Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, including Japan. Although the development of imaging modalities has made the early diagnosis of HCC possible, surgically resectable cases are relatively uncommon because of hepatic function reserve and/or an advanced stage at presentation. Several modalities, such as transcatheter arterial chemoembolization, percutaneous ethanol injection, microwave coagulation therapy and radiofrequency ablation are reportedly useful in treating patients with non-resectable disease. However, unfortunately, many HCC patients have tumor recurrence. The overall prognosis of patients with HCC is very poor, and treatment of the advanced form is still problematic. In this article, we review the clinical efficacy and toxicity of enteric-coated tegafur/uracil in the treatment of patients with advanced non-resectable HCC.
Keywords: Advanced hepatocellular carcinoma, Tumor dormancy, Enteric-coated tegafur/uracil, Chemotherapy, Portal vein tumor thrombus, Lung metastasis
INTRODUCTION
Chronic liver disease predisposes patients to hepatocellular carcinoma (HCC), therefore, a high-risk group can be identified[1]. Progress in diagnostic imaging studies has facilitated the relatively early diagnosis of HCC. In many patients, however, the disease is already advanced at the time of detection. Patients with recurrence after local treatment and those with far-advanced HCC should receive effective systemic chemotherapy. In this review, we outline the mechanism of action and outcomes of enteric-coated tegafur/uracil (UFT-E Taiho Pharmaceutical, Co. Ltd., Tokyo, Japan) used as systemic chemotherapy in patients with HCC.
DEVELOPMENT OF UFT
Heidelberger et al[2] synthesized 5-fluorouracil in 1957. Since that time, fluoropyrimidine antimetabolites have been used as broad-spectrum anticancer drugs to treat various types of tumors. However, the serum half-life of 5-fluorouracil is very short (8-12 min)[3,4]. Therefore, many derivatives have been developed to maintain high serum concentrations of 5-fluorouracil, enhance response, and reduce toxicity. In 1968, Hiller et al[5] synthesized tegafur, a prodrug of 5-fluorouracil, which is gradually converted to 5-fluorouracil by cytochrome P450 2A6. Subsequently, UFT was developed to enhance the tumor specificity and effectiveness of 5-fluorouracil. In 1978, Fujii et al[6] conducted a series of experiments on combination therapy with fluoropyrimidines and pyrimidines and reported that the antitumor activity of tegafur was most strongly enhanced by uracil. They also found that combinations of tegafur and uracil in certain ratios enhanced the antitumor activity of tegafur, without increasing toxicity. A combination of tegafur and uracil in a molar ratio of 1:4 was experimentally shown to be optimal.
These findings led to the development of UFT. This preparation produces and maintains high concentrations of 5-fluorouracil and its active metabolites in tumors, and has specific characteristics not obtained with tegafur or 5-fluorouracil alone. In some patients, however, the conventional formulation of UFT capsules is associated with adverse upper gastrointestinal effects such as loss of appetite, nausea and vomiting, and requires dose reduction or treatment withdrawal. To reduce such adverse effects, UFT-E was developed from 1987 to 1988. Subsequently, the therapeutic usefulness of UFT-E has been confirmed in clinical studies[7,8]. In 1990, a new drug application was submitted. UFT-E was approved in 1992 and is now widely used.
