Abstract
Background
Cancer immunotherapy refers to an array of strategies intended to treat progressive tumors by augmenting a patient’s anti-tumor immune response. As immunotherapy is eventually incorporated into oncology treatment paradigms, it is important to understand how these therapies interact with established cancer treatments such as chemotherapy or Radiotherapy (RT). To address this, we utilized a well-established, autochthonous murine model of prostate cancer to test whether RT could augment (or diminish) the CD4 T cell response to a tumor vaccine.
Methods
Transgenic mice that develop spontaneous prostate cancer (TRAMP) which also express a unique tumor associated antigen (Influenza hemagglutinin) under the control of a prostate-specific promoter were given local RT in combination with immunotherapy. The immunological outcome of this combinatorial strategy was assayed by monitoring the effector response of adoptively transferred, prostate-specific CD4 T cells.
Results
Neither RT nor immunotherapy alone was capable of priming an anti-tumor immune response in animals with evolving tumors. The combination of immunotherapy with RT resulted in anti-tumor T cell activation – this effect was profoundly dependent on the relative timing of RT and immunotherapy. Anti-tumor immune responses occurred when immunotherapy was administered 3-5 weeks post-RT, but such responses were undetectable when immunotherapy was administered either earlier (peri-radiothrerapy) or later.
Conclusions
The therapeutic temporal window of immunotherapy post-RT suggests that highly aggressive, immuno-suppressive tumors might be most sensitive to immunotherapy in a fairly narrow time window; these results should help to guide future development of clinical combinatorial strategies.
Keywords: Radiation, Immunotherapy, TRAMP, T Cell, vaccine
INTRODUCTION
Although RT has been widely thought of as immuno-suppressive (1,2), there is mounting evidence that demonstrates otherwise (3). The abscopal effect, in which local radiation therapy mediates regression of distant lesions outside of the original treatment portal, was recently shown to be dependent on an adaptive immune response, as this effect could not be induced in nude (T and B cell deficient) mice (4). RT can enhance the expression of MHC Class I on tumor surfaces thereby sensitizing them to tumor-specific cytotoxic lymphocytes (5). Additionally, the efficacy of cytotoxic (chemo- and radiotherapy) therapies are partially dependent upon components of the innate immune system (6). These data suggest that under certain conditions RT alone might generate a significant anti-tumor immune response (3). Several groups have attempted to combine RT with various methods to stimulate an anti-tumor immune response, including cytokines (7-9), monocolonal antibodies (10), and adoptive transfer of T cells (11). In terms of prostate cancer, Gulley et. al. recently reported a Phase I clinical trial in which a PSA-targeted vaccine (ProstVac VF) was combined with RT in men with localized disease. The combination was safe, well-tolerated, and led to significant T cell responses in the majority of treated patients (12).
The combination of RT with immunotherapy is particularly applicable to the case of high-risk localized prostate cancer, for which the major primary treatment option is RT. While generally effective, the 5 year biochemical relapse rate for these high-risk men is approximately 50%, suggesting that combining RT with additional treatment modalities might further abate morbidity and mortality for these patients (13). While many potential treatment options might be considered, immunotherapy represents an attractive option to improve clinical outcome without the potential side-effects typically associated with cytotoxic chemotherapy or androgen-ablation (14). However, for immunotherapy to be eventually incorporated into the treatment of patients with prostate cancer, careful studies must be performed to determine an efficacious regimen (timing and dose), especially since conventional cytotoxic therapies (i.e. RT and chemotherapy) may generally be immunosuppressive (15).
Standard implanted tumor models are not ideal for such studies as they generally fail to properly model the physiological setting in which tolerogenic tumors arise over extended time periods. This limitation can be partially addressed by using transgenic cancer models (16). In order to model immunotherapy for prostate cancer, we have established a variant of the TRAMP (Transgenic Adenocarcinoma of the Mouse Prostate) animal (17), in which a model tumor antigen is co-expressed with the SV-40 large T antigen that drives tumorigenesis (18). To accomplish this, we used the minimal rat probasin promoter to drive prostate and prostate-cancer restricted expression of influenza hemagglutinin (HA), which serves as a tumor associated antigen (TAA) in these double-transgenic animals. Like parental TRAMP animals, these (Pro-HA×TRAMP) mice develop an autochthonous cancer characterized by prostate intraepithelial neoplasia (PIN) by week 12, adenocarcinoma by week 20, lymph node metastases by week 24, and distant metastases after 30 weeks (19).
