Abstract
AIM: To identify the cholecystokinin (CCK)-A receptors (CCK-AR) on the guniea pig gallbladder interstitial cells of cajal (ICC) and to study CCK-8 induced gallbladder muscle strip contractions through the CCK-AR.
METHODS: The existence of CCK-AR was examined by immunohistofluorescence on sectioned tissue and cultured cells. In vitro contractile response of guinea pig gallbladder muscle strips and the strips with ICC removed were also studied with CCK-8 receptors added.
RESULTS: In tissue sections, intensely CCKAR-immunoreactive interstitial cells were found mainly in the muscular layers. In cultured cell sections, distinctive double staining of C-kit and CCK-AR ICCs were found. When we removed the ICC of the gallbladder, CCK-8 induced muscle strip contraction dose response curve significantly shifted to the right.
CONCLUSION: We proved that both the existence of CCK-AR on the guinea pig gallbladder ICC and CCK evoked contraction are mediated through direct action on CCK-AR on the gallbladder ICC.
Keywords: Gallbladder, Interstitial cells of cajal, Cholecystokinin-A receptor, C-kit, Immunohistofluo-rescence
INTRODUCTION
Cholecystokinin (CCK) is released from mucosal endocrine cells in the proximal small intestine in response to a meal[1], and it is classically known to stimulate the gallbladder contraction. The CCK-induced gallbladder contractions were once believed to be brought about by direct stimulation of gallbladder smooth muscle. The CCK receptors have been identified by using isolated gallbladder muscle membranes and autoradiographical analysis[2–4]. Two CCK receptors, CCK-A (CCK-AR) and CCK-B receptors (CCK-BR) have been identified[5]. It is well established that CCK-AR and CCK-BR regulate a number of physiological functions, e.g. gallbladder contraction[6], pancreatic enzyme release[7], gastric acid secretion[8] and pyloric sphincter closure[9,10]. In 1892, a special kind of cell which is now called “interstitial cells of Cajal” (ICC) in the gastrointestinal tract was first described by the famous Spanish neuro-histologist Ramony Cajal[11]. It has been approved that ICC play an important role in gastrointestinal tissues by generating and propagating electrical slow waves to gastrointestinal muscles, and it mediates signals from the enteric nervous system[12–15]. ICCs were found in the murine[16], Homo sapiens[17] and guinea pig gallbladders[18]. Both the CCK-AR and CCK-BR mediate the contraction of the guinea pig ileum[19–21], whereas the guinea pig gallbladder contraction is mediated solely by CCK-AR[22]. It is considered that CCK-induced gallbladder contractions are controlled through CCK-ARs both on the vagal nerve in stimulating endogenous release of acetylcholine and on the gallbladder to directly stimulate muscle contraction in dogs[23]. The recent availability of antibodies directed against the CCK-AR has made it possible to identify the receptor by immunocytochemistry[24] and immunohistochemistry[25]. It has been reported that some ICCs in the rat pylorus express CCK-ARs[26]. Therefore, we used an immunohistochemical approach to localize the CCK-AR in the gallbladder. Surprisingly, we found that the interstitial cells of Cajal express CCKARs to a high degree. We also measured the effect of atropine, tetrodotoxin and CCK on the gallbladder muscle strips, and the contractions of the muscle strips with ICC removed in the guinea pig. This is the first study in which the CCK-induced gallbladder contraction through CCK-AR on the gallbladder ICC was investigated using the guinea pig as the experimental animal.
MATERIALS AND METHODS
Animals
Adult guinea-pigs (250-300 g) were obtained by mail from Animal Experiment Center of Wuhan University. The animal-testing protocol used in the present investigation was approved by the Institutional Animal Care and the Use Committee of the Wuhan University.
Chemicals
All the chemicals and bovine serum albumin were obtained from Sigma. The rat monoclonal antibodies against C-kit were purchased from R&D. The Rabbit anti-CCK-AR polyclonal antibody was purchased from Chemicon. M199 were purchased from Sigma. All the secondary antibodies were bought from Beijing Zhongshan Biotechnology CO., LTD.