MECHANISM OF ACTION OF UFT
The mechanism of action of UFT is shown in Figures 1 and 2. The anticancer activity of UFT is derived from 5-fluorouracil, to which tegafur is gradually converted. As for the mechanism of action of 5-fluorouracil, 5-fluoro-2'-deoxyuridine 5'-monophosphate (FdUMP), the active metabolite of 5-fluorouracil, competes with 2'-deoxyuridine 5'-monophosphate (dUMP) and inhibits thymidylate synthase, thereby blocking DNA synthesis. 5-fluorouridine 5'-triphosphate (FUTP) is incorporated into RNA, which disrupts RNA function (in vitro)[9,10]. The antitumor activity of 5-fluorouracil depends mainly on inhibition of DNA synthesis. 5-fluorouracil is a time-dependent anticancer drug; therefore, prolonged exposure of cancer cells to even low concentrations of 5-fluorouracil results in antitumor activity. Dihydropyrimidine dehydrogenase (DPD), the main metabolizing enzyme of 5-fluorouracil, is present throughout the body, including the liver, epithelium of the gastrointestinal tract, and peripheral leukocytes, but is most abundant in the liver[11]. After intravenous administration, > 90% of the dose of 5-fluorouracil is metabolized during the first pass through the liver[12]. Tumors contain DPD, which metabolizes 70% of the 5-fluorouracil that enters tumor tissue. Therefore, outcomes of 5-fluorouracil monotherapy in patients with HCC are poor. On the other hand, uracil is a pyrimidine-based analogue, a component of nucleic acids. Uracil alone has no appreciable antitumor activity or toxicity, but has a high affinity for DPD in the liver and tumors, thereby inhibiting DPD and preventing the metabolism of 5-fluorouracil. This mechanism provides the theoretical basis for UFT, a combination of uracil and tegafur that produces prolonged concentrations of 5-fluorouracil in serum, thereby enhancing antitumor activity against HCC and reducing toxicity[13]. Previous studies have reported that γ-hydroxy butyrate (GHB), the metabolite of the protecting group of tegafur, γ-butyrolactone (GBL), and high-dose tegafur inhibit neovascularization[14]. UFT-E is thus expected to be effective against HCC.
Figure 1.
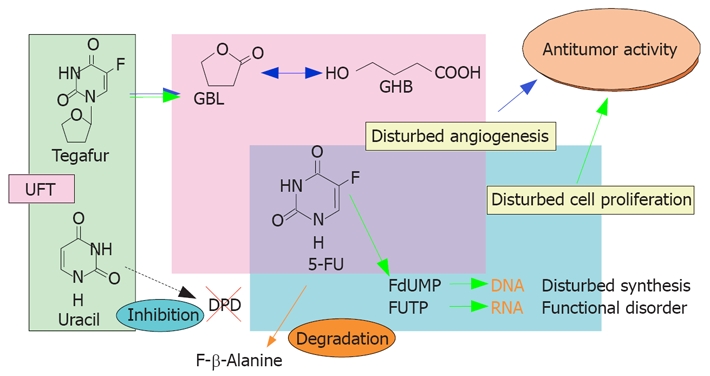
Mechanism of action of UFT.
Figure 2.
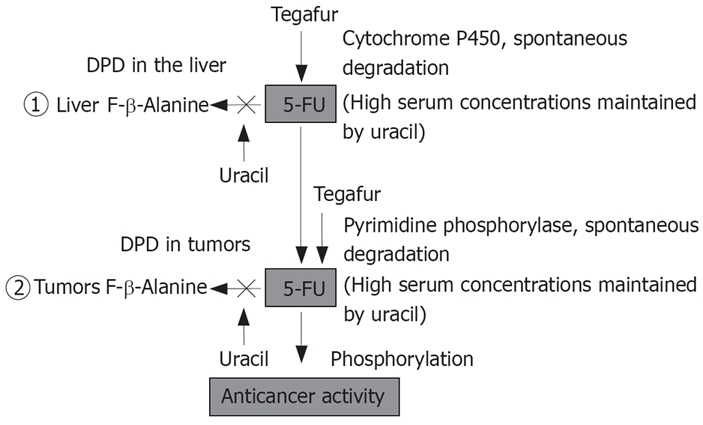
Biochemical modulation of UFT.
