Abstract
Anti-tumor necrosis factor alpha (TNF-α) inhibitors are effective in the treatment of various inflammatory rheumatic conditions. Increased risks of serious infections are the major issues concerning the long-term safety of these agents. We present a case of a young male Behcet’s patient whose disease was complicated by cytomegalovirus (CMV) colitis. Colitis started 10 d after the third Infliximab dose and responded to the cessation of TNF blocking treatment and administration of ganciclovir. Tumor necrosis factor alpha and interferon gamma act at several levels in combating viral infections. CMV infections should be kept in mind and included in the differential diagnosis of severe gastrointestinal symptoms in patients receiving anti-TNF agents.
Keywords: Tumor necrosis factor alpha inhibitors, Adverse effects, Virus diseases
INTRODUCTION
Anti-tumor necrosis factor alpha (TNF-α) inhibitors are relatively new drugs in the rheumatic arena which are effective in the treatment of various inflammatory rheumatic conditions. Increased risk of infections and possible risk of lymphoma with the use of TNF targeting therapies are the major issues concerning the long-term safety of these agents[1]. The association of anti- TNF-α therapy and serious infections has generally been attributed to an increased risk of bacterial (especially microbacteria) and opportunistic fungal microorganisms[2]. In recent years, an increasing number of studies, mainly based on case reports, have reported an increased risk of serious viral infections in patients treated with anti-TNF agents.
In this report, we describe a case of severe, biopsy proven cytomegalovirus (CMV) colitis in a patient with Behcet's disease (BD) whose symptoms started 10 d after the third Infliximab (INF; Remicade®, Schering-Plough) infusion.
CASE REPORT
A 25-year-old man presented with diarrhea and weight loss for two months. He was diagnosed as neuro BD 4 years ago according to recurrent oral and genital ulcerations, uveitis, urinary incontinence and seizures. He was treated with various immunosuppressive drugs including monthly cyclophosphamide infusions, interferon and a combination of cyclosporine and azathioprine. Although these medications were used, his eye involvement could not be controlled and visual acuity decreased to no light perception in the left eye and 10/100 in the right eye. Because of this, INF at a dose of 5 mg/kg was started. Remission of ocular symptoms was observed after two infusions. Ten days after the third dose of INF (52 d after the first infusion), the patient developed abdominal discomfort, anorexia, epigastric pain and diarrhea occurring seven to ten times per day with watery stools, but without any associated blood or mucus. He had no history of chronic diarrhea before INF administration. His symptoms continued to worsen and he lost six kilograms during the past two months. He did not use any medications other than INF, colchicine (1.5 mg/d) and carbamazepine (600 mg/d). Physical examination showed epigastric tenderness on palpation, with hyperactive bowel sounds. Admission laboratory studies were significant for increased C-reactive protein (CRP = 14.4 mg/L). Stool examination was negative for parasites and Clostridium difficile toxin. Repeated stool cultures were also negative for enteric pathogens. Colonoscopic examination revealed diffuse redness and edematous mucosa and multiple ulcers throughout the colon including rectum and terminal ileum (Figure 1). Histological examination of the biopsy specimen identified cytomegalovirus (CMV) by specific immunohistochemistry (Figure 2). Serological testing revealed a positive CMV immunoglobulin (Ig) G and a negative IgM antibody. CMV pp65 antigenemia as determined by immunofluorescence was also negative. Infliximab was stopped and the patient was given ganciclovir (600 mg/d IV). On the 14th d of treatment, stool frequency decreased to 4-5 per day and repeated colonoscopy revealed edematous mucosa without ulcers. Immunohistochemical staining for CMV turned negative on histopathological examination of the repeat biopsy specimen. Ganciclovir was continued for an additional 14 d. The patient gained 5 kg and his stool frequency decreased to two per day. In a follow-up period of 30 mo, he did not reveal any recurrence of colitis symptoms. TNF targeting treatments were not considered during this period.
Figure 1.
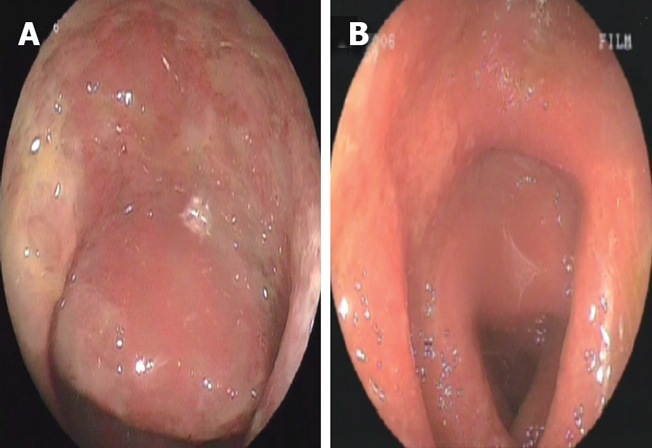
Colonoscopic appearance of the mucosa before (A) and during the 14 d of anti-viral treatment (B). The presence of edema and redness is seen in both images. Note the ulcers in the sigmoid colon before the treatment as shown in A.