In this study we utilize this unique transgenic model to study the combination of RT with tumor vaccines. We show that RT as a single modality does not mitigate T cell tolerance to any significant degree. However, when RT is combined with active immunotherapy (“vaccination”), T cell tolerance is significantly mitigated. This diminution of tolerance shows a strong time-dependence and suggests that immunotherapy should most likely be administered post-RT, in a time period 3-5 weeks after the completion of RT.
METHODS AND MATERIALS
Animals
Non-transgenic (B10.D2) mice were obtained from The Jackson Laboratory. TRAMP mice express SV40 under the minimal probasin promoter, and, on the C57BL/6 background develop autochthonous prostate cancer that is prominent in the dorsal and ventral lobes (17,19). Pro-HA mice express Hemagglutinin (HA) under the minimal probasin promoter, and express HA in a prostate-restricted mannner (18). C3-HA mice express HA under the C3 promoter, which results in expression of HA in a number of epithelial tissues including lung and prostate (20). Both the Pro-HA and the C3-HA mice are on the B10.D2 genetic background. Pro-HA×TRAMP mice represent double transgenic animals resulting from backcrossing the TRAMP transgenics onto a Pro-HA background >8 generations. These mice are characterized by the development of prostate cancer and the expression of HA as a prostate(tumor)-specific antigen (18). The 6.5 mouse is a TCR Transgenic line that expresses a TCR specific for a HA peptide bound to MHC Class II (I-Ad) (21). 6.5 TCR Transgenic mice were also backcrossed >8 generations onto the B10.D2 genetic background. In these studies, CD4 T cells from 6.5 mice are used as a readout to determine whether antigen recognition is tolerogenic or immunogenic.
Radiation Therapy
Animals were irradiated using a Varian 6EX linear accelerator outfitted with stereotactic RT collimators (Varian medical systems, Palo Alto, CA) with three doses of 10 Gy. Each dose was separated by five days; the three doses were given within a span of ten days. A one centimeter tissue bolus was placed on the pelvis of anesthetized (2,2,2-tribromoethanol, 350 mg/Kg) mice, and a 1.5 cm diameter beam was delivered. For some experiments, a Shepherd Mark I cesium irradiator (J.L. Shepherd, San Fernando, CA) was used to deliver radiation to the pelvis of anesthetized mice. For cesium radiation therapy, mice were shielded with lead (lower extremities, abdomen, thorax, and head) and a single dose of 15 Gy was delivered via a collimated beam to the pelvis.
Adoptive T Cell Transfer
CD4+ T cells were enriched from the spleen and lymph nodes of 6.5 TCR Tg mice using magnetic separation (Miltenyi #130-090-860). For proliferation and functional studies, enriched CD4+ T cells were labeled with CFSE (2.5 mM) (Invitrogen #C34554), and 2×106 HA-specific cells were adoptively transferred by tail vein injection into recipient mice. For an activation control, non-transgenic mice adoptively transferred with CD4 cells were injected i.p. with 106 pfu of Vaccinia-HA. Five days following adoptive transfer, para-aortic (prostate draining) and axillary lymph nodes were harvested from recipient mice. Cells were restimulated in culture for 5 hours in the presence of HA Class II peptide (SFERFEIFPK) (10 mg/mL) and Brefeldin A (10 μg/mL). Cells were then fixed and permeabilized with BD Cytofix/Cytoperm kit (#554714). Cells were stained with anti-CD4, anti-Thy1.1, and anti-interferon(IFN)-γ (all antibodies obtained from BD Biosciences), and analyzed using a FacsCalibur flow cytometer (BD Biosciences).
Tumor Vaccine
Recombinant Vaccinia-HA (Vacc-HA) was used as a tumor vaccine in our prostate cancer model (Pro-HA×TRAMP) as HA is expressed exclusively in the prostate/prostate-cancer. This construct has been previously described (22).
T Cell Tolerance Model
10 days prior to vaccination, mice received an adoptive T cell transfer of a small number (105) of HA-specific T cells. By utilizing a small number of T cells, we insure that antigen is not limiting in the generation of tolerance (18). To assay for tolerance, mice were injected i.p. with 106 pfu of Vacc-HA 10 days post adoptive transfer. Functional response to vaccination was assayed by expansion of the tumor(HA)-specific (adoptively transferred) CD4 T cells and IFN-γ secretion. Cells were harvested from the para-aortic and axillary lymph nodes five days after the vaccination and subject to a 5 hour ex vivo stimulation with HA Class II peptide (10 mg/mL) and Brefeldin A (10 μg/mL) and stained for CD4, Thy1.1, and IFN-γ as above.