REGIMENS OF UFT
Recent controlled studies have reported the usefulness of postoperative adjuvant chemotherapy with UFT, given continuously in a high daily dose (400 mg/m2) for 1-2 years. In a representative study of patients with resected rectal cancer, the 3-year recurrence-free survival rate was 78% in patients who received postoperative adjuvant chemotherapy with UFT (400 mg/m2 per day) for 1 year, and 60% in those who underwent surgery alone. The 3-year survival rate was 91% in the UFT group and 81% in the surgery alone group. The rates of recurrence-free and overall survival were significantly improved by postoperative adjuvant chemotherapy with UFT[15]. In this study, because UFT was given at a high dose of 400 mg/m2 per day (in two divided doses), adequate concentrations of 5-fluorouracil in serum might have been maintained, which inhibited the growth of small amounts of residual tumor cells. Severe myelosuppression and gastrointestinal disorders associated with 5-fluorouracil were rare. This was attributed to the fact bone marrow cells and gastrointestinal mucosal cells were rescued by treatment with UFT, given for five consecutive days followed by two days rest. However, in patients with HCC, long-term treatment with oral anticancer drugs may negatively affect liver function as a side effect because of underlying chronic liver disease. Oral anticancer drugs should thus be administered cautiously. The next section describes the use of a reduced dose of UFT-E, equivalent to two-thirds to less than half of the usual dose, in patients with HCC.
TREATMENT OF HCC WITH UFT-E
Effectiveness of UFT-E for stage IVA HCC
We have previously described our own experience with a patient who had stage IVA HCC, in whom all tumors disappeared after monotherapy with UFT-E, with no evidence of recurrence[16]. Subsequently, we assessed the usefulness of monotherapy with UFT-E in 28 patients with stage IVA HCC who could not undergo hepatectomy, interventional radiology, or intra-arterial infusion chemotherapy[17]. In a study that compared patients who received UFT-E with those who did not, the survival rate was significantly higher in the UFT-E group (Figure 3). Moreover, survival was significantly longer in the UFT-E group than in the untreated group among patients who had a complete or partial response, as well as among those with no change or progressive disease (Figure 4)[17]. Although patients with clinical disease progression tended to have poor outcome, multivariate analysis showed that treatment with UFT-E contributes to improved survival, which indicates UFT-E is effective (Table 1)[17]. These results indicate administration of UFT-E improves the survival time by inducing a cytostatic phase, rather than by tumor reduction[18].
Figure 3.
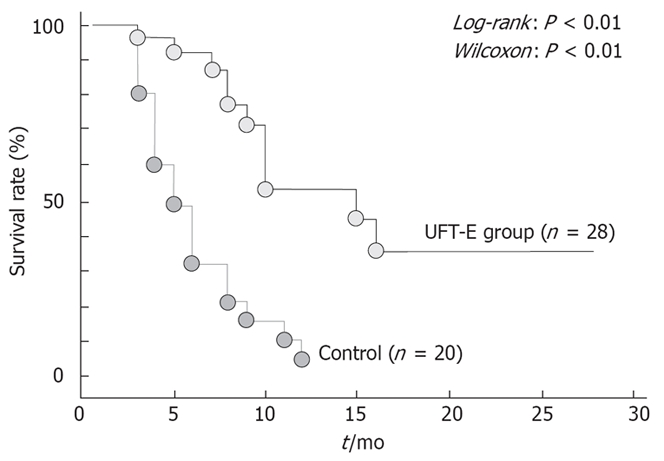
Cumulative survival rates in patients with stage IVA HCC who received UFT-E and those who did not.
Figure 4.