Figure 2.
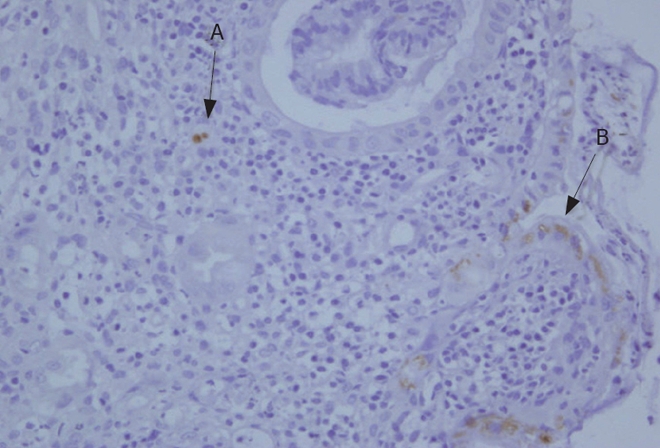
Colonic endothelial cells showing positive nuclear (A) and cytoplasmic (B) immunostaining of the CMV antigen.
DISCUSSION
We present a case of a young male Behcet’s patient whose disease was complicated by CMV colitis. Colitis started 10 d after the third INF dose and responded to the cessation of TNF blocking treatment and administration of ganciclovir.
CMV is a member of the herpesviridae family which is prevalent among the general population with an overall seroprevalence of 30%-70% in developed countries[3]. CMV causes a variety of clinical syndromes in immunocompromised patients. Pneumonitis, retinitis and gastrointestinal (GI) CMV disease are the mostly encountered clinical manifestations[3]. GI tract may be affected anywhere from the mouth to the anus. Esophagus and colon are the frequently involved sites. Ulcerations, erosions, and mucosal hemorrhage are the primary macroscopic findings[4]. The clinical signs and symptoms vary depending upon the involved areas. Patients with colonic CMV disease may present with diarrhea with or without blood, abdominal pain, urgency and tenesmus accompanied with systemic symptoms such as fever, malaise anorexia and weight loss[4]. Diagnosis of CMV infection is based upon the presence of CMV in clinical specimens demonstrated by conventional tissue culture or rapid culture with confirmation by specific monoclonal antibodies, or by detection of the pp65 CMV antigen in peripheral blood leukocytes[5].
Severe CMV infections during TNF targeting treatments have been reported including retinitis[6], hepatitis[7], duodenitis[8], ileitis[9], colitis[10] and disseminated CMV infection[11]. All these cases received concomitant immunosuppressive treatment in addition to INF. Except for our patient and patients who developed retinitis, all other reported CMV infections developed in subjects who had primary GI problems, such as inflammatory bowel disease, common variable immunodeficiency sprue and indeterminate colitis.
BD may affect the intestine. Intestinal involvement commonly accompanies ulcerative lesions in the small and large bowel. The lesions are most commonly found in the terminal ileum and the cecum and less frequently in the colon. Rectal and anal involvement is quite rare[12]. In our patient, clinical symptomatology mimicked intestinal BD. However, diffuse involvement of the colon including the rectum and development of symptoms after the initiation of TNF inhibitory treatment suggested an infectious etiology. Following histopathological examination of the biopsies, which revealed the causative microorganism, colonic CMV disease was diagnosed. Reactivation of the virus was thought since CMV serology was positive for IgG and negative for IgM.
Tumor necrosis factor alpha and interferon gamma act at several levels in combating viral infections. TNF exhibits its antiviral activities against both DNA and RNA viruses by enhancing the induction of antiviral state in uninfected cells and by selectively killing virus-infected cells[13]. CD8+ T cells are the major defense against viruses by direct cytolysis of target cells mediated by perforin release and Fas, or by secreting cytokines such as TNF and interferon-γ and expressing chemokines that attract inflammatory cells to the sites of infection[14]. It has been shown that treatment with monoclonal antibodies directing against TNF-α is associated with a progressive loss of CD4+ and CD8+ T cells[15]. In addition, Infliximab treatment has been reported to be associated with decreased levels of TNF-α and interferon-γ[16]. On the other hand, TNF levels are increased during CMV infection and CD4+ T cells specific for the human CMV result in early gene products[17]. Up-regulation of TNF gene expression by CMV[18] is the major cause for the increased TNF. It has been shown that increased TNF production in response to CMV infection displays anti-CMV activity in vitro[19]. Furthermore, recombinant TNF-α possesses anti-CMV activity[17].
