Abstract
AIM: To explore the correlation between the mRNAs and protein expression of gastrin (GAS), somatostatin (SS) and apoptosis index (AI), apoptosis regulation gene Fas/FasL and caspases in large intestinal carcinoma (LIC).
METHODS: Expression of GAS and SS mRNAs were detected by nested RT-PCR in 79 cases of LIC. Cell apoptosis was detected by molecular biology in situ apoptosis detecting methods (TUNEL). Immunohistochemical staining for GAS, SS, Fas/FasL, caspase-3 and caspase-8 was performed according to the standard streptavidin-biotin-peroxidase (S-P) method.
RESULTS: There was a significant positive correlation between mRNA and protein expression of GAS and SS (GASrs=0.99, P < 0.01; SSrs = 0.98, P < 0.01). There was significant difference in positive expression rates of GAS, SS mRNAs and protein among different histological differentiation, histological types and Dukes’ stage of LIC. The AI in GAS high and moderate expression groups was significantly lower than that in low expression groups (3.75 ± 2.38 vs 7.82 ± 2.38, P < 0.01; 5.51 ± 2.66 vs 7.82 ± 2.38, P < 0.01), and the AI in SS high and moderate expression groups was significantly higher than that in low expression groups (9.03 ± 1.76 vs 5.35 ± 3.00, P < 0.01; 7.44 ± 2.67 vs 5.35 ± 3.00, P < 0.01). There was a significant negative correlation between the integral ratio of GAS to SS and the AI (rs = -0.41, P < 0.01). The positive expression rate of FasL in GAS high and moderate expression groups was higher than that in low expression group (90.9% and 81.0% vs 53.2%, P < 0.05). The positive expression rates of Fas, caspase-8 and caspase-3 in SS high (90.0%, 90.0% and 100%) and moderate (80.0%, 70.0%, 75.0%) expression groups were higher than that in low expression group (53.1%, 42.9%, 49.0%) (90.0% and 80.0% vs 53.1%, P < 0.05; 90.0% and 70.0% vs 42.9%, P < 0.05; 100.0% and 75.0% vs 49.0%, P < 0.05). There was a significant positive correlation between the integral ratio of GAS to SS and the semiquantitative integral of FasL (rs = 0.32, P < 0.01).
CONCLUSION: GAS and SS play important roles in the regulation and control of cell apoptosis in LIC, and the mechanism may be directly related to the aberrant expression of Fas/FasL. The GAS and SS will be valuable targets of the biological behavior of LIC.
Keywords: Large intestinal carcinoma, Gastrin, Somatostatin, Apoptosis index, Fas, FasL, Caspase
INTRODUCTION
Large intestinal cancer (LIC) is one of the most common digestive tract malignant tumors in the world, with a high incidence rate in North America, Western Europe, Australia, New Zealand and France, and is the second leading cause of gastrointestinal cancer-related mortality worldwide[1–4]. In China, it now ranks fourth[5]. Although great progress in understanding the molecular aspects of LIC has been made and several therapeutic agents have been developed, it is still difficult to cure the tumor. It is posing a serious threat to public health and remains a major killer among Chinese patients. The general survival rate of LIC patients does not exceed 40%. Thereby, it is very important to know what kind of cell factor can influence cell apoptosis, which will enrich the etiology theory of tumor. In addition, while studying the mechanism of loss of control of tumor cell apoptosis from signal transduction pathway, we may find a new way to treat malignant tumors. Previous studies have demonstrated that the occurrence of LIC is directly related to the abnormal expression of gastrointestinal hormones such as gastrin (GAS), somatostatin (SS), etc[6,7]. Some studies found that GAS was able to promote growth and inhibit apoptosis of LIC cells, but the role of SS was opposite[8–10]. However, the detailed molecule mechanism by which GAS and SS mediate cell apoptosis of LIC is not fully known. We used nested RT-PCR method to detect the expression of GAS and SS mRNAs in LIC tissues, and TUNEL method to detect cell apoptosis, and immunohistochemical staining S-P method to detect the protein expression of GAS, SS, Fas/FasL, caspase-3 and caspase-8. The aim of this study was to explore the correlation between the mRNAs and protein expression of GAS and SS, and between the protein expression of GAS, SS and apoptosis index (AI), and apoptosis regulation gene Fas/FasL and caspases in LIC, and to further confirm whether GAS and SS could mediate LIC cell apoptosis mainly via affecting the expression of Fas/FasL.
MATERIALS AND METHODS
Clinical data
Seventy-nine cases of cancer tissue samples were randomly and retrospectively selected from patients with LIC hospitalized in our hospital from January 2004 to October 2006. All patients were confirmed as having LIC by clinical pathology. Among them, 36 were cases of rectum cancer, and 43 were cases of colorectal carcinoma. Thirty-seven were females and 42 were males. The median age was 50.8 ± 11.2 years, with a range of 21-79 years. There were 27 patients with well differentiated carcinoma, 28 patients with moderately differentiated carcinoma, and 24 patients with poorly differentiated carcinoma (inclusive of undifferentiated carcinoma). Among them, there were 40 cases of ulcerative type, 32 protruded type, 7 infiltrating type, 19 papillary adenocarcinoma, 35 glandular adenocarcinoma, 10 mucoid carcinoma, 6 signet-ring cell carcinoma and 9 undifferentiated carcinoma. The clinical stage was determined according to the Dukes’ stage. Dukes’ stages A and B were found in 36 patients, and stages C and D in 43 patients.
Main reagents
The polyclonal rabbit antibodies against human SS protein and human GAS, monoclonal rabbit antibodies against human Fas and FasL, caspase-3, caspase-8, and immunohistochemical staining kit and in situ cell apoptosis detection kit II were all purchased from Beijing Zhongshan Biological Technology Co, Ltd. Trizol liquid, M-MLV reverse transcriptase, SK312, Taq DNA polymerase, RNAsin, oligo (dt) 15 and dNTPs primers were purchased from Shanghai Sangon Biological Engineering Technology and Service Co. Ltd and PCR primers were synthesized by the same company.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from frozen tissue specimens (50-100 mg) using TRIzol reagent according to the protocol provided by the manufacturer. Total RNA was primed with an Oligo (dT) 15 oligonucleotide and reverse-transcribed following the manufacturer’s instructions.