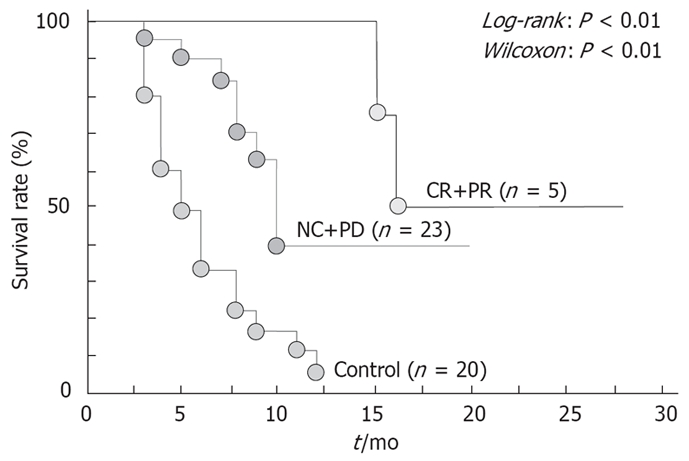
Cumulative survival rates in patients with stage IVA hepatocellular carcinoma according to response to treatment. CR: Complete response; PR: Partial response; NC: No change; PD: Progressive disease.
Table 1.
Multivariate analysis of prognostic factors in patients with stage IVA hepatocellular carcinoma
| Variable | No. of patients | Risk ratio | 95% CI | P value |
| Treatment | ||||
| Tegafur/uracil | 28 | 0.170 | 0.069-0.441 | < 0.001 |
| Control | 20 | 1 | ||
| Cirrhosis | ||||
| Yes | 43 | 2.592 | 0.541-12.423 | NS (P = 0.235) |
| No | 5 | 1 | ||
| Sex | ||||
| Men | 35 | 0.751 | 0.289-1.955 | NS (P = 0.557) |
| Women | 13 | 1 | ||
| Age | ||||
| ≥ 65 | 22 | 0.678 | 0.307-1.499 | NS (P = 0.337) |
| < 65 | 26 | 1 | ||
| AFP | ||||
| ≥ 100 | 23 | 1.741 | 0.724-4.188 | NS (P = 0.216) |
| < 100 | 25 | 1 | ||
| Serum bilirubin | ||||
| ≥ 2.0 | 18 | 0.573 | 0.211-1.557 | NS (P = 0.275) |
| < 2.0 | 30 | 1 | ||
| Serum albumin | ||||
| ≥ 3.0 | 28 | 1.290 | 0.525-3.171 | NS (P = 0.579) |
| < 3.0 | 20 | 1 | ||
| Tumor thrombus | ||||
| Yes | 20 | 3.516 | 0.927-8.661 | NS (P = 0.062) |
| No | 28 | 1 | ||
| Okuda staging | ||||
| I/II | 42 | 0.368 | 0.099-1.370 | NS (P = 0.136) |
| III | 6 | 1 |
NS: Not significant.
Effectiveness of UFT-E for far-advanced HCC
HCC with portal vein tumor thrombosis (PVTT): We have previously reported that UFT-E is therapeutically useful in patients with stage IVA HCC[16,17]. However, in patients with advanced HCC accompanied by PVTT, outcome is extremely poor, and UFT-E monotherapy is apparently not beneficial. Combination therapy with UFT-E has, therefore, been tried. Recently, Kusunoki et al[19,20] have reported that pharmacokinetic modulating chemotherapy, based on the concept that the benefit of continuous venous 5-fluorouracil infusion can be potentiated by low-dose oral UFT, is useful for a variety of cancers.
We have previously reported on patients with various types of cancer who had a complete response to pharmacokinetic modulating chemotherapy with UFT-E and continuous intravenous infusion of 5-fluorouracil[21–27]. Such regimens have recently been used to increase tissue 5-fluorouracil concentrations in patients with advanced gastric cancer or colorectal cancer. We, therefore, modified the regimen for intra-arterial infusion chemotherapy with epirubicin, etoposide, and cisplatin (EEP therapy) described by Takayasu et al[28] to treat HCC patients with PVTT. Our modified EEP regimen consisted of intra-arterial infusion chemotherapy with epirubicin (30 mg), carboplatin (200 mg), and etoposide (60 mg), given once weekly, followed by a 24-h infusion of 5-fluorouracil (500 mg) as standard therapy, plus continuous treatment with UFT-E (200 mg/d). We have previously described a patient with UFT-E who had a complete response to this regimen. We then studied outcomes in several similar patients. Despite PVTT, all patients could receive chemotherapy on an outpatient basis. The mean survival from the beginning of intra-arterial infusion chemotherapy, excluding the period of previous treatment, was 457.2 d (Figure 5). Further studies are needed to determine the optimal dosage and treatment intervals. However, preliminary evidence indicates modified EEP therapy, including oral anticancer treatment with UFT-E, might be a useful treatment strategy for advanced HCC accompanied by PVTT (Vp3, Vp4)[29].