RESULTS
External beam radiation therapy in a transgenic model of prostate cancer
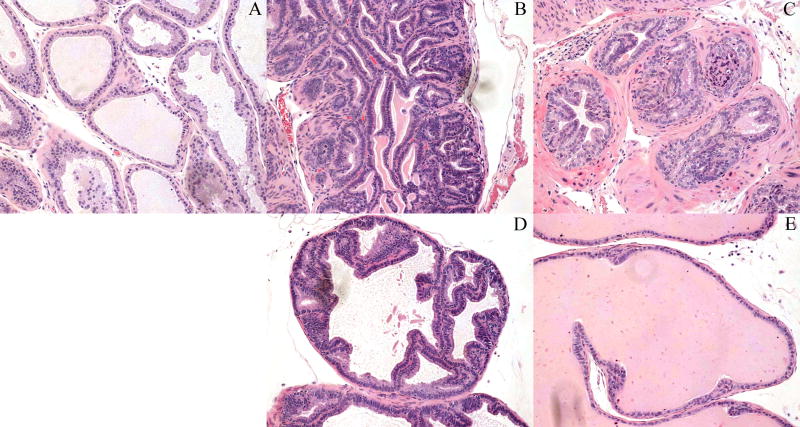
To establish an experimental system for combinatorial studies, we first set out to model RT in Pro-HA×TRAMP mice. 20-24 week old mice were anesthetized and irradiated using a calibrated cesium source; at this age mice have adenocarcinoma with metastases to the prostate draining (para-aortic) lymph nodes (19). Five weeks following RT prostate lobes were harvested from irradiated and unirradiated animals and stained with H&E (Figure 1). Unirradiated non-transgenic (wild-type) animals demonstrated normal epithelial cellularity, with normal nuclear euchromasia, and a low nuclear-to-cytoplasmic ratio (Figure 1a). Conversely, unirradiated Pro-HA×TRAMP mice demonstrated hypercellularity, nuclear hyperchromasia, and an increased nuclear-to-cytoplasmic ratio: all consistent with well-described neoplastic transformation (Figure 1b, d). RT resulted in several histological changes in the Pro-HA×TRAMP prostate gland. The dorsal lobes demonstrated diminished gland size, fibrotic reactions surrounding the glands, and relative hypochromasia of the epithelial nuclei (Figure 1c). A response to RT was observed in the ventral lobes as well; this consisted of a reversion to an almost normal glandular architecture (Figure 1e). A moderate, but statistically significant survival benefit was also noted (data not shown).
Figure 1. Pathological diminishing of local tumor burden post-RT.
Five weeks following RT prostate lobes were harvested from irradiated and unirradiated Pro-HA×TRAMP mice and non-transgenic control mice. 200X magnification of H&E stained (a) dorsal lobe non-transgenic, (b) dorsal lobe unirradiated Pro-HA×TRAMP, (c) dorsal lobe irradiated Pro-HA×TRAMP, (d) ventral lobe unirradiated Pro-HA×TRAMP, and (e) ventral lobe irradiated Pro-HA×TRAMP.
Radiation therapy results in decreased T cell recognition of a tumor-associated antigen
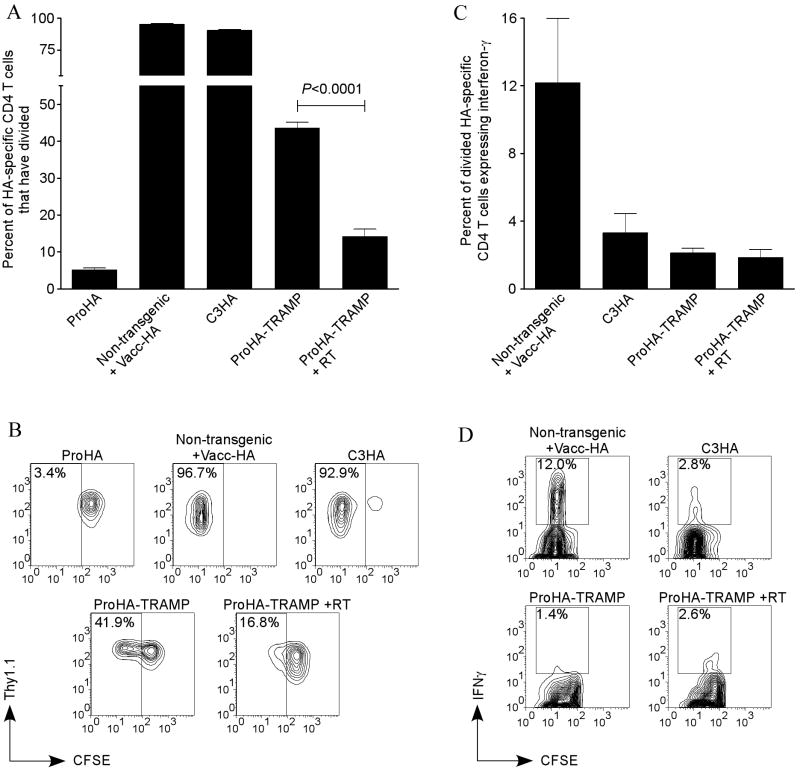
To determine the effects of RT on immune recognition and function, we delivered a single dose of 15 Gy to the pelvis of Pro-HA×TRAMP mice using a calibrated cesium source, and adoptively transferred 2×106 HA-specific cells into these mice 21 days post-RT. Additionally, HA-specific cells were adoptively transferred into age-matched Pro-HA animals (these mice express HA in their prostates, but do not develop prostate cancer); non-transgenic (wild-type) mice given an HA-specific tumor vaccine (Vacc-HA); C3-HA (a transgenic mouse strain that expresses HA in multiple epithelial tissues including prostate and lung); and Pro-HA×TRAMP that did not receive RT (20). Proliferation of HA-specific CD4 T cells was analyzed by CFSE dilution (as labeled cells divide, CFSE is successively diluted such that each daughter cell has half of the amount, and therefore half the fluorescence, of the parent cell). In these studies, the adoptively transferred T cells were distinguished from host T cells by the congenic marker Thy1 (CD90); adoptively transferred T cells express Thy1.1 (CD90.1), while host T cells all express Thy1.2 (CD90.2).