PCR amplification
The primers of GAS, SS and β-actin were synthesized according to the primer design principles, all primers spanned an intron to control against amplification of genomic DNA sequences. Two point five μL first strand cDNA was amplified in 25 μL total volume. The first PCR amplifications were performed in a UNO II thermocycler (Perkin-Elmer, USA) under the following conditions. After an initial denaturation for 5 min at 94°C, samples were subjected to 30 cycles, each amplification consisting of denaturation at 94°C for 30 s, primer annealing at 60°C for 45 s, extension at 72°C for 45 s, followed by a final extension of 6 min 72°C. Conditions and parameters of the second PCR amplifications were the same with the first PCR amplifications except for primers. The length of final PCR products was 174 base pair (bp) (GAS) and 231 bp (SS), respectively. At last, 6 μL of reaction product was analyzed by electrophoresis on a 1.5% agarose gel followed by ethidium bromide staining and digital camera photography (Table 1, Figure 1).
Table 1.
Primers used for nested RT-PCR amplification of GAS and SS
| Name | Primer sequence | PCR conditions | Size (bp) |
| GAS | 1: 5’-TATGTGCTGATCTTTGCACTGGCT-3’ (sense: 6307-6330) | 94°C, 30 s | 282 |
| 2: 5’-CTCATCCTCAGCACTGCGGCGGCC-3’ (antisense: 6718-6695) | 60°C, 45 s | ||
| 72°C, 45 s | |||
| GAS | 3: 5’-GAGCTACCCTGGCTGGAGCAGCAG-3’ (sense: 6415-6438) | 94°C, 30 s | 174 |
| 2: 5’-CTCATCCTCAGCACTGCGGCGGCC-3’ (antisense: 6718-6695) | 60°C, 45 s | ||
| 72°C, 45 s | |||
| SS | 1: 5’-ATGCTGTCCTGCCGCCTCCAG-3’ (sense: 106-126) | 94°C, 30 s | 348 |
| 2: 5’-ACAGGATGTGAAAGTCTTCCA-3’ (antisense: 1330-1310) | 60°C, 45 s | ||
| SS | 3: 5’-GCTGCTGCCGCGGGGAAGCAG-3’ (sense: 223-243) | 94°C, 30 s | 231 |
| 2: 5’-ACAGGATGTGAAAGTCTTCCA-3’ (antisense: 1330-1310) | 60°C, 45 s | ||
| 72°C, 45 s |
Figure 1.
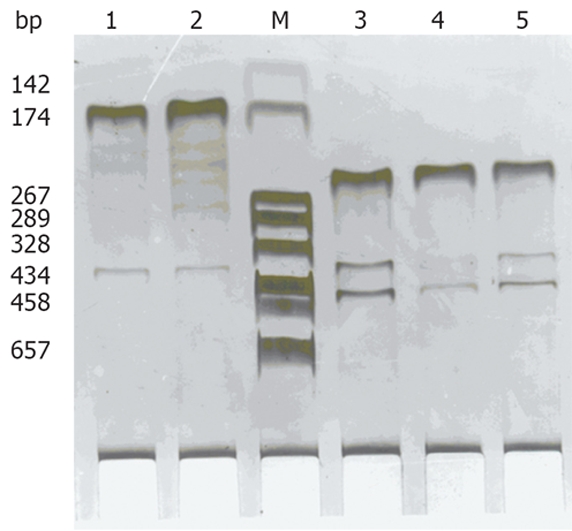
Expression of GAS, SS mRNA by nested RT-PCR analysis in LIC tissue. Lane M: DNA marker DL 2000; Lane 1-2: Positive expression of GAS; Lane 3-5: Positive expression of SS.
Detection of cell apoptosis
Cell apoptosis was detected using molecular biology in situ apoptosis detecting methods (TUNEL). The sections were fixed and embedded conventionally. Dnase (1 mg/L) was used to digest the positive control specimen for 10 min before put into TUNEL response mixture. Only label liquor was added into the negative control response mixture. The detailed manipulation was conducted according to the introductions for users. Cells with brown or yellow nuclei were assumed as apoptotic cells. The number of apoptotic cells and total cancer cells was counted under light microscope at 400 × magnification in 10 fields of vision and the average values were obtained for the calculation of apoptosis index (AI) according to the formula: AI = (apoptotic cells/total cancer cells) × 100%.
Immunohistochemical staining
Specimens obtained at surgery were routinely fixed in 10% neutral formalin and embedded in paraffin. Serial 4 μm thick sections were cut. Immunohistochemical staining for Fas, FasL, caspase-3, caspase-8, GAS and SS was performed by the standard streptavidin-biotin-peroxidase (S-P) method. Positive pancreatic tissue and stomach antrum mucous membrane were used as a positive control for SS and GAS. Positive controls for Fas, FasL, caspase-3 and caspase-8 were purchased from Shanghai Sangon Biological Engineering Technology and Service Co. Ltd. PBS 0.01 mol/L as a negative control replaced the primary antibody. The detailed manipulation of S-P immunohistochemical method was used according to its manual.
Evaluation of scores
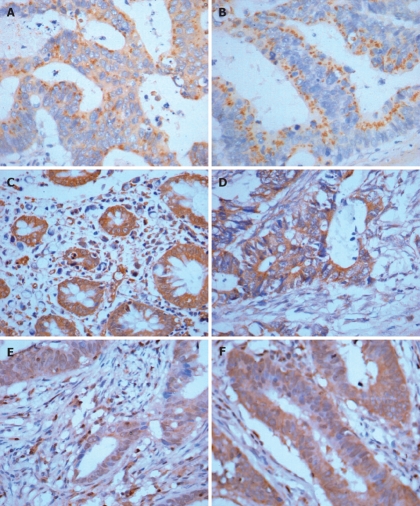
Brown-yellow staining mainly in cell plasma, partly in cell membranes was considered positive SS and GAS protein expression. When SS and GAS protein expression was scored, both the extent and intensity of immunopositivity were considered. The intensity of staining was scored as follows: 0, no staining; 1, weak-yellow; 2, brown-yellow; and 3, brown-black. The extent of positive cells was scored as follows (100 cells were counted by two independent observers, who did not know the clinicopathological features of these LIC): 1, positive staining cells < 5%; 2, positive staining cells in 5%-10%; 3, positive staining cells in 10%-20%; and 4, positive staining cells > 20%. The final score was determined by multiplying the intensity and extent of positivity scores, yielding a range from 1 to 12. According to the semi-quantitative integral evaluation, SS and GAS were divided into three groups: Scores 1-3 was defined as the low expression group, 4-6 as the moderate expression group, and 7-12 as the high expression group (Figure 2A and B).