In conclusion, TNF-α in line with interferon-γ acts at several levels in combating viral infections. Blocking the action of TNF-α with novel medications may predispose these patients to serious viral diseases. CMV infections should be kept in mind and included in the differential diagnosis of severe gastrointestinal symptoms in patients receiving Infliximab.
Peer reviewer: Burton I Korelitz, MD, Department of Gastroenterology, Lenox Hill Hospital, 100 East 77th Street, 3 Achelis, New York 10021, United States
S- Editor Zhong XY L- Editor Wang XL E- Editor Lu W
References
- 1.Cush JJ. Unusual toxicities with TNF inhibition: heart failure and drug-induced lupus. Clin Exp Rheumatol. 2004;22:S141–S147. [PubMed] [Google Scholar]
- 2.Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor alpha therapy. Arthritis Rheum. 2003;48:3013–3022. doi: 10.1002/art.11301. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- 4.Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119:924–935. doi: 10.7326/0003-4819-119-9-199311010-00010. [DOI] [PubMed] [Google Scholar]
- 5.Reusser P. Cytomegalovirus Infection. In: Cohen J, Powderly WG, editors. Cohen & Powderly: Infectious Diseases. 2nd: Mosby, An Imprint of Elsevier; 2004. pp. 1175–1177. [Google Scholar]
- 6.Haerter G, Manfras BJ, de Jong-Hesse Y, Wilts H, Mertens T, Kern P, Schmitt M. Cytomegalovirus retinitis in a patient treated with anti-tumor necrosis factor alpha antibody therapy for rheumatoid arthritis. Clin Infect Dis. 2004;39:e88–e94. doi: 10.1086/425123. [DOI] [PubMed] [Google Scholar]
- 7.Mizuta M, Schuster MG. Cytomegalovirus hepatitis associated with use of anti-tumor necrosis factor-alpha antibody. Clin Infect Dis. 2005;40:1071–1072. doi: 10.1086/428672. [DOI] [PubMed] [Google Scholar]
- 8.Medlicott SA, Coderre S, Horne G, Panaccione R. Multimodal immunosuppressant therapy in steroid-refractory common variable immunodeficiency sprue: a case report complicating cytomegalovirus infection. Int J Surg Pathol. 2006;14:101–106. doi: 10.1177/106689690601400120. [DOI] [PubMed] [Google Scholar]
- 9.Kohara MM, Blum RN. Cytomegalovirus ileitis and hemophagocytic syndrome associated with use of anti-tumor necrosis factor-alpha antibody. Clin Infect Dis. 2006;42:733–734. doi: 10.1086/500262. [DOI] [PubMed] [Google Scholar]
- 10.Papadakis KA, Tung JK, Binder SW, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:2137–2142. doi: 10.1111/j.1572-0241.2001.03949.x. [DOI] [PubMed] [Google Scholar]
- 11.Helbling D, Breitbach TH, Krause M. Disseminated cytomegalovirus infection in Crohn's disease following anti-tumour necrosis factor therapy. Eur J Gastroenterol Hepatol. 2002;14:1393–1395. doi: 10.1097/00042737-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Bayraktar Y, Ozaslan E, Van Thiel DH. Gastrointestinal manifestations of Behcet's disease. J Clin Gastroenterol. 2000;30:144–154. doi: 10.1097/00004836-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Wong GH, Goeddel DV. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. Nature. 1986;323:819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 14.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 15.Baert FJ, D'Haens GR, Peeters M, Hiele MI, Schaible TF, Shealy D, Geboes K, Rutgeerts PJ. Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology. 1999;116:22–28. doi: 10.1016/s0016-5085(99)70224-6. [DOI] [PubMed] [Google Scholar]
- 16.Zou J, Rudwaleit M, Brandt J, Thiel A, Braun J, Sieper J. Down-regulation of the nonspecific and antigen-specific T cell cytokine response in ankylosing spondylitis during treatment with infliximab. Arthritis Rheum. 2003;48:780–790. doi: 10.1002/art.10847. [DOI] [PubMed] [Google Scholar]
- 17.Davignon JL, Castanie P, Yorke JA, Gautier N, Clement D, Davrinche C. Anti-human cytomegalovirus activity of cytokines produced by CD4+ T-cell clones specifically activated by IE1 peptides in vitro. J Virol. 1996;70:2162–2169. doi: 10.1128/jvi.70.4.2162-2169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geist LJ, Monick MM, Stinski MF, Hunninghake GW. The immediate early genes of human cytomegalovirus upregulate tumor necrosis factor-alpha gene expression. J Clin Invest. 1994;93:474–478. doi: 10.1172/JCI116995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbein G, O'Brien WA. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc Soc Exp Biol Med. 2000;223:241–257. doi: 10.1177/153537020022300305. [DOI] [PubMed] [Google Scholar]