Five days following adoptive transfer, lymphocytes were harvested from the prostate draining lymph nodes. HA-specific cells (CD4+Thy1.1+) adoptively transferred into Pro-HA mice did not proliferate (decrease CFSE fluorescence), indicating low levels of prostate antigen recognition from the normal prostate, and confirming our earlier studies suggesting the prostate gland is immunologically ignored in the absence of tumorigenesis (Figures 2a, 2b top left panel) (18). Conversely, non-transgenic mice administered an HA-specific tumor vaccine (Vacc-HA) demonstrated a robust immune response, with almost all of the adoptively transferred HA-specific CD4 cells dividing (Figures 2a, 2b top middle panel). This group serves as a control for productive T cell activation. HA-specific cells collected from C3-HA mice also divided, indicative of HA antigen recognition, and consistent with the widespread expression of the HA transgene in these animals (Figures 2a, 2b top right panel) (20). Consistent with our previously published data (18), HA-specific CD4 cells divided significantly in the prostate draining lymph node of Pro-HA×TRAMP mice compared to antigen bearing non-tumor mice (Pro-HA) (Figures 2a, 2b bottom left panel). Interestingly, treatment of Pro-HA×TRAMP with RT did not result in an increase in T cell recognition of prostate cancer, but rather mediated a statistically significant reduction in recognition as assayed by CFSE dilution (Figures 2a, 2b bottom right panel).
Figure 2. Decrease in tumor recognition by tumor-specific T cells following pelvic irradiation (cesium).
20 week Pro-HA×TRAMP mice were given a single dose of 15 Gy. 35 days post-radiation, 2×106 CFSE-labeled HA-specific CD4 T cells were adoptively transferred into irradiated Pro-HA×TRAMP and age-matched unirradiated controls (Pro-HA, vaccinated non-transgenic, C3-HA, and Pro-HA×TRAMP). Adoptively transferred HA-specific cells were Thy1.1 congenic, which served as the marker used to distinguish them from endogenous (Thy1.2) T cells. (a) The percent of HA-specific cells in the prostate draining lymph node that had divided by day 5 post-adoptive transfer. (b) Representative FACS plots showing CFSE-division of cells gated on CD4+Thy1.1+ for Pro-HA (top left), vaccinated non-transgenic (top middle), C3-HA (top right), Pro-HA×TRAMP (bottom left), and irradiated Pro-HA×TRAMP (bottom right). (c, d) T cell activation was assessed by IFN-γ expression in divided cells. (d) Representative FACS plots are gated on CD4+Thy1.1+CFSELow cells of vaccinated non-transgenic mice (top left), C3-HA (top right), Pro-HA×TRAMP (bottom left), and irradiated Pro-HA×TRAMP (bottom right). Significant differences between irradiated and unirradiated Pro-HA×TRAMP groups are given on the graphs. For all experimental groups n=5.