Experimental procedures
Demonstration of CCK-AR on the gallbladder tissue by immunohistofluorescence: The gallbladder was fixed with 4% paraformaldehyde at 4°C for 16 h. Following fixation, the tissues were embedded and cut for immunohistochemistry. Next, they were washed and incubated with normal bovine serum albumin for 10 min. They were subsequently incubated with rat anti-kit monoclonal antibody, with rabbit anti-CCK-AR polyclonal antibody added to the anti-kit solution at 4°C for 24 h. The sections were incubated with the secondary antibody after the first antibody. CCK-AR polyclonal antibody was visualized using the goat anti-rabbit IgG conjugated to Cy3 and the C-kit was visualized using the rabbit anti-rat antibody conjugated to FITC for 2 h at room temperature. A negative control without primary antibody was performed.
Demonstration of CCK-AR on the gallbladder ICCs by immunohistofluorescence: The surrounding tissue of guinea pig’s gallbladder was cleaned off and cut longitudinally. The tissue was pinned to the base of a dish filled with Sylgard elastomer with the mucosal side facing up. The mucosa was removed by sharp dissection, and the muscular layer tissues were cut out and incubated at 37°C for 23 min in a Ca2+-free physiological saline solution (PSS mmol/L: NaCl 135.0, KCl 5.0, glucose 10.0, Hepes 10.0, and MgCl2 1.2; pH set to 7.4 with NaOH) containing type II collagenase (1.2 mg/mL), soybean trypsin inhibitor (2.0 mg/mL) and bovine serum albumin (BSA; 2.0 mg/mL). The pieces of tissue were then rinsed five times with Ca2+- free PSS and gently triturated with a wide-bore glass pipette to yield the isolated cells. Aliquots of the suspension of the cells were then placed in glass coverslips, and they were left at 4°C for 45-60 min to attach onto the bottom of coverslips for culturing. Cells were washed four times with Krebs-Ringer buffer (KRB mmol/L: NaCl 118.5, KCl 4.5, MgCl2 1.2, NaHCO3 23.8, KH2PO4 1.2, dextrose 11.0 and CaCl2 2.4; pH at 7.4) containing penicillin (200 U/mL), streptomycin (200 mg/mL) and amphotericin B (0.5 mg/mL), and they were placed in M199 medium (Sigma) containing similar concentrations of antibiotics and antimycotic. The cells were incubated at 37°C (90% humidity and 95% O2 - 5% CO2) for 1 d. Cells were fixed with 4% paraformaldehyde at 4°C for 16 h, and then it was washed and incubated with normal bovine serum albumin for 10 min. Tissues were processed with protocol identical to the cells. The cells and tissues were examined under the TCS SP2 MP laser scanning confocal microscope (Leica, Germany). With an excitation beam (488 nm) produced by argon, the emitted fluorescence was captured using Leica Confocal software running on Windows XP work station.
Contractile response of the gallbladder muscle strip to CCK-8: (1) CCK-Induced Contractions on Common Muscle Strips. The adult guinea-pigs sent in were killed by cervical dislocation. The gallbladder was removed and opened, and it was washed several times in Krebs solution to remove bile. Four strips of each gallbladder measuring approximately 0.2 cm × 0.8 cm were mounted in organ baths containing Krebs solution (30 mL) with the following composition (mmol/L): NaCl 120.35, KCl 5.9, MgCl2 2.5, NaH2PO4 1.2, NaHCO3 15.5, CaCl2 2.5, and glucose 11.5; pH 7.4. The organ bath was maintained at 37°C and gassed with 95% O2 and 5% CO2. A resting 1 g pre-load was applied to each muscle strip and was allowed to equilibrate for 1 h, and during which time the Krebs solution was changed every 20 min. During the equilibration period, the tension was adjusted to 1 g when required. Mechanical activity was recorded using isometric transducers (World Precision Instruments, Stevenage, Herts, U.K.). Tension was continuously