Figure 2.
Strong expressions of GAS, SS, Fas, FasL, caspase-3 and caspase-8 in LIC tissues. A: Strong GAS expression in LIC. S-P × 400; B: Strong SS expression in LIC. S-P × 400; C: Strong Fas expression in SS high expression group. S-P × 400; D: Strong FasL expression in GAS high expression group of colorectal carcinoma. S-P × 400; E: Strong caspase-3 expression in SS high expression group. S-P × 400; F: Strong caspase-8 expression in SS high expression group. S-P × 400.
The positive Fas and FasL protein expression was defined with brown-yellow staining mainly in cell membranes, or cell plasma, while positive caspase-3 and caspase-8 protein expression was defined with brown-yellow staining mainly in cell plasma. The degree of Fas, FasL, caspase-3 and caspase-8 staining was estimated by semi-quantitative evaluation and categorized by the extent and intensity of staining[11]. The intensity of staining was scored as: 0, negative; 1, weak-yellow; 2, brown-yellow; and 3, brown-black. The extent of positive cells was scored as: 0 = positive staining cells in 0%-5%, 1 = positive staining cells in 6%-25%, 2 = positive staining cells in 26%-50%, 3 = positive staining cells in 51%-75%, and 4 = positive staining cells > 75%. Combined staining score was used to evaluate the results of Fas, FasL, caspase-3 and caspase-8 staining. The final score was determined by adding the intensity and extent of staining scores, yielding a range from 0 to 7. Scores 1-2 were defined as negative expression (-), 3 as weak staining (+), 4 as moderately staining (++), and ≥ 5 as strong staining (+++) (Figure 2C-F).
Statistical analysis
Statistical evaluation was performed using Chi-square test and q test to differentiate the positive rates of different groups, and using Spearman test to analyze the correlation between the ratio of GAS to SS and the integral of Fas, FasL, caspase-3 and caspase-8. All data were analyzed with statistical package for social science (SPSS) version 10.0. P < 0.05 was considered statistically significant.
RESULTS
Expression of GAS, SS mRNA and protein
The positive expression rates of GAS and SS mRNA were 46.8% (36/79), 41.8% (33/79), respectively. The positive expression rates of GAS and SS protein were 40.5% (32/79) and 38.0% (30/79), respectively. There was a significant positive correlation between mRNA and protein expression of GAS and SS (P < 0.01, GASrs = 0.99; P < 0.01, SSrs = 0.98). There was significant difference in positive expression rates of GAS, SS mRNAs and protein among different histological differentiation, histological types and Dukes’ stage of LIC, as shown in Table 2.
Table 2.
Correlation between clinicopathological factors and GAS and SS mRNA and protein expression in LIC
| Groups | n |
mRNA |
Protein |
||||||||||
| GAS (+) | χ2 | P | SS (+) | χ2 | P | GAS (+) | χ2 | P | SS (+) | χ2 | P | ||
| Differentiation | |||||||||||||
| Well | 27 | 8b | 10.47 | < 0.01 | 18 | 6b | 10.23 | < 0.01 | 17 | 5.24 | < 0.05 | ||
| Moderate | 28 | 11a | 6.68 | < 0.05 | 10c | < 0.05 | 10a | 4.95 | < 0.05 | 9c | 11.24 | < 0.01 | |
| Poor | 24 | 18 | 5d | 10.78 | < 0.01 | 16 | 4d | ||||||
| Histological types | |||||||||||||
| Papillary | 19 | 6e | 4.80 | < 0.05 | 13 | 4e | 6.22 | < 0.05 | 12 | ||||
| Tubular | 35 | 13e | 4.40 | < 0.05 | 14 | 11e | 4.38 | < 0.05 | 14 | ||||
| Mucinous and signet-ring | 16 | 11 | 4g | 6.56 | < 0.05 | 10 | 3g | 6.99 | < 0.05 | ||||
| Undifferentiated | 9 | 7 | 2g | 5.44 | < 0.05 | 7 | 1g | 7.33 | <0.05 | ||||
| Dukes’ stage | |||||||||||||
| A and B | 36 | 12i | 4.84 | < 0.05 | 20i | 5.17 | < 0.05 | 10i | 4.44 | < 0.05 | 18i | 4.06 | < 0.05 |
| C and D | 43 | 25 | 13 | 22 | 12 | ||||||||
P < 0.05,
P < 0.01 vs poor differentiation;
P < 0.05,
P < 0.01 vs well differentiation;
P < 0.05 vs mucinous and signet-ring and undifferentiated;
P < 0.05 vs papillary;
P < 0.05 vs Dukes’ stages C and D.
Apoptosis index
The AI in GAS high and moderate expression groups was significantly lower than in low expression groups (qhigh vs low = 6.71, P < 0.01; qmiddle vs low = 4.60, P < 0.01), and the AI in SS high and moderate expression groups was significantly higher than in low expression groups (qhigh vs low = 5.66, P < 0.01; qmiddle vs low = 4.21, P < 0.01). There was a significant negative correlation between the integral ratio of GAS to SS and the AI (rs = -0.41, P < 0.01) (Table 3).
Table 3.
Comparison of AI in GAS and SS high, moderate and low expression groups of LIC (mean ± SD)
| Groups | n | A I (mean ± SD) | F | P |
| SS | ||||
| Low | 10 | 5.35 ± 3.00 | ||
| Moderate | 20 | 7.44 ± 2.67b | 17.63 | < 0.01 |
| High | 49 | 9.03 ± 1.76b | ||
| GAS | ||||
| Low | 47 | 7.82 ± 2.38 | ||
| Moderate | 21 | 5.51 ± 2.66d | 16.08 | < 0.01 |
| High | 11 | 3.75 ± 2.38d |
P < 0.01 vs low SS group;
P < 0.01 vs low GAS group.