Figure 5.
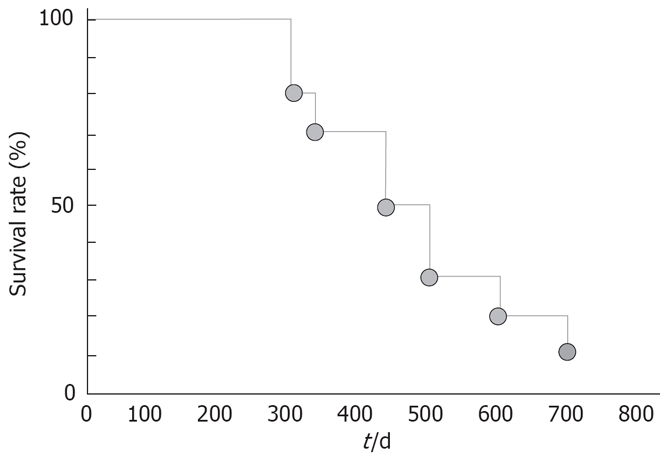
Cumulative survival rates in patients with tumor occlusion of the portal vein who received modified EEP therapy.
Effectiveness of UFT-E against stage IVB HCC with extrahepatic metastasis: Systemic chemotherapy is usually given to patients who have HCC with extrahepatic metastasis. However, drug recommendations based on scientific evidence are currently not available. Although the effectiveness of UFT-E has been confirmed in patients with stage IVA HCC, monotherapy with UFT-E does not improve outcome in patients who have stage IVB disease with distant metastasis, usually an indication for systemic chemotherapy. We have previously reported a case of HCC with multiple lung metastases that had a complete response to combination therapy with UFT-E plus docetaxel/cisplatin[30]. At present, additional patients with distant metastasis are being studied. Long-term studies of larger numbers of patients are needed to assess the clinical significance of combination therapy including oral anticancer drugs.
TOXIC EFFECTS OF UFT
Toxic effects of UFT include severe liver dysfunction and diarrhea. Leukoencephalopathy has been reported as a neurological effect, and pigmentation as a skin symptom. Loss of appetite, nausea and vomiting commonly occur in clinical practice. These adverse signs and symptoms may require a reduction in the dose of UFT or even the cessation of treatment in some patients[31]. In a study that compared the incidence of toxicity between UFT capsules and UFT-E granules, which were developed to reduce upper gastrointestinal symptoms and thereby improve compliance, patients who received UFT-E granules had a lower incidence of adverse upper gastrointestinal symptoms[31]. Grade 1 stomatitis developed in three patients with stage IVA HCC who received monotherapy with UFT-E. However, hepatic function reserve was undisturbed. Monotherapy with UFT-E thus contributed to an improved quality of life. Ikeda et al[32] conducted a controlled study to compare outcome between patients who received UFT-E adjuvant chemotherapy before transcatheter arterial embolization and those who did not. Outcomes did not significantly differ between the groups, but ascites and hepatic encephalopathy developed in the UFT-E group. However, in our studies, no patient had UFT-E-induced liver failure. In patients with tumor occlusion of the portal vein, who received combination chemotherapy, toxic effects such as stomatitis and leukopenia developed, but treatment could be continued after a rest period.