monitored and recorded using a MacLab data acquisition system (AD Instruments Ltd., Hastings, U.K.). A dose-response curve for CCK-8 was constructed by adding CCK-8 to the medium in a cumulative way, and the effect of pretreatment with 10-6 mol/L atropine, 3 × 10-7 mol/L tetrodotoxin (TTX) on CCK-8 induced gallbladder contraction was also examined. (2) CCK-Induced Contractions on Muscle Strips with ICCs Removed. Methylene blue staining is a nonspecific but effective and time-dependent staining method for ICCs. After the removal of the mucosa, muscle strips were washed with fresh oxygenated Krebs solution (in mmol/L: NaCl 120.35, KCl 5.9, MgCl2 2.5, NaH2PO4 1.2, NaHCO3 15.5, CaCl2 2.5, and glucose 11.5; pH at 7.4) and then incubated in Krebs solution containing 50 μmol/L methylene blue at 37°C bubbled with 95% O2 -5% CO2 (v/v) for 40 min in the dark with intermittent visual checking of the staining intensity. After staining, the muscle strips were immediately put under the light (50 mW/cm2) for 5 min. Next, CCK-induced contractions on muscle strips with ICCs removed were done as mentioned above for the common muscle strips. Results were expressed as mean ± SE. (3) Transmisson Electron Microscopy. We used transmission electron microscopy to identify the effect of methylene blue with light on gallbladder ICC. The muscle trips removed ICC were fixed in 2.5% GA in 0.1 mol/L (PB) for 2 h or overnight. They were then washed in 0.1 mol/L PB (2 × 15 min) and post-fixed with 2% osmium tetroxide in 0.1 mol/L PB (2 h). After another wash with 0.1 mol/L PB (2 × 15 min), they were dehydrated through ascending grades of ethanol (50, 75, 95 and 2 × 100%, 15-20 min each) and embedded in an epoxy resin, Quetol-812. Ultra-thin sections were cut using a Reichert OMU4 ultramicrotome with a diamond-cutting knife. Sections were picked up on 200 mesh copper grids and stained with 2% uranyl acetate in 30% ethanol, and this was followed by Sato's lead staining. Grids with sections were viewed under the OPTON EM10C transmission electron microscope.
RESULTS
Demonstration of CCK-AR on the gallbladder
Tissue sections immunohistofluorescence: In cross sections of the gallbladder, intensely CCK-AR immunoreactive interstitial cells were found mainly in the muscular layers. Labeling in the most intensely stained cells was already optimal at an antibody concentration of 1:8000. At low antibody concentrations, there were very little staining found on smooth muscle cells of the gallbladder muscle, yet, at higher concentrations, the gallbladder muscle showed considerable immunoreactivity, particularly in its inner portions (Figure 1).
Figure 1.
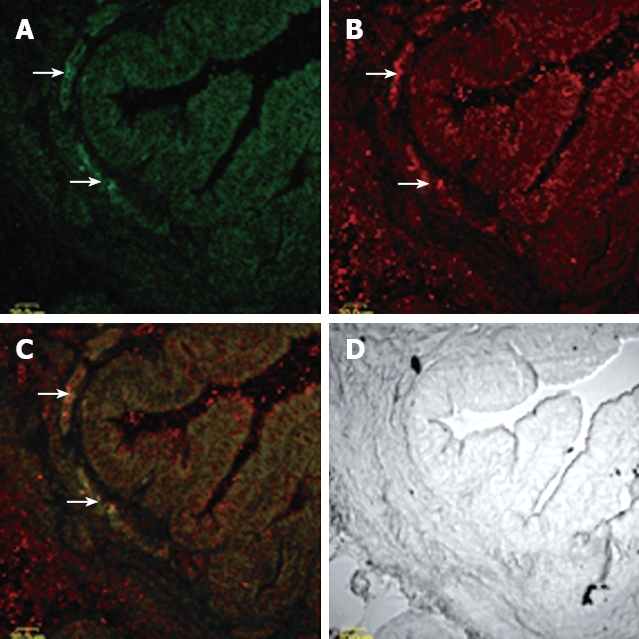
Overview of CCKAR-immunoreactive ICC seen on cross section of guinea pig gallbladder. A: Low-magnification montage showing C-kit positive cells (arrow) distributed over a band of gallbladder muscle adjacent to the mucosa; B: Low-power montage showing CCKAR-immunoreactive cells distribute mainly in the muscular layers of guinea pig gallbladder; C: Compound micrographs from (A) and (B). Some, but not all C-kit immunoreactive (green) ICC colocalize CCKAR (red), meanwhile, not all CCKAR-immunoreactive cells are ICC; D: Phase-contrast micrographs of the same gallbladders wall at the same magnification.
Cultured cells immunohistofluorescence: CCK-AR immunoreactive ICC in the muscle of the gallbladder differed in the complexity and shape of their labeling processes (Figure 2). In one extreme end, the processes showed multiplicity and the results were frequently subdivided into fine filaments and peaks (Figure 2A-D). In another end, ICCs possessed only three smooth processes and two labeled ICC were aggregated into the network (Figure 2E-H).