Fas, FasL, caspase-3 and caspase-8 expression in GAS and SS high, moderate and low expression groups of LIC
The positive expression rate of FasL in GAS high (10/11) and moderate (17/21) expression groups was higher than that in low expression group (26/47) (χ2high vs low = 6.24, P < 0.05; χ2moderate vs low = 4.74, P < 0.05). There were no significant differences in positive expression rates of Fas, caspase-3 and caspase-8 among GAS high, moderate and low expression groups (P > 0.05). The positive expression rates of Fas, caspase-8 and caspase-3 in SS high (9/10, 9/10 and 10/10) and moderate (16/20, 14/20 and 15/20) expression groups was higher than that in low expression group (26/49, 21/49 and 24/49) (χ2high vs low = 5.48, 5.62 and 6.89, P < 0.05; χ2middle vs low = 4.32, 4.19 and 3.91, P < 0.05). There was no significant difference in positive expression rate of FasL among SS high, moderate and low expression groups (P > 0.05) (Table 4). There was a significant positive correlation between the integral ratio of GAS to SS and the semi-quantitative integral of FasL (rs = 0.32, P < 0.01). But, there was no correlation between the integral ratio of GAS to SS and Fas, caspase-3 and caspase-8 (P > 0.05).
Table 4.
Comparison of positive rates of Fas, FasL, caspase-3 and caspase-8 protein in GAS and SS high, moderate and low expression groups of LIC
| Groups | n |
Fas |
FasL |
Caspase-3 |
Caspase-8 |
||||
| + (%) | - | + (%) | - | + (%) | - | + (%) | - | ||
| GAS | |||||||||
| High | 11 | 7 (63.6) | 4 | 10 (90.9)a | 1 | 9 (81.8) | 2 | 8 (72.7) | 3 |
| Moderate | 21 | 16 (76.2) | 5 | 7 (81.0)a | 4 | 14 (66.7) | 7 | 13 (61.9) | 8 |
| Low | 47 | 37 (78.7) | 10 | 26 (53.2) | 21 | 29 (61.7) | 18 | 28 (59.6) | 19 |
| SS | |||||||||
| High | 10 | 9 (90.0)c | 1 | 8 (80.0) | 2 | 10 (100.0)c | 0 | 9 (90.0)c | 1 |
| Moderate | 20 | 16 (80.0)c | 4 | 14 (70.0) | 6 | 15 (75.0)c | 5 | 14 (70.0)c | 6 |
| Low | 49 | 26 (53.1) | 23 | 32 (65.3) | 17 | 24 (49.0) | 25 | 21 (42.9) | 28 |
P < 0.05 vs GAS low expression group;
P < 0.05 vs SS low expression group.
DISCUSSION
LIC arises mainly from mutations in somatic cells. However, conversion of normal to cancer cells is not the result of a single mutation. It is achieved through a multi-step process that is closely associated with the accumulation of multiple gene changes including both oncogenes and tumor suppressor genes[12–16]. Uncontrolled cell proliferation and apoptosis are the main hallmarks of LIC. Apoptosis is a unique physiological mechanism that it can not only eliminate discrete cells in normal development, host defense and maintain the body in well stable condition, also play an important role in regulating and controlling tumor occurrence, development and treatment[17,18]. Impaired apoptosis has been implicated in the development of many human diseases, including cancer. It has been proved that occurrence of cancers is due to the loss of control of normal apoptosis and the disturbance of balance between cell proliferation and apoptosis[19–21]. Two main signaling pathways initiate the apoptotic program in mammalian cells[22]. The cell-extrinsic pathway, namely, death receptor signaling pathway triggers apoptosis in response to activation by their respective ligand of the tumor necrosis factor (TNF) family of death receptors, including TNF-RI for TNFa, Fas or CD95 for FasL, and death receptor 4 (DR4) or 5 (DR5) for TNF-related apoptosis-inducing ligand (TRAIL). On the other hand, the cell-intrinsic pathway, namely, mitochondria signaling pathway triggers apoptosis in response to DNA damage, loss of survival factors, or other types of cell distress. Both pathways involve the activation of cysteine proteases called caspased, which are constitutively expressed in the cytosol as proenzymes, and are activated to mature proteases by cleavage, which triggers the activation of the caspase-8 and caspase-9, followed by the activation of caspase-3. Caspase-3 is one of the key executioners in the apoptosis pathway. Caspase-3 activation is thought to be a major step in the apoptotic signal transduction cascade that commits cells to suicide. Caspase-3 cleavage during apoptosis leads to the proteolysis of several cytosolic and nuclear proteins and precedes DNA fragmentation. However, some recent studies have shown that activation of Fas/FasL signaling pathway is regulated by many kinds of cell factors, including GAS and SS[23–25].
Many studies have pointed out that some tissue growths are regulated by hormones, and these tissues turned into tumors are still controlled by hormones[26–28]. Gastrointestinal hormones such as GAS and SS regulate the secretion, motility, absorption, blood flow and cell nutrition of the digestive tract. Abnormality of their secretion often affected the normal functions of digestive tract, even produced clinical symptoms or syndromes[29]. Xie et al[30] found that the disordered gastrointestinal hormones play a crucial role in the pediatric chronic gastritis and duodenal ulcer. There seems to be an increasing tendency in the expressions of GAS and SS in children with chronic gastritis and duodenal ulcer. In recent years, some studies have demonstrated that there is a high correlation between the aberrant expressions of GAS, SS and the occurrence and development of LIC[31–33]. D’Onghia et al[34] found that GAS serum levels were slightly higher in patients with colorectal cancer than in healthy controls. GAS levels were higher in patients carrying left colorectal cancer than the others, and higher in early stage than in late stage. Cao et al[35] found that GAS 17 can increase colorectal cancer cells’ invasion, the mechanism of which is probably that GAS 17 makes FAK-Tyr397 to be phosphorylated and localized to lamellipodia, causing the formation of FAK-Src-p130 (Cas)-Dock180 signaling complex when it is bound to its receptor CCK-2 and activation of Rac. In this study, we found that there was a significant positive correlation between mRNA and protein expression of GAS and SS. The positive expression rate of GAS and SS mRNA and protein varied greatly in different histological type, differentiation degree, and Dukes’ stage. The higher mRNA and protein expression of GAS was, the poorer of tissue differentiation degree and clinical stages. For example, the expression GAS in poorly differentiated LIC, in particular, in mucinous adenocarcinomas and signet-ring cell carcinoma was prominently higher than the others, with poor prognosis. However, the action of SS was opposite. The results indicate that the expressions of GAS and SS were closely related to the biological behavior of LIC. It is obvious that the GAS and SS will be valuable targets of the biological behavior of LIC.