CD4 cells that encounter their cognate antigen are subject to disparate differentiation pathways dependent on the context of antigen recognition. Activation involves differentiation of naïve CD4 T cells into “effector” cells that promote either cell mediated immune responses or antibody mediated immune responses against an antigen. Activated CD4 T cells secrete a number of effector cytokines including IL-2, TNF-α, and IFN-γ (23-25). Among these, IFN-γ is the most highly regulated, and hence serves as a standard readout for T cell effector function (26). In these experimental models, expression of IFN-γ correlates with TNF-α expression (data not shown). CD4 T cells that recognize cognate antigen in the absence of appropriate “co-stimulation” are not activated upon division, but rather are “tolerized” and are characterized by a lack of IFN-γ production and are refractory to further stimulation (27). Using standard intracellular staining techniques, adoptively transferred, HA-specific antigen experienced (CD4+Thy1.1+CFSELow) cells were analyzed for effector function. HA-specific CD4 cells that respond to Vacc-HA in non-transgenic mice demonstrated robust IFN-γ expression indicative of activation (Figures 2c, 2d top left panel). However, divided cells obtained from C3-HA mice did not express IFN-γ, consistent with previous data demonstrating “non-productive” division in response to self-antigen in this context (Figures 2c, 2d top right panel) (20). T cells harvested from tumor-bearing Pro-HA×TRAMP mice did not express IFN-γ (Figures 2c, 2d bottom left panel), again consistent with our previous data demonstrating “non-productive” division in these animals (18). Radiation treatment of Pro-HA×TRAMP mice did not mitigate this effect and permit activation of HA-specific CD4 cells (Figures 2c, 2d bottom right panel). Taken together, our data demonstrating a lack of significant proliferation and effector cytokine production by HA-specific T cells adoptively transferred to treated Pro-HA×TRAMP mice suggests that radiation therapy alone does not sufficiently prime T cell specific anti-tumor immunity in response to antigen recognition in the context of prostate cancer.
Radiation therapy mitigates tumor-mediated T cell tolerance
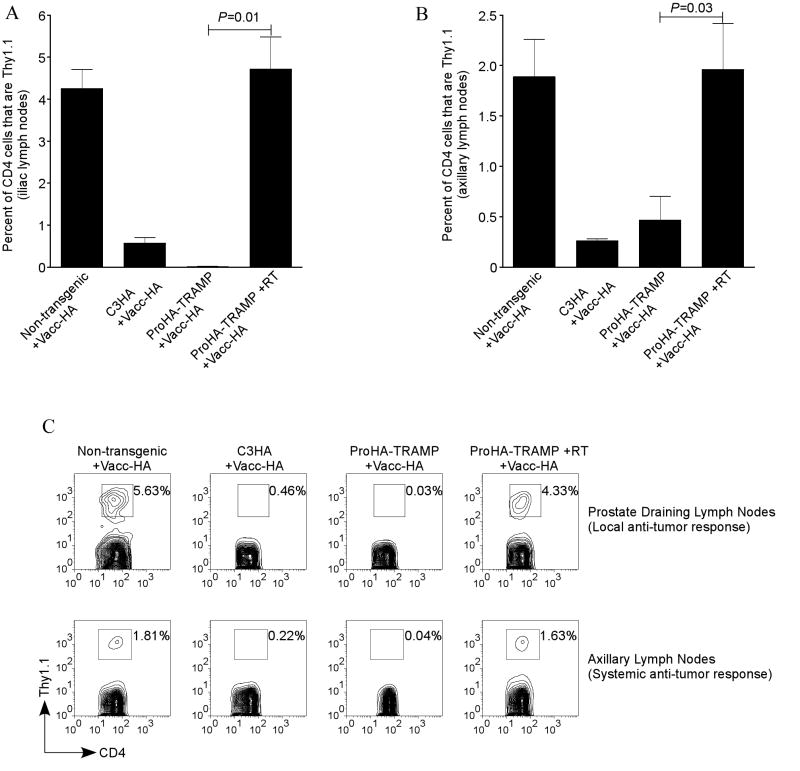
We next sought to determine whether the combination of RT and tumor vaccination of tumor-bearing mice could mitigate tolerance to a tumor associated antigen. As previously described (18), we performed these studies using a smaller number (105) of adoptively transferred HA-specific CD4 T cells for a readout, in order to insure that limiting concentrations of antigen would not affect tolerance. Tolerance is assayed by administering a tumor-specific vaccine (Vacc-HA) and determining the expansion of the adoptively transferred T cells by FACS analysis. As one of several controls, we utilized ProHA mice – in these animals HA is expressed in a prostate-restricted pattern in the absence of cancer, and immune recognition of HA in these animals is essentially negligible (functional ignorance)(18). For a positive control, HA-specific CD4 T cells were adoptively transferred into non-transgenic mice and challenged with Vacc-HA. In the absence of endogenous or tumor-associated antigen, these cells are not tolerized, and expand significantly (Figures 3a-c). In the prostate draining lymph nodes, HA-specific cells represented 4-5% of the CD4 population in vaccinated non-transgenic mice (Figures 3a, 3c top left panel). Although these data represent a systemic response to vaccination, HA-specific T cells make up a lower percentage of the CD4 population in the axillary lymph nodes, most likely due to the fact that vaccine is administered intraperitoneally (Figures 3b, 3c bottom left panel). In contrast, HA-specific cells adoptively transferred into C3-HA or Pro-HA×TRAMP mice failed to respond to the HA-targeted tumor-vaccine (Figures 3a-c), demonstrating tolerance induction, Treatment of Pro-HA×TRAMP mice with radiation therapy 5 weeks prior to T cell transfer significantly mitigated this tolerance (Figures 3a-c). This response was most evident in the para-aortic lymph nodes (Figures 3a, 3c top right panel), which represent the tumor-draining lymph nodes in this model. Importantly, a response to the tumor vaccine was also noted systemically (Figure 3b, 3c bottom right panel). These data suggest that RT mitigates specific T cell tolerance to autochthonous tumors under certain conditions and that this diminution of tolerance is both systemic and local.