Figure 2.
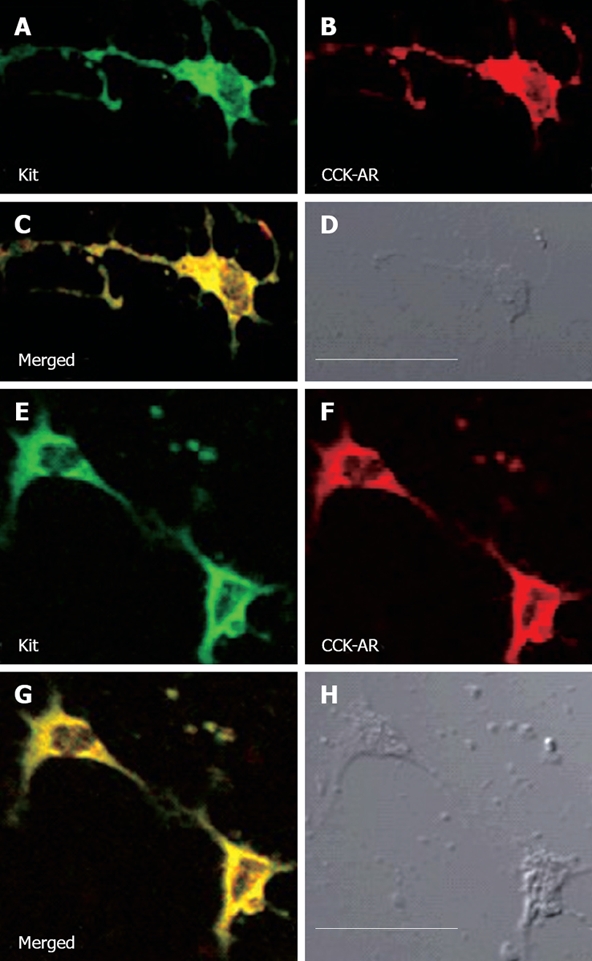
Colocalization of CCKAR immunoreactivity with markers for ICC, kit in guinea pig gallbladder cultured ICC. CCK-AR immunoreactive ICC in the muscle of the gallbladder is distinctive of their labled processes. ICC possessed several processes (A-D); C-kit immunoreactive (green) ICC colocalize CCKAR (red); C: Compound micrographs from (A) and (B); D: Phase-contrast micrographs of the same gallbladder ICC at the same magnification; Often two or more labled ICC were aggregrated into a network (E-H), making it difficult to discern the individual cells; C-kit immunoreactive (green) ICCs colocalize CCKAR (red); G: Compound micrographs from (E) and (F); H: Phase-contrast micrographs of the same gallbladder ICCs at the same magnification; All these ICCs were big and had a large and angular nucleus; Bar (in D) 30 μm for (A-D) and (in (H)) 30 μm for (E-H).
CCK-8-induced contractions on gallbladder muscle strips
The contractile responses of muscle strip to CCK-8 was observed at 10-10 mol/L, and they reached the maximum at 10-6 mol/L as shown in Figures 3 and 4. CCK-8 stimulated tonic contraction in a concentration-dependent manner. The dose response curve for CCK-8 was not affected by pretreatment of the muscle strips with atropine (10-6 mol/L) or TTX (3 × 10-7 mol/L) (Figure 3). The purpose of using atropine and TTX is to eliminate the effect of nervous system on the contractions of the guniea pig gallbladder. When we removed the ICCs from the gallbladder muscle strip, it significantly shifted the dose response curve to the right (Figure 4).
Figure 3.
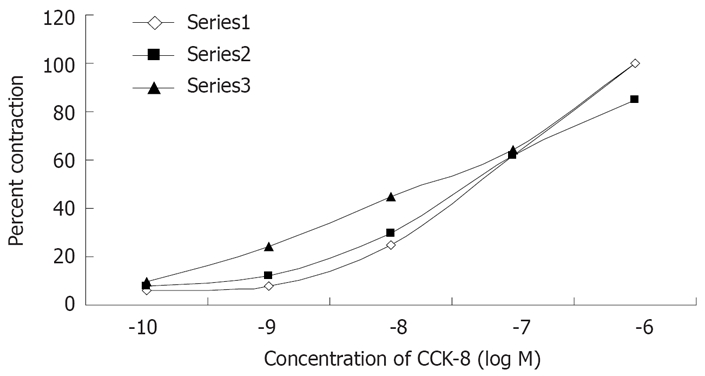
Effect of atropine and TTX on CCK-8 induced contraction of gallbladder muscle strips. The addition of atropine (10-6 mol/L) or TTX (3 × 10-7 mol/L) to the medium had no effect on the control dose-response curve for CCK-8. Each value is the mean ± SE obtained in 5 observations and observed at 10-10, 10-9, 10-8, 10-7, 10-6 mol/L. Series 1: CCK-8+TTX; Series 2: CCK-8+atropine; Series 3: CCK-8 alone.