Tumor escape mechanisms in LIC result from dysregulation of the cell apoptosis signaling pathways, which are mediated by death ligands. Recently, great progress has been made in understanding the regulated signaling pathway mechanisms of GAS and SS. Some studies showed that the abnormal expressions of GAS and SS were closely related to cell apoptosis of LIC. GAS could promote cell proliferation and inhibit cell apoptosis. However, the action of SS was opposite in LIC[36,37]. Our studies showed that the expression of GAS protein in LIC was higher and the AI was lower, and the higher expression of SS protein, the higher AI. Despite abundant evidence that GAS may play an integral role in promoting tumor growth in the stomach and malignancies in the GI tract, the precise mechanisms about gastrin-restrained, somatostatin-induced apoptosis are still largely unknown. To elucidate the mechanisms by which GAS and SS might influence apoptotic signaling pathway, we analyzed them effects on the expression of Fas, FasL, caspase-3 and caspase-8 protein in human LIC tissue.
GAS is a gastrointestinal (GI) peptide that possesses potent trophic effects on most of the normal and neoplastic mucosa of the GI tract. GAS is mainly secreted from GAS secreting cells (G cells) in the antrum mucosa or upper small intestine and large intestine. Medulla oblongata and dorsal nuclei of vagus nerves in central nervous system also secrete GAS[38,39]. Recent reports indicate that chronic hypergastrinaemia increases the risk of colorectal cancer and cancer growth, and that interruption of the effects of GAS could be a potential target in the treatment of colorectal cancer[40]. Recent data have shown that GAS is not only able to induce cell proliferation, but also inhibit LIC cell apoptosis. However, the underlying mechanisms that GAS inhibits cell apoptosis, is still worthy of further elucidation. Wu et al[41] recent studies found that down-regulation of GAS gene expression results in the activation of caspase 9 and 3 in gastrin-dependent human colon cancer cells inducing cell apoptosis. Cui et al[42] found that GAS can induce apoptosis in gastric epithelial cells via GAS/CCK-2 receptor and synergized with FasL stimulation and contribute to the development of gastric carcinogenesis. In this study, we found that the level of GAS protein expression was higher and the positive expression rate of FasL was higher in LIC tissues, and expression of GAS protein was not related to expression of Fas, caspase-3 and caspase-8. The results indicate that the mechanisms of GAS inhibiting LIC cell apoptosis might be via up-regulation of expression of FasL inducing apoptosis with Fas-expressing T cells, making LIC cells escape from immune surveillance and immunocyte attack.
SS is a multifunctional hormone, which is secreted from SS secreting cells (D cells). D cells are distributed mainly in human central and peripheral nervous system, and in the gastrointestinal tract, including the large intestine. SS inhibits multiple functions, including exocrine and endocrine secretions, inflammation, and angiogenesis, as well as cell proliferation and tumorigenesis, as shown in normal or tumoral cell models[43–46]. SS acts as an inhibitory peptide of various secretory and proliferative responses. The diverse biological effects of SS are mediated through a family of five SS receptors (sst1-sst5) that belong to the family of G-protein-coupled receptors and that regulate diverse signal transduction pathways including adenylate cyclase, phospholipase C-β, phospholipase A2, guanylate cyclase, ionic conductance channels, and tyrosine phosphatase[47,48]. The mechanisms of the inhibition are the combined interaction of SS and its analogs with SST1-5R in tumor tissues, either directly inhibiting division and proliferation of tumor cells or inhibiting the activities of growth factors such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), etc[49–51], thus counteracting tumorigenesis and tumor cell proliferation. The ability of SS and its stable analogues to promote tumor cell apoptosis has been demonstrated in various cell types including mammary, prostatic, gastric, pancreatic, colorectal, and small cell lung cancer cells. However, the underlying mechanisms of SS induced cell apoptosis are still poorly understood. Guillermet et al[52] showed that sst2-dependent activation and cell sensitization to death ligand-induced apoptosis involved activation of the executioner caspases are key factors in both death ligand-or mitochondria-mediated apoptosis. Sst2 affected pathways by up-regulating expression of TRAIL and TNFalpha receptors, DR4 and TNFRI, respectively, and sensitizing the cells to death ligand-induced initiator capase-8 activation; by down-regulating expression of the antiapoptotic mitochondrial Bcl-2 protein; and by enhancing TNFalpha-mediated activation of NF-kappaB, resulting in JNK inhibition and subsequent executioner caspase activation and cell death. Kang et al[53] found that SS was able to induce peritoneal macrophages apoptosis by a Bax- and NO-independent p53 accumulation, and through Fas and caspase-8 activation pathways. Recent reports have demonstrated that SS analogs octreotide was able to induce human somatotroph tumor cell apoptosis by activating SST2 and also induce an increase in caspase-3 levels[54,55]. In this study, we found that the higher the integral of SS was, the higher the positive expression rate of Fas, caspase-3, caspase-8, and expression of SS protein was foreign to expression of FasL. Our results indicated that SS was able to induce over-expression of Fas, caspase-3 and caspase-8 protein, and the mechanisms of SS promoting LIC cell apoptosis can be via up-regulation of expression of Fas or through increasing caspase-3 levels and sensitivity of death receptor signaling pathway.
In this study, we found that the ratio of GAS to SS had an effect on biological characteristics such as malignant type, tissue differentiation and clinical stages of LIC. The increased ratio of GAS to SS is an event of significance in LIC occurrence and development[56,57]. Our results indicated that there was a positive correlation between the ratio of GAS to SS and the semi-quantitative integral of FasL, and negative correlation between the integral ratio of GAS to SS and the AI. Furthermore, the expression of GAS and SS proteins was directly related to the expression of Fas, FasL, caspase-3, 8 in LIC.
In conclusion, the regulation and control of GAS and SS in LIC cell apoptosis may be directly related to the aberrant expression of Fas/FasL. The GAS and SS will be valuable targets of the biological behavior of LIC.