CONCLUSION
The effectiveness of UFT-E should be evaluated at different stages of HCC. At the same time, different dosages and treatment with UFT-E regimens, including rest periods, should be assessed, rather than administering anticancer drugs continuously. Unlike other types of cancer, HCC is usually associated with cirrhosis. Long-term treatment with anticancer drugs may therefore adversely affect liver function. Further studies are needed to determine optimal regimens, doses, and treatment periods for anticancer drugs. Other clinical questions, such as whether systemic chemotherapy with UFT-E is an effective adjuvant therapy after liver transplantation, remain to be answered.
Peer reviewer: Susumu Ohwada, Associate Professor, Department of Surgery, Gunma University Graduate School of Medicine, 3-39-15 Shoma-Machi, Maebashi 371-8511, Japan
S- Editor Liu JN L- Editor Kerr C E- Editor Yin DH
References
- 1.Ishikawa T, Ichida T, Yamagiwa S, Sugahara S, Uehara K, Okoshi S, Asakura H. High viral loads, serum alanine aminotransferase and gender are predictive factors for the development of hepatocellular carcinoma from viral compensated liver cirrhosis. J Gastroenterol Hepatol. 2001;16:1274–1281. doi: 10.1046/j.1440-1746.2001.02616.x. [DOI] [PubMed] [Google Scholar]
- 2.Heidelberger C, Chaudhuri NK, Dannerberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179:663–666. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- 3.Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;16:215–237. doi: 10.2165/00003088-198916040-00002. [DOI] [PubMed] [Google Scholar]
- 4.Baker SD, Khor SP, Adjei AA, Doucette M, Spector T, Donehower RC, Grochow LB, Sartorius SE, Noe DA, Hohneker JA, et al. Pharmacokinetic, oral bioavailability, and safety study of fluorouracil in patients treated with 776C85, an inactivator of dihydropyrimidine dehydrogenase. J Clin Oncol. 1996;14:3085–3096. doi: 10.1200/JCO.1996.14.12.3085. [DOI] [PubMed] [Google Scholar]
- 5.Hiller SA, Zhuk RA, Lidak MJ, Zidermane AA. Br Patent. 1968;1:391–395. [Google Scholar]
- 6.Fujii S, Ikenaka K, Fukushima M, Shirasaka T. Effect of uracil and its derivatives on antitumor activity of 5-fluorouracil and 1-(2-tetrahydrofuryl)-5-fluorouracil. Gann. 1978;69:763–772. [PubMed] [Google Scholar]
- 7.Takahashi H, Kamano T. [Clinical results of UFT enteric-coated granule therapy under cooperative study (phase II study). Tokyo Cancer Chemotherapy Cooperative Study Group] Gan To Kagaku Ryoho. 1990;17:2043–2049. [PubMed] [Google Scholar]
- 8.Kikuchi K, Wakui A. [Cooperative research of UFT E phase II study. Cooperative Study Group of UFT E in Tohoku Area] Gan To Kagaku Ryoho. 1990;17:2183–2190. [PubMed] [Google Scholar]
- 9.Heidelberger C, Kaldor G, Mukherjee KL, Danneberg PB. Studies on fluorinated pyrimidines. XI. In vitro studies on tumor resistance. Cancer Res. 1960;20:903–909. [PubMed] [Google Scholar]
- 10.Hartmann KU, Heidelberger C. Studies on fluorinated pyrimidines. XIII. Inhibition of thymidylate synthetase. J Biol Chem. 1961;236:3006–3013. [PubMed] [Google Scholar]
- 11.Naguib FN, el Kouni MH, Cha S. Enzymes of uracil catabolism in normal and neoplastic human tissues. Cancer Res. 1985;45:5405–5412. [PubMed] [Google Scholar]