Figure 3. Mitigation of T cell tolerance following pelvic irradiation (cesium).
20 week Pro-HA×TRAMP mice were given a single dose of 15 Gy. Five weeks post-radiation 105 HA-specific CD4 T cells (Thy1.1) were adoptively transferred into recipient mice (Thy1.2); this was followed by a tumor vaccine 2 weeks after the adoptive transfer. The response to the vaccine was assessed by expansion of the Thy1.1 CD4 population. (a) The local response to the tumor vaccine in the prostate draining lymph node. (b) Systemic response to the tumor vaccine in the axillary lymph nodes. (c) Representative FACS plots of the tumor vaccine induced T cell expansion in the (top row) prostate draining lymph nodes and the (bottom row) axillary lymph nodes for non-transgenic (far left), C3-HA (second from left), Pro-HA×TRAMP (second from right), and irradiated Pro-HA×TRAMP (far right). The differences in expansion between the irradiated and control Pro-HA×TRAMP mice were statistically significant in the para-aortic and the axillary lymph nodes, p=0.01 and p=0.03, respectively. For all experimental groups n=10.
A temporal window of efficacy for immunotherapy administered with RT
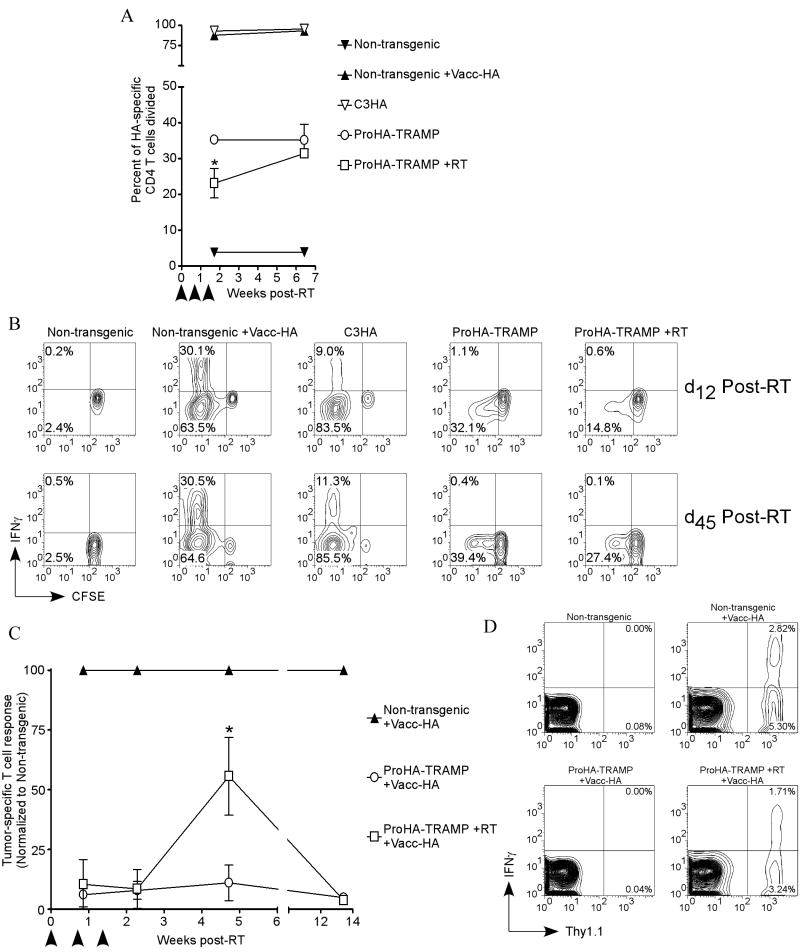
To understand the temporal constraints of combining immunotherapy with radiation therapy we performed time course studies analyzing the immune response to cancer using RT as a treatment modality. For these studies, we once again turned to our large-number T cell transfers, in which T cell recognition is assayed by CFSE dilution and effector cytokine production. These studies were performed both in the absence (Figures 4a and 4b) as well as in the presence (Figures 4c and 4d) of tumor-specific vaccination with Vacc-HA. For these more preclinical studies, we utilized fractionated radiation delivered via a clinical instrument in an attempt to more accurately model current radiotherapy techniques.