COMMENTS
Background
Although great progress in understanding the molecular aspects of large intestinal cancer (LIC) has been made and several therapeutic agents have been developed, it is still difficult to cure the tumor. The general survival rate of LIC patients does not exceed 40%. Thereby it is highly important to know that what kind of cell factor can influence cell apoptosis, which will enrich the etiology theory of tumor. Despite abundant evidence that gastrin may play an integral role in promoting tumor growth in the large intestine, the precise mechanisms about gastrin-restrained and somatostatin-induced apoptosis are still largely unknown.
Research frontiers
Recent some studies have shown that activation of Fas/FasL signaling pathway is regulated by many kinds of cell factors, including gastrin (GAS) and somatostatin (SS).
Innovations and breakthroughs
Some studies found that GAS was able to inhibit apoptosis in LIC cell, but the role of SS was opposite. However, the detailed molecule mechanism by which GAS and SS mediate cell apoptosis of LIC is not fully known. In this study, the authors analyzed their effects on the expression of Fas, FasL, caspase-3 and caspase-8 protein. To further confirm whether GAS and SS could mediate LIC cell apoptosis mainly via affecting the expression of Fas/FasL, will help us find a new way to treat malignant tumors.
Applications
The GAS and SS will be valuable targets of the biological behavior of LIC.
Terminology
GAS: It is mainly secreted from GAS secreting cells (G cells) in antrum mucosa or upper small intestine and large intestine. Medulla oblongata and dorsal nuclei of vagus nerves in central nervous system also secrete GAS. SS: It is secreted from SS secreting cells (D cells). D cells are distributed mainly in human central and peripheral nervous system, and in the gastrointestinal tract. Fas/FasL: It belongs to the ligand of the tumor necrosis factor (TNF) family of death receptors. Caspase: Mammalian cysteine aspartate proteases (caspase) can be divided into initiator (e.g. caspases 2, 8, 9, 10) and effector (caspases 3, 4, 5, 6, 7, 11, 12, and 13) enzymes.
Peer review
This paper demonstrates that GAS and SS play important roles in the regulation and control of cell apoptosis in LIC, and the mechanism may be directly related to the aberrant expression of Fas/FasL, which will enrich the etiology theory of LIC. In addition, it also provides a way to evaluate targets of the biological behavior of LIC.
Supported by The Natural Science Foundation of Educational Bureau of Anhui Province, No. 2006kj115c, 2005kj302, and Natural Science Foundation of Anhui Province, No. 03043704
Peer reviewers: Rene Lambert, Professor, International Agency for Research on Cancer, 150 Cours Albert Thomas, Lyon 69372 cedex 8, France; Takayuki Yamamoto, MD, Inflammatory Bowel Disease Center, Yokkaichi Social Insurance Hospital, 10-8 Hazuyamacho, Yokkaichi 510-0016, Japan
S- Editor Zhu WL L- Editor Ma JY E- Editor Liu Y
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411:390–395. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 3.Tsukuma H, Ajiki W. [Descriptive epidemiology of colorectal cancer--international comparison] Nippon Rinsho. 2003;61 Suppl 7:25–30. [PubMed] [Google Scholar]
- 4.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 5.Zhang MG, Li JT, Yang HY, Zhao HC. Clinical analysis on 1143 cases of large intestine carcinoma. Zhonghua Yixue Zazhi. 2003;83:2087–2088. [Google Scholar]
- 6.Saga T, Tamaki N, Itoi K, Yamazaki T, Endo K, Watanabe G, Maruno H, Machinami R, Koizumi K, Ichikawa T, et al. [Phase III additional clinical study of 111In-pentetreotide (MP-1727): diagnosis of gastrointestinal hormone producing tumors based on the presence of somatostatin receptors] Kaku Igaku. 2003;40:185–203. [PubMed] [Google Scholar]
- 7.Cobb S, Wood T, Ceci J, Varro A, Velasco M, Singh P. Intestinal expression of mutant and wild-type progastrin significantly increases colon carcinogenesis in response to azoxymethane in transgenic mice. Cancer. 2004;100:1311–1323. doi: 10.1002/cncr.20094. [DOI] [PubMed] [Google Scholar]
- 8.Beales IL, Ogunwobi O. Glycine-extended gastrin inhibits apoptosis in colon cancer cells via separate activation of Akt and JNK pathways. Mol Cell Endocrinol. 2006;247:140–149. doi: 10.1016/j.mce.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 9.Ogunwobi OO, Beales IL. Glycine-extended gastrin stimulates proliferation and inhibits apoptosis in colon cancer cells via cyclo-oxygenase-independent pathways. Regul Pept. 2006;134:1–8. doi: 10.1016/j.regpep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Mao JD, Wu P, Xia XH, Hu JQ, Huang WB, Xu GQ. Correlation between expression of gastrin, somatostatin and cell apoptosis regulation gene bcl-2/bax in large intestine carcinoma. World J Gastroenterol. 2005;11:721–725. doi: 10.3748/wjg.v11.i5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fromowitz FB, Viola MV, Chao S, Oravez S, Mishriki Y, Finkel G, Grimson R, Lundy J. ras p21 expression in the progression of breast cancer. Hum Pathol. 1987;18:1268–1275. doi: 10.1016/s0046-8177(87)80412-4. [DOI] [PubMed] [Google Scholar]
- 12.Joyce T, Pintzas A. Microarray analysis to reveal genes involved in colon carcinogenesis. Expert Opin Pharmacother. 2007;8:895–900. doi: 10.1517/14656566.8.7.895. [DOI] [PubMed] [Google Scholar]
