of graft-vs.-host disease in liver transplant patients. Liver Transpl. 2007;13:516–522. doi: 10.1002/lt.21082. [DOI] [PubMed] [Google Scholar]
- 5.Kiuchi T, Harada H, Matsukawa H, Kasahara M, Inomata Y, Uemoto S, Asonuma K, Egawa H, Maruya E, Saji H, Tanaka K. One-way donor-recipient HLA-matching as a risk factor for graft-versus-host disease in living-related liver transplantation. Transpl Int. 1998;(11 Suppl 1):S383–S384. doi: 10.1007/s001470050503. [DOI] [PubMed] [Google Scholar]
- 6.Kamei H, Oike F, Fujimoto Y, Yamamoto H, Tanaka K, Kiuchi T. Fatal graft-versus-host disease after living donor liver transplantation: differential impact of donor-dominant one-way HLA matching. Liver Transpl. 2006;12:140–145. doi: 10.1002/lt.20573. [DOI] [PubMed] [Google Scholar]
- 7.Soejima Y, Shimada M, Suehiro T, Hiroshige S, Gondo H, Takami A, Yasue S, Maehara Y. Graft-versus-host disease following living donor liver transplantation. Liver Transpl. 2004;10:460–464. doi: 10.1002/lt.20101. [DOI] [PubMed] [Google Scholar]
- 8.Nemoto T, Kubota K, Kita J, Shimoda M, Rokkaku K, Tagaya N, Fujiwara T, Sunakawa M. Unusual onset of chronic graft-versus-host disease after adult living-related liver transplantation from a homozygous donor. Transplantation. 2003;75:733–736. doi: 10.1097/01.TP.0000053401.91094.CA. [DOI] [PubMed] [Google Scholar]
- 9.Schmuth M, Vogel W, Weinlich G, Margreiter R, Fritsch P, Sepp N. Cutaneous lesions as the presenting sign of acute graft-versus-host disease following liver transplantation. Br J Dermatol. 1999;141:901–904. doi: 10.1046/j.1365-2133.1999.03166.x. [DOI] [PubMed] [Google Scholar]
- 10.Meves A. Acute graft-versus-host disease after liver transplantation diagnosed by fluorescent in situ hybridization testing of skin biopsy specimens. J Am Acad Dermatol. 2006;55:642–646. doi: 10.1016/j.jaad.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 11.Schlitt HJ, Kanehiro H, Raddatz G, Steinhoff G, Richter N, Nashan B, Ringe B, Wonigeit K, Pichlmayr R. Persistance of donor lymphocytes in liver allograft recipients. Transplantation. 1993;56:1001–1007. doi: 10.1097/00007890-199310000-00042. [DOI] [PubMed] [Google Scholar]
- 12.Collins RH, Jr, Anastasi J, Terstappen LW, Nikaein A, Feng J, Fay JW, Klintmalm G, Stone M. J. Brief report: donor-derived long-term multilineage hematopoiesis in a liver-transplant recipient. N Engl J Med. 1993;328:762–765. doi: 10.1056/NEJM199303183281104. [DOI] [PubMed] [Google Scholar]
- 13.Alexander SI. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med. 2008;358:369–374. doi: 10.1056/NEJMoa0707255. [DOI] [PubMed] [Google Scholar]
- 14.Domiati-Saad R, Klintmalm GB, Netto G, Agura ED, Chinnakotla S, Smith DM. Acute graft versus host disease after liver transplantation: patterns of lymphocyte chimerism. Am J Transplant. 2005;5:2968–2973. doi: 10.1111/j.1600-6143.2005.01110.x. [DOI] [PubMed] [Google Scholar]
- 15.Wu D, Vu Q, Nguyen A, Stone JR, Stubbs H, Kuhlmann G, Sholl LM, Iafrate AJ. In situ genetic analysis of cellular chimerism. Nat Med. 2009;15:215–219. doi: 10.1038/nm.1862. [DOI] [PubMed] [Google Scholar]