Figure 4. Therapeutic window post-radiation (linear accelerator) for optimal tumor vaccine response.
20 week Pro-HA×TRAMP mice were irradiated using a linear accelerator using the three dose regimen described previously. (a,b) 2×106 HA-specific CD4 T cells were adoptively transferred into the irradiated Pro-HA×TRAMP mice as well as age-matched unirradiated controls (non-transgenic, tumor vaccinated non-transgenic, C3-HA, and Pro-HA×TRAMP). Cells were adoptively transferred on either day 12 or day 45 following the initial dose of radiation. Five days after the adoptive transfers HA-specific lymphocytes were harvested and analyzed for (a) division (arrow heads correspond to RT) and (b) T cell activation (IFN-γ). (c) 105 HA-specific CD4 T cells were adoptively transferred into irradiated Pro-HA×TRAMP mice and unirradiated age-matched controls (non-transgenic and Pro-HA×TRAMP) on day 6, day 16, day 33, or day 94 (the reference, day 0, was the initial dose of radiation). Two weeks following the adoptive transfer, mice were given a tumor vaccine, and the response to the vaccine was analyzed on day 5 post-vaccination by T cell expansion (arrow heads correspond to RT). (d) Representative FACS data from day 33 demonstrates that HA-specific (Thy1.1+) CD4 cells that proliferate in response to the tumor vaccine concomitantly upregulates IFN-γ. *p<0.05.
In the absence of vaccination, HA-specific cells adoptively transferred into non-transgenic mice neither proliferated nor produced cytokines at any time point (Figures 4a, 4b far left panels). In contrast, non-transgenic mice given the HA-specific tumor vaccine proliferated and produced the effector cytokine IFN-γ (Figures 4a, 4b second panels from left). HA-specific CD4 T cells adoptively transferred into C3-HA and the Pro-HA×TRAMP proliferated at both time points (Figure 4a), but were functionally “non-productive” in that they failed to upregulate effector cytokine (Figure 4b). Consistent with our prior data (Figure 2a), therapeutic RT resulted in a decrease in CD4 T cell recognition of tumor-associated antigen by week 2 post-RT (Figure 4a). By approximately 7 weeks post RT, the immune recognition approached that observed in the un-irradiated Pro-HA×TRAMP control group (Figure 4a), consistent with expected disease progression (data not shown). Despite this significant proliferation, HA-specific T cells adoptively transferred to either irradiated or unirradiated Pro-HA×TRAMP mice did not produce the effector cytokine IFN-γ at any time point (Figure 4b).
We next performed these time-course studies in combination with tumor-specific vaccination. When HA-specific CD4 T cells were adoptively transferred into RT treated Pro-HA×TRAMP mice prior to the completion of RT (day 6) or immediately after completion of RT (day 16), the T cells were tolerized as demonstrated by a lack of proliferative response to the specific vaccine (Figure 4c). However, when HA-specific CD4 T cells were adoptively transferred approximately three weeks (day 33) following RT, significant proliferation and cytokine production were noted (Figures 4c, 4d). This proliferation and cytokine production was vaccine-dependent, and was not noted in RT-treated Pro-HA×TRAMP mice that were not vaccinated (Figures 4a, 4b). This mitigation of vaccine tolerance was transient however, and was not noted when T cells were adoptively transferred 14 weeks post-RT. Taken together, these data suggest that RT of prostate tumors may mitigate tolerance to tumor-vaccination, but that the window over which this occurs is discrete.