- 13.John R, El-Rouby NM, Tomasetto C, Rio MC, Karam SM. Expression of TFF3 during multistep colon carcinogenesis. Histol Histopathol. 2007;22:743–751. doi: 10.14670/HH-22.743. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Michor F, Iwasa Y, Lengauer C, Nowak MA. Dynamics of colorectal cancer. Semin Cancer Biol. 2005;15:484–493. doi: 10.1016/j.semcancer.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Lièvre A, Laurent-Puig P. [Colorectal carcinogenesis: update] Rev Prat. 2004;54:143–150. [PubMed] [Google Scholar]
- 17.Kim JS, Lee YC, Nam HT, Li G, Yun EJ, Song KS, Seo KS, Park JH, Ahn JW, Zee O, et al. Apicularen A induces cell death through Fas ligand up-regulation and microtubule disruption by tubulin down-regulation in HM7 human colon cancer cells. Clin Cancer Res. 2007;13:6509–6517. doi: 10.1158/1078-0432.CCR-07-1428. [DOI] [PubMed] [Google Scholar]
- 18.Fernández-Cebrián JM, Nevado Santos M, Vorwald Kuborn P, Pardo de Lama M, Martín-Cavanna J, Pacheco Martínez P, Fernández Escudero B, Ramos Fernández M. Can the clinical outcome in stage II colon carcinomas be predicted by determination of molecular marker expression? Clin Transl Oncol. 2007;9:663–670. doi: 10.1007/s12094-007-0119-z. [DOI] [PubMed] [Google Scholar]
- 19.Søreide K. [Genetics and molecular classification of colorectal cancer] Tidsskr Nor Laegeforen. 2007;127:2818–2823. [PubMed] [Google Scholar]
- 20.He L, Li X, Luo HS, Rong H, Cai J. Possible mechanism for the regulation of glucose on proliferation, inhibition and apoptosis of colon cancer cells induced by sodium butyrate. World J Gastroenterol. 2007;13:4015–4018. doi: 10.3748/wjg.v13.i29.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lev-Ari S, Kazanov D, Liberman E, Ben-Yosef R, Arber N. Down-regulation of PGE2 by physiologic levels of celecoxib is not sufficient to induce apoptosis or inhibit cell proliferation in human colon carcinoma cell lines. Dig Dis Sci. 2007;52:1128–1133. doi: 10.1007/s10620-006-9619-x. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 23.Cui G, Takaishi S, Ai W, Betz KS, Florholmen J, Koh TJ, Houghton J, Pritchard DM, Wang TC. Gastrin-induced apoptosis contributes to carcinogenesis in the stomach. Lab Invest. 2006;86:1037–1051. doi: 10.1038/labinvest.3700462. [DOI] [PubMed] [Google Scholar]
- 24.Xie XZ, Wang ZM, Zhang HY, Wang L, Gao BH, Li XM, Hu WG. Expression of gastrin, somatostatin, PCNA and Fas-L in the mucosa of gastric antrum of children with chronic gastritis and duodenal ulcer. Zhonghua Er Ke Za Zhi. 2006;44:774–777. [PubMed] [Google Scholar]
- 25.Guillermet J, Saint-Laurent N, Rochaix P, Cuvillier O, Levade T, Schally AV, Pradayrol L, Buscail L, Susini C, Bousquet C. Somatostatin receptor subtype 2 sensitizes human pancreatic cancer cells to death ligand-induced apoptosis. Proc Natl Acad Sci USA. 2003;100:155–160. doi: 10.1073/pnas.0136771100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt M, Fink D, Lang U, Kimmig R. [Hormone replacement therapy: curse or blessing?] Gynakol Geburtshilfliche Rundsch. 2006;46:165. doi: 10.1159/000095724. [DOI] [PubMed] [Google Scholar]
- 27.Lam PM, Chung TK, Haines C. Where are we with postmenopausal hormone therapy in 2005? Gynecol Endocrinol. 2005;21:248–256. doi: 10.1080/09513590500279733. [DOI] [PubMed] [Google Scholar]
- 28.Slattery ML, Sweeney C, Murtaugh M, Ma KN, Wolff RK, Potter JD, Caan BJ, Samowitz W. Associations between ERalpha, ERbeta, and AR genotypes and colon and rectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2936–2942. doi: 10.1158/1055-9965.EPI-05-0514. [DOI] [PubMed] [Google Scholar]
- 29.Cho KH, Lee HS, Ku SK. Changes in gastric endocrine cells in Balb/c mice bearing CT-26 carcinoma cells: an immunohistochemical study. Eur J Histochem. 2006;50:293–300. [PubMed] [Google Scholar]
- 30.Xie XZ, Wang ZM, Zhang HY, Wang L, Gao BH, Li XM, Hu WG. [Expression of gastrin, somatostatin, PCNA and Fas-L in the mucosa of gastric antrum of children with chronic gastritis and duodenal ulcer] Zhonghua Erke Zazhi. 2006;44:774–777. [PubMed] [Google Scholar]
- 31.Tejeda M, Gaál D, Hullán L, Hegymegi-Barakonyi B, Kéri G. Evaluation of the antitumor efficacy of the somatostatin structural derivative TT-232 on different tumor models. Anticancer Res. 2006;26:3477–3483. [PubMed] [Google Scholar]
- 32.Singh P. Role of Annexin-II in GI cancers: interaction with gastrins/progastrins. Cancer Lett. 2007;252:19–35. doi: 10.1016/j.canlet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schally AV, Szepeshazi K, Nagy A, Comaru-Schally AM, Halmos G. New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs. Cell Mol Life Sci. 2004;61:1042–1068. doi: 10.1007/s00018-004-3434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Onghia V, Leoncini R, Carli R, Santoro A, Giglioni S, Sorbellini F, Marzocca G, Bernini A, Campagna S, Marinello E, et al. Circulating gastrin and ghrelin levels in patients with colorectal cancer: correlation with tumour stage, Helicobacter pylori infection and BMI. Biomed Pharmacother. 2007;61:137–141. doi: 10.1016/j.biopha.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Cao J, Yu JP, Zhou L, Song WC, Luo HS, Yu HG. [Molecular mechanism of gastrin increasing colon cancer cells' invasion] Zhonghua Yixue Zazhi. 2007;87:1704–1708. [PubMed] [Google Scholar]