Figure 4.
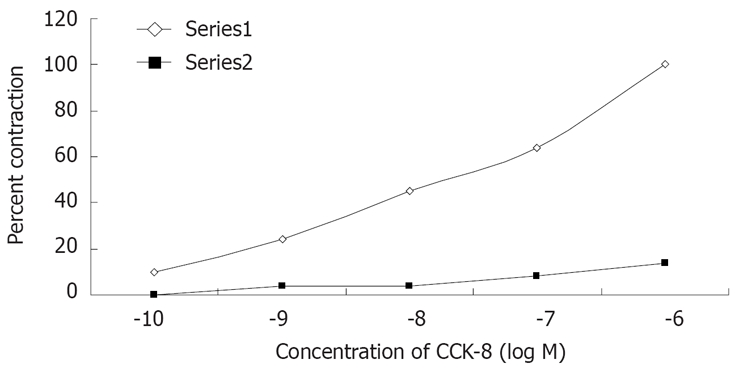
Effects of CCK-8 on contraction of gallbladder muscle strips in vitro. Removing the ICC of the gallbladder significantly shifted the control dose-response curve to the right. Each value is the mean ± SE obtained in 5 observations and observed at 10-10, 10-9, 10-8, 10-7, 10-6 mol/L. Series 1: Common gallbladder muscle strip; Series 2: Gallbladder muscle strip removed ICC.
Transmission electron microscopy
Methylene blue with light destroyed the structure of the gallbladder ICCs. The ICCs were swollen, and they showed low contrast for the cytoplasm. Cells became smaller and processes diminished. All these changes result in the reduction of contractile function of the gallbladder (Figure 5).
Figure 5.
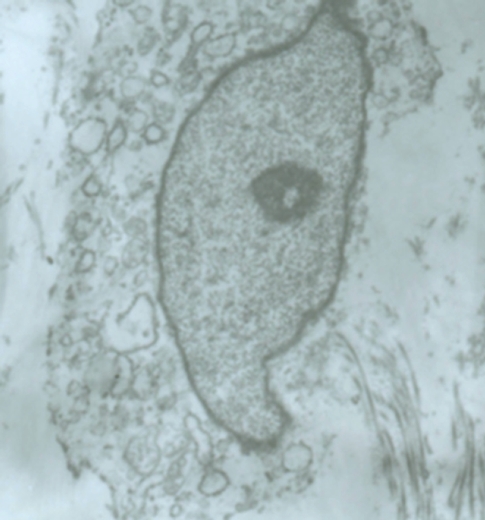
The effect of methylene blue with light on gallbladder ICC. After incubation with methylene and light, ICC on gallbladder was swollen and had low cytoplasmic, and the nucleolus became smaller and processes diminished.
DISCUSSION
In this study, we demonstrated that: (1) CCK-AR are present on the gallbladder interstitial cells of Cajal; (2) The gallbladder contractions evoked by CCK-8 through the CCK-AR on the gallbladder ICC. This is the first report on on ICC of guniea pig in vitro studies on the mechanism of gallbladder contraction evoked by CCK-8 through CCK-AR.
The study has clearly shown that CCK-AR are present on gallbladder ICCs by in vitro study with immunohisto-chemistry, immunocytochemistry and muscle strip trials. The CCK receptors on the gallbladder have been demonstrated previously by autoradiography in the guniea pig. In this study, we have clearly shown that CCK-AR exists in the gallbladder smooth muscle layer in the guniea pig, and to the best of our knowledge, the CCK-AR has not yet been demonstrated by any other studies. When we initially used high primary antibody concentrations, the entire gallbladder exhibited immunoreactivity. With lower antibody concentration of 1:8000, there were little immunoreactivity associated with the smooth muscle, but intense staining of ICC in the gallbladder sections. CCK may bind to CCK-ARs on both smooth muscle and ICC.