- 12.Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;16:215–237. doi: 10.2165/00003088-198916040-00002. [DOI] [PubMed] [Google Scholar]
- 13.Yonekura K, Basaki Y, Chikahisa L, Okabe S, Hashimoto A, Miyadera K, Wierzba K, Yamada Y. UFT and its metabolites inhibit the angiogenesis induced by murine renal cell carcinoma, as determined by a dorsal air sac assay in mice. Clin Cancer Res. 1999;5:2185–2191. [PubMed] [Google Scholar]
- 14.Basaki Y, Yonekura K, Chikahisa L, Okabe S, Hashimoto A, Miyadera K, Aoyagi K, Yamada Y. [Anti-angiogenic activities of UFT and its metabolites, GHB and GBL, in the dorsal air sac (DAS) model in mice] Gan To Kagaku Ryoho. 2000;27:93–98. [PubMed] [Google Scholar]
- 15.Akasu T, Moriya Y, Ohashi Y, Yoshida S, Shirao K, Kodaira S. Adjuvant chemotherapy with uracil-tegafur for pathological stage III rectal cancer after mesorectal excision with selective lateral pelvic lymphadenectomy: a multicenter randomized controlled trial. Jpn J Clin Oncol. 2006;36:237–244. doi: 10.1093/jjco/hyl014. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa T, Ichida T, Ishimoto Y, Yokoyama J, Nomoto M, Ebe Y, Usuda H, Naito M, Asakura H. Complete remission of multiple hepatocellular carcinomas associated with hepatitis C virus-related, decompensated liver cirrhosis by oral administration of enteric-coated tegafur/uracil. Am J Gastroenterol. 1999;94:1682–1685. doi: 10.1111/j.1572-0241.1999.01163.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa T, Ichida T, Sugitani S, Tsuboi Y, Genda T, Sugahara S, Uehara K, Inayoshi J, Yokoyama J, Ishimoto Y, et al. Improved survival with oral administration of enteric-coated tegafur/uracil for advanced stage IV-A hepatocellular carcinoma. J Gastroenterol Hepatol. 2001;16:452–459. doi: 10.1046/j.1440-1746.2001.02352.x. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y, Nishioka K. Survival without tumor shrinkage: re-evaluation of survival gain by cytostatic effect of chemotherapy. J Natl Cancer Inst. 1995;87:1262–1263. [PubMed] [Google Scholar]
- 19.Kusunoki M, Yanagi H, Kotera H, Noda M, Yamamura T. Effects of pharmacokinetic modulating chemotherapy using oral UFT and continuous venous 5FU infusion on the prognosis of irradiated rectal carcinomas with p53 overexpression. Int J Oncol. 1998;13:653–657. doi: 10.3892/ijo.13.4.653. [DOI] [PubMed] [Google Scholar]
- 20.Kusunoki M, Yanagi H, Noda M, Yamamura T. The usefulness of pharmacokinetic modulating chemotherapy (UFT plus 5FU) in the treatment of unresectable colorectal carcinomas. Oncol Rep. 1999;6:547–552. doi: 10.3892/or.6.3.547. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa T, Sato S, Matsuzawa J, Mita Y, Matsui S, Tashiro K, Tashiro S, Matsuki H. [A case of successful management of nonresectable pancreas cancer with liver metastasis by intra-arterial infusion chemotherapy with angiotensin-II and administration of tegafur/uracil] Gan To Kagaku Ryoho. 2001;28:521–525. [PubMed] [Google Scholar]
- 22.Ishikawa T, Mita Y, Kobayashi M, Tashiro K, Tashiro S, Matsuki H. [A case of nonresectable scirrhous type gastric cancer treated by hypertensive subselective chemotherapy with pharmacokinetic modulating chemotherapy] Gan To Kagaku Ryoho. 2001;28:1137–1140. [PubMed] [Google Scholar]