- 36.Beales IL, Ogunwobi O. Glycine-extended gastrin inhibits apoptosis in colon cancer cells via separate activation of Akt and JNK pathways. Mol Cell Endocrinol. 2006;247:140–149. doi: 10.1016/j.mce.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 37.Qiu CZ, Wang C, Huang ZX, Zhu SZ, Wu YY, Qiu JL. Relationship between somatostatin receptor subtype expression and clinicopathology, Ki-67, Bcl-2 and p53 in colorectal cancer. World J Gastroenterol. 2006;12:2011–2015. doi: 10.3748/wjg.v12.i13.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali MA, Nyberg F, Chandranath SI, Dhanasekaran S, Tariq S, Petroianu G, Hasan MY, Adeghate EA, Adem A. Distribution of neuroendocrine cells in the small and large intestines of the one-humped camel (Camelus dromedarius) Neuropeptides. 2007;41:293–299. doi: 10.1016/j.npep.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Rindi G, Solcia E. Endocrine hyperplasia and dysplasia in the pathogenesis of gastrointestinal and pancreatic endocrine tumors. Gastroenterol Clin North Am. 2007;36:851–865, vi. doi: 10.1016/j.gtc.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Niv Y, Delpre G, Sperber AD, Sandbank J, Zirkin H. Hyperplastic gastric polyposis, hypergastrinaemia and colorectal neoplasia: a description of four cases. Eur J Gastroenterol Hepatol. 2003;15:1361–1366. doi: 10.1097/00042737-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Wu H, Owlia A, Singh P. Precursor peptide progastrin(1-80) reduces apoptosis of intestinal epithelial cells and upregulates cytochrome c oxidase Vb levels and synthesis of ATP. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1097–G1110. doi: 10.1152/ajpgi.00216.2003. [DOI] [PubMed] [Google Scholar]
- 42.Cui G, Takaishi S, Ai W, Betz KS, Florholmen J, Koh TJ, Houghton J, Pritchard DM, Wang TC. Gastrin-induced apoptosis contributes to carcinogenesis in the stomach. Lab Invest. 2006;86:1037–1051. doi: 10.1038/labinvest.3700462. [DOI] [PubMed] [Google Scholar]
- 43.Williams GT. Endocrine tumours of the gastrointestinal tract-selected topics. Histopathology. 2007;50:30–41. doi: 10.1111/j.1365-2559.2006.02570.x. [DOI] [PubMed] [Google Scholar]
- 44.Badway AC, Blake AD. Somatostatin: a hormone for the heart? Curr Vasc Pharmacol. 2005;3:125–131. doi: 10.2174/1570161053586958. [DOI] [PubMed] [Google Scholar]
- 45.Ueberberg B, Tourne H, Redman A, Walz MK, Schmid KW, Mann K, Petersenn S. Differential expression of the human somatostatin receptor subtypes sst1 to sst5 in various adrenal tumors and normal adrenal gland. Horm Metab Res. 2005;37:722–728. doi: 10.1055/s-2005-921092. [DOI] [PubMed] [Google Scholar]
- 46.Pichler R, Pichler J, Mustafa H, Nussbaumer K, Zaunmüller T, Topakian R. Somatostatin-receptor positive brain stem glioma visualized by octreoscan. Neuro Endocrinol Lett. 2007;28:250–251. [PubMed] [Google Scholar]
- 47.Guillermet-Guibert J, Lahlou H, Cordelier P, Bousquet C, Pyronnet S, Susini C. Physiology of somatostatin receptors. J Endocrinol Invest. 2005;28:5–9. [PubMed] [Google Scholar]
- 48.Arena S, Pattarozzi A, Massa A, Esteve JP, Iuliano R, Fusco A, Susini C, Florio T. An intracellular multi-effector complex mediates somatostatin receptor 1 activation of phospho-tyrosine phosphatase eta. Mol Endocrinol. 2007;21:229–246. doi: 10.1210/me.2006-0081. [DOI] [PubMed] [Google Scholar]
- 49.Wilkinson-Berka JL, Wraight C, Werther G. The role of growth hormone, insulin-like growth factor and somatostatin in diabetic retinopathy. Curr Med Chem. 2006;13:3307–3317. doi: 10.2174/092986706778773086. [DOI] [PubMed] [Google Scholar]
- 50.Sall JW, Klisovic DD, O'Dorisio MS, Katz SE. Somatostatin inhibits IGF-1 mediated induction of VEGF in human retinal pigment epithelial cells. Exp Eye Res. 2004;79:465–476. doi: 10.1016/j.exer.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Zatelli MC, Piccin D, Vignali C, Tagliati F, Ambrosio MR, Bondanelli M, Cimino V, Bianchi A, Schmid HA, Scanarini M, et al. Pasireotide, a multiple somatostatin receptor subtypes ligand, reduces cell viability in non-functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Endocr Relat Cancer. 2007;14:91–102. doi: 10.1677/ERC-06-0026. [DOI] [PubMed] [Google Scholar]
- 52.Guillermet J, Saint-Laurent N, Rochaix P, Cuvillier O, Levade T, Schally AV, Pradayrol L, Buscail L, Susini C, Bousquet C. Somatostatin receptor subtype 2 sensitizes human pancreatic cancer cells to death ligand-induced apoptosis. Proc Natl Acad Sci USA. 2003;100:155–160. doi: 10.1073/pnas.0136771100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang BN, Jeong KS, Park SJ, Kim SJ, Kim TH, Kim HJ, Ryu SY. Regulation of apoptosis by somatostatin and substance P in peritoneal macrophages. Regul Pept. 2001;101:43–49. doi: 10.1016/s0167-0115(01)00264-6. [DOI] [PubMed] [Google Scholar]
- 54.Capello A, Krenning EP, Bernard BF, Breeman WA, van Hagen MP, de Jong M. Increased cell death after therapy with an Arg-Gly-Asp-linked somatostatin analog. J Nucl Med. 2004;45:1716–1720. [PubMed] [Google Scholar]
- 55.Ferrante E, Pellegrini C, Bondioni S, Peverelli E, Locatelli M, Gelmini P, Luciani P, Peri A, Mantovani G, Bosari S, et al. Octreotide promotes apoptosis in human somatotroph tumor cells by activating somatostatin receptor type 2. Endocr Relat Cancer. 2006;13:955–962. doi: 10.1677/erc.1.01191. [DOI] [PubMed] [Google Scholar]
- 56.Wu P, Tu JS, Riu J, Hang H, Hang WB, Yuan P. To study the correlation between expression of gastrin, somatostatin and cell proliferation, apoptosis in colorectal carcinoma. Zhonghua Shiyan Waike Zazhi. 2003;20:947. [Google Scholar]
- 57.Wu P, Mao JD, Yan JY, Rui J, Zhao YC, Li XH, Xu GQ. Correlation between the expressions of gastrin, somatostatin and cyclin and cyclin-depend kinase in colorectal cancer. World J Gastroenterol. 2005;11:7211–7217. doi: 10.3748/wjg.v11.i45.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]