We demonstrate here that a population of guniea pig gallbladder ICC strongly expresses CCK-AR immunoreactivity. Identification of ICC was based on their typical morphological traits such as clusters of mitochondria, well-developed smooth endoplasmic reticulum, dense body, lysosomes, distinctive caveolae and abundant fibers around the lysosomes. In addition, co-localization of C-kit double labeling with CCK-AR showed that only a subpopulation was localized mainly to a contiguous band of circular muscle of the guniea pig.
In the gallbladder, ICCs intimately contact with smooth muscle cells with gap junction, and they are responsible for the generation of smooth muscle rhythmic activity[18]. The finding that gallbladder ICCs strongly express CCK-AR suggests a CCK-induced gallbladder activity. ICCs may mediate some of the effect of CCK on gallbladder pressure. It was previously shown that the gallbladder muscle strip contractions evoked by CCK are mediated by a direct myogenic action[27,28] and inhibited by a specific CCK-AR antagonist in some species[29]. The next question is how CCK action is mediated to the gallbladder through the CCK-AR on the ICC. When we removed the ICCs in the gallbladder, the CCK-8 induced contraction dose response curve shifted significantly to the right. Liu[30] showed that intense illumination after incubation with methylene blue resulted in specific lesions of dogs’ ICCs, and it was verified by electron microscopy. The ICCs were swollen and had low cytoplasmic contrast. They had barely discernible mitochondria with few caveloae, and the plasma membranes was punctuated so that they were presumably ruptured. All changes in ICCs result in the abolishing of the slow wave. The effects of methylene blue with light in ICC were specific only to ICCs, but not the associated smooth muscle cells. In our study, the contractile ability of the guinea pig gallbladder muscle strips without the evocation of CCK-8 was sharply weakened. This phenomenon implied that CCK-8 evoked the contraction of the gallbladder through the gallbladder ICC.
Immunohistofluorescence and muscle strip trials proved that CCK acted not only on the CCK-AR on the smooth muscle, but also on the CCK-AR on the gallbladder ICC. This may be the first indication that ICC do not only generate slow-wave pacemaker activity and mediate effect of enteric neurotransmitters, but also mediate the effects of circulating hormones on smooth muscle activity.
In conclusion, we have shown that the CCK-evoked contractions are mediated through direct action on CCK-AR on the gallbladder ICC.
COMMENTS
Background
It has been convinced that the interstitial cells of Cajal (ICC) in rat gastric pylorus expresses CCK-ARs to a high degree. The fact that some ICC in the rat pylorus expresses CCK-ARs suggests the possibility that circulating CCK can influence gastrointestinal motility via ICC. It is well known that CCK regulates the guniea pig gallbladder contraction by CCK-AR. The ICC were also found in the murine, human and guinea pig gallbladder. It deserved us to study that if there are CCK-ARs on the guniea pig gallbladder ICC and how CCK regulates gallbladder contraction through CCK-AR on ICC.
Research frontiers
ICC does not only generate slow-wave pacemaker activity and mediate effects of enteric neurotransmitters, but also mediate effects of circulating hormones such as CCK on smooth muscle activity.
Innovations and breakthroughs
In the present study, it was demonstrated that: (1) CCK-AR is present on the gallbladder interstitial cells of Cajal; (2) The gallbladder contrations evoked by CCK-8 through the CCK-AR on the gallbladder ICC. This is the first report on guniea pig in vitro studies on the mechanism of gallbladder contraction evoked by CCK-8 through CCK-AR on ICC.
Applications
It has been shown that CCK evoked contraction is mediated through direct action on CCK-AR on the gallbladder ICC. This maybe some use of study in gallbladder motility disorders, such as cholelithiasis.
Peer review
This manuscript demonstrates CCK-AR receptor immunoreactivity in the gallbladder of the guinea pig. Furthermore, the authors demonstrate that administration of CCK8 to gallbladder muscle strips caused muscle contraction. This study is of important clinical significance and should be of interest to the readers.