- 23.Ishikawa T, Nomura K, Baba Y, Hayashi S, Oota H, Yoshida T, Kamimura T. [A case of advanced gastric cancer with liver and intra-abdominal lymph node metastasis treated by hypertensive selective chemotherapy with pharmacokinetic modulating chemotherapy] Gan To Kagaku Ryoho. 2003;30:1151–1155. [PubMed] [Google Scholar]
- 24.Ishikawa T, Mizuno K, Togashi T, Watanabe K, Seki K, Ohta H, Yoshida T, Kamimura T. [A case of advanced gastric cancer with bone metastasis and severe DIC responding to hypertensive subselective chemotherapy with pharmacokinetic modulating chemotherapy] Gan To Kagaku Ryoho. 2005;32:523–527. [PubMed] [Google Scholar]
- 25.Ishikawa T, Mizuno K, Togashi T, Watanabe K, Seki K, Ohta H, Yoshida T, Kamimura T. [Modified pharmacokinetic modulating chemotherapy for progressive gastric cancer accompanied by peritoneal dissemination] Gan To Kagaku Ryoho. 2005;32:469–472. [PubMed] [Google Scholar]
- 26.Ishikawa T, Mizuno K, Watanabe K, Baba Y, Oota H, Yoshida T, Kamimura T. [A case of successful management of nonresectable pancreas cancer with liver metastasis by intra-arterial infusion chemotherapy with gemcitabine hydrochloride, 5-FU, CDDP and administration of tegafur/uracil] Gan To Kagaku Ryoho. 2004;31:1555–1558. [PubMed] [Google Scholar]
- 27.Ishikawa T, Ishikawa N, Oota H, Yoshida T, Honma A, Kamimura T, Takeda K, Ishikawa N, Ozaki T. [A case of common bile duct cancer responding to MMC leucovorin, 5-FU, and UFT combination chemotherapy and radiation] Gan To Kagaku Ryoho. 1996;23:773–777. [PubMed] [Google Scholar]
- 28.Takayasu Y. [Hepatic arterial infusion chemotherapy for hepatocellular carcinoma by EEP regimen] Nippon Rinsho. 2001;59 Suppl 6:624–628. [PubMed] [Google Scholar]
- 29.Ishikawa T, Imai M, Kamimura H, Tsuchiya A, Togashi T, Watanabe K, Seki K, Ohta H, Yoshida T, Kamimura T. Improved survival for hepatocellular carcinoma with portal vein tumor thrombosis treated by intra-arterial chemotherapy combining etoposide, carboplatin, epirubicin and pharmacokinetic modulating chemotherapy by 5-FU and enteric-coated tegafur/uracil: a pilot study. World J Gastroenterol. 2007;13:5465–5470. doi: 10.3748/wjg.v13.i41.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa T, Ichida T, Yokoyama J, Matsuda Y, Watanabe T, Asakura H. Complete disappearance of pulmonary metastases in a case of hepatocellular carcinoma treated with docetaxel-based systemic chemotherapy. J Gastroenterol Hepatol. 2004;19:1423–1426. doi: 10.1111/j.1440-1746.2004.03737.x. [DOI] [PubMed] [Google Scholar]
- 31.Ohyama M, Matsumura M, Katsuta K, Nobori T, Matsuyama H, Fukami K, Kiyota R, Yano H, Shima T, Ogawa K. [A comparative study of UFT enteric-coated granules with UFT capsules on the occurrence of side effects in patients with head and neck cancers--a special attention to the upper gastrointestinal tract disorders] Gan To Kagaku Ryoho. 1990;17:1211–1216. [PubMed] [Google Scholar]
- 32.Ikeda K, Saitoh S, Koida I, Tsubota A, Arase Y, Chayama K, Kumada H. A prospective randomized evaluation of a compound of tegafur and uracil as an adjuvant chemotherapy for hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Am J Clin Oncol. 1995;18:204–210. doi: 10.1097/00000421-199506000-00005. [DOI] [PubMed] [Google Scholar]