Supported by The Natural Science Foundation of Hubei Province, No. 2006AA412c
Peer reviewer: Sharon DeMorrow, Assistant Professor, Division of Research and Education, Scott and White Hospital and The Texas A&M University System, Health Science Center College of Medicine, Temple, Texas 76504, United States
S- Editor Li DL L- Editor Li M E- Editor Ma WH
References
- 1.Lewis LD, Williams JA. Regulation of cholecystokinin secretion by food, hormones, and neural pathways in the rat. Am J Physiol. 1990;258:G512–G518. doi: 10.1152/ajpgi.1990.258.4.G512. [DOI] [PubMed] [Google Scholar]
- 2.Schjoldager B, Shaw MJ, Powers SP, Schmalz PF, Szurszewski J, Miller LJ. Bovine gallbladder muscularis: source of a myogenic receptor for cholecystokinin. Am J Physiol. 1988;254:G294–G299. doi: 10.1152/ajpgi.1988.254.3.G294. [DOI] [PubMed] [Google Scholar]
- 3.von Schrenck T, Moran TH, Heinz-Erian P, Gardner JD, Jensen RT. Cholecystokinin receptors on gallbladder muscle and pancreatic acinar cells: a comparative study. Am J Physiol. 1988;255:G512–G521. doi: 10.1152/ajpgi.1988.255.4.G512. [DOI] [PubMed] [Google Scholar]
- 4.Tokunaga Y, Cox KL, Coleman R, Concepcion W, Nakazato P, Esquivel CO. Characterization of cholecystokinin receptors on the human gallbladder. Surgery. 1993;113:155–162. [PubMed] [Google Scholar]
- 5.West SD, Mercer DW. Cholecystokinin-induced gastropro-tection: a review of current protective mechanisms. Dig Dis Sci. 2004;49:361–369. doi: 10.1023/b:ddas.0000020487.59974.4b. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava A, Pandey SN, Dixit M, Choudhuri G, Mittal B. Cholecystokinin receptor A gene polymorphism in gallstone disease and gallbladder cancer. J Gastroenterol Hepatol. 2008;23:970–975. doi: 10.1111/j.1440-1746.2007.05170.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Otani M, Jia DM, Fukumitsu K, Yoshikawa H, Akiyama T, Otsuki M. Differential mechanism and site of action of CCK on the pancreatic secretion and growth in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285:G681–G687. doi: 10.1152/ajpgi.00312.2002. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Zhao CM, Hakanson R, Samuelson LC, Rehfeld JF, Friis-Hansen L. Altered control of gastric acid secretion in gastrin-cholecystokinin double mutant mice. Gastroenterology. 2004;126:476–487. doi: 10.1053/j.gastro.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Scheurer U, Varga L, Drack E, Burki HR, Halter F. Measurement of cholecystokinin octapeptide-induced motility of rat antrum, pylorus, and duodenum in vitro. Am J Physiol. 1983;244:G261–G265. doi: 10.1152/ajpgi.1983.244.3.G261. [DOI] [PubMed] [Google Scholar]
- 10.Scheurer U, Varga L, Drack E, Burki HR, Halter F. Mechanism of action of cholecystokinin octapeptide on rat antrum, pylorus, and duodenum. Am J Physiol. 1983;244:G266–G272. doi: 10.1152/ajpgi.1983.244.3.G266. [DOI] [PubMed] [Google Scholar]
- 11.Junquera C, Martinez-Ciriano C, Castiella T, Serrano P, Azanza MJ, Junquera SR. Immunohistochemical and ultrastructural characteristics of interstitial cells of Cajal in the rabbit duodenum. Presence of a single cilium. J Cell Mol Med. 2007;11:776–787. doi: 10.1111/j.1582-4934.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- 13.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki N, Prosser CL, Dahms V. Boundary cells between longitudinal and circular layers: essential for electrical slow waves in cat intestine. Am J Physiol. 1986;250:G287–G294. doi: 10.1152/ajpgi.1986.250.3.G287. [DOI] [PubMed] [Google Scholar]
- 15.Wang XY, Sanders KM, Ward SM. Intimate relationship between interstitial cells of cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res. 1999;295:247–256. doi: 10.1007/s004410051231. [DOI] [PubMed] [Google Scholar]
- 16.Sun X, Yu B, Xu L, Dong W, Luo H. Interstitial cells of Cajal in the murine gallbladder. Scand J Gastroenterol. 2006;41:1218–1226. doi: 10.1080/00365520600708800. [DOI] [PubMed] [Google Scholar]
- 17.Hinescu ME, Ardeleanu C, Gherghiceanu M, Popescu LM. Interstitial Cajal-like cells in human gallbladder. J Mol Histol. 2007;38:275–284. doi: 10.1007/s10735-007-9099-0. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie B, Balemba OB, Nelson MT, Ward SM, Mawe GM. Morphological and physiological evidence for interstitial cell of Cajal-like cells in the guinea pig gallbladder. J Physiol. 2007;579:487–501. doi: 10.1113/jphysiol.2006.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucaites VL, Mendelsohn LG, Mason NR, Cohen ML. CCK-8, CCK-4 and gastrin-induced contractions in guinea pig ileum: evidence for differential release of acetylcholine and substance P by CCK-A and CCK-B receptors. J Pharmacol Exp Ther. 1991;256:695–703. [PubMed] [Google Scholar]
- 20.Dal Forno G, Pietra C, Urciuoli M, van Amsterdam FT, Toson G, Gaviraghi G, Trist D. Evidence for two cholecystokinin receptors mediating the contraction of the guinea pig isolated ileum longitudinal muscle myenteric plexus. J Pharmacol Exp Ther. 1992;261:1056–1063. [PubMed] [Google Scholar]
- 21.Patel M, Spraggs CF. Functional comparisons of gastrin/cholecystokinin receptors in isolated preparations of gastric mucosa and ileum. Br J Pharmacol. 1992;106:275–282. doi: 10.1111/j.1476-5381.1992.tb14328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bishop LA, Gerskowitch VP, Hull RA, Shankley NP, Black JW. Combined dose-ratio analysis of cholecystokinin receptor antagonists, devazepide, lorglumide and loxiglumide in the guinea-pig gall bladder. Br J Pharmacol. 1992;106:61–66. doi: 10.1111/j.1476-5381.1992.tb14293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonobe K, Sakai T, Satoh M, Haga N, Itoh Z. Control of gallbladder contractions by cholecystokinin through chole-cystokinin-A receptors in the vagal pathway and gallbladder in the dog. Regul Pept. 1995;60:33–46. doi: 10.1016/0167-0115(95)00117-0. [DOI] [PubMed] [Google Scholar]
- 24.Fischer de Toledo C, Roettger BF, Morys-Wortmann C, Schmidt WE, Miller LJ. Cellular handling of unoccupied and agonist-stimulated cholecystokinin receptor determined by immunolocalization. Am J Physiol. 1997;272:G488–G497. doi: 10.1152/ajpgi.1997.272.3.G488. [DOI] [PubMed] [Google Scholar]
- 25.Sternini C, Wong H, Pham T, De Giorgio R, Miller LJ, Kuntz SM, Reeve JR, Walsh JH, Raybould HE. Expression of cholecystokinin A receptors in neurons innervating the rat stomach and intestine. Gastroenterology. 1999;117:1136–1146. doi: 10.1016/s0016-5085(99)70399-9. [DOI] [PubMed] [Google Scholar]
- 26.Patterson LM, Zheng H, Ward SM, Berthoud HR. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res. 2001;305:11–23. doi: 10.1007/s004410100402. [DOI] [PubMed] [Google Scholar]
- 27.Amer MS. Studies with cholecystokinin in vitro. 3. Mechanism of the effect on the isolated rabbit gall bladder strips. J Pharmacol Exp Ther. 1972;183:527–534. [PubMed] [Google Scholar]
- 28.Yau WM, Makhlouf GM, Edwards LE, Farrar JT. Mode of action of cholecystokinin and related peptides on gallbladder muscle. Gastroenterology. 1973;65:451–456. [PubMed] [Google Scholar]
- 29.Gully D, Frehel D, Marcy C, Spinazze A, Lespy L, Neliat G, Maffrand JP, Le Fur G. Peripheral biological activity of SR 27897: a new potent non-peptide antagonist of CCKA receptors. Eur J Pharmacol. 1993;232:13–19. doi: 10.1016/0014-2999(93)90722-t. [DOI] [PubMed] [Google Scholar]
- 30.Liu LW, Thuneberg L, Huizinga JD. Selective lesioning of interstitial cells of Cajal by methylene blue and light leads to loss of slow waves. Am J Physiol. 1994;266:G485–G496. doi: 10.1152/ajpgi.1994.266.3.G485. [DOI] [PubMed] [Google Scholar]