DISCUSSION
Our data indicate that there is a therapeutic effect of RT in this autochthonous mouse model of prostate cancer, and establish an experimental system in which we are able to model the interaction between immunotherapy and RT. As has been noted, the immune response to an antigen is directed by a number of factors, including the “maturation” state of the professional antigen presenting cells—dendritic cells (DCs). In general, apoptotic cells do not mature or activate DCs; this is likely an evolutionary adaptation to hinder autoreactive immune responses (28). Conversely, DCs that uptake antigen in the presence of “danger signals” mature and mediate an effector immune response (29). A recent study demonstrated that the efficacy of RT is partially dependent on TLR-4 signaling in DCs, but this study did not address adaptive immune responses against pre-established tumors (6). Various groups have augmented radio-immunotherapy against pre-established tumors with the addition of DC maturation agents such as Flt3-L and GM-CSF (30-32). Furthermore, Vaccinia virus that is employed in our tumor vaccine has been shown to activate DCs in vivo, evidenced by increase of surface expression of MHC Class I and the co-stimulatory molecules CD40, CD80, and CD86 (33). Paradoxically MHC Class II expression is decreased, but DCs are still capable of activating CD4 T cells.
In our autochthonous tumor model, tumor-specific CD4 T cells adoptively transferred post-RT were not activated and failed to express the effector cytokines IFN-γ and TNF-α. However, they divided less than T cells adoptively transferred into tumor-bearing animals that were unirradiated. This decrease in division without upregulation of effector cytokines suggests that the effect of RT on the interaction between the immune system and the tumor was decreased antigen presentation through direct, RT-mediated cytotoxic effects on the tumor resulting in a decreased tumor burden. This notion is further supported by pathologic data which demonstrated a decrease in the cellularity of the prostate glands post-RT. However, it should be noted an indirect effect on tumor infiltrating antigen presenting cells cannot be ruled out in this experimental system as a previous study demonstrated an altered peptide repertoire as a result of RT (5).
The prostate tumors in Pro-HA×TRAMP mice are tolerigenic, and adoptively transferred CD4 T cells are tolerized and fail to proliferate in response to tumor vaccination (10,18,34). Interestingly, RT decreased tumor-mediated proliferation of adoptively transferred CD4 T cells, but conversely augmented the response to the tumor vaccine. This augmentation of vaccine response is likely due to the decrease in tolerogenic antigen presentation, as the adoptively transferred CD4 T cells failed to upregulate effector cytokines in the absence of vaccination. The observed response to vaccine post-RT could also be a result of a relative depletion of tumor-specific suppressor (CD4+Foxp3+) T cells (35), especially if those T cells preferentially resided in the prostate draining lymph nodes and were preferentially radio-sensitive. Although we used a focused external beam of radiation, a moderate diameter beam was employed to guarantee prostate targeting. Due to the extremely close proximity of the prostate draining lymph nodes to the gland itself in mice, it cannot be ruled out that the prostate draining lymph nodes were directly affected by the RT. However, a careful analysis of the numbers of T cells, B cells and DC in the prostate-draining lymph nodes of treated mice did not reveal significant differences in cell numbers or percentages (data not shown).
Our data further demonstrate that the response to tumor vaccine was transient, with an observed peak in the response between 3 and 5 weeks post-RT. This mitigation of tolerance was abrogated by week 14 post-RT. Importantly, the response to vaccine was poor in the peri-RT and immediate post-therapy time frame. These data suggest that for aggressive, tolerogenic tumors, immunotherapy might prove most efficacious at a time point after RT when the tumor burden is at its nadir. As survival experiments in the TRAMP (C57BL/6) mice typically last longer than a year, we chose to first establish the timing of the combination (radio- immunotherapy) therapy before commencing the long-term experiments.
As RT is a standard treatment for many tumor types, novel immunotherapeutic regimens must be optimized with RT. Here we demonstrate that while RT itself might play a therapeutic role against aggressive tumors; RT alone did not appear to mediate an active anti-cancer immune response. Moreover, immunotherapy with a tumor vaccine consisting of a recombinant pox virus expressing a tumor associated antigen did not mitigate T cell tolerance in this model when the vaccine was employed as a single modality. Only when RT was combined with immunotherapy in an appropriate sequence was tumor-induced immune tolerance mitigated. We are currently translating these data in to a clinical trial setting, in which immunotherapy is administered 4 weeks after definitive, fractionated RT in men with prostate cancer.
Acknowledgments
C.G.D. is a Damon Runyon-Lilly Clinical Investigator. T.J.H. is a UNCF GlaxoSmithKline Scholarship Recipient. D.M.P. is a Januey Scholar and holds the Seraph Chair for Cancer Research. This work was also supported by NIH grants and funds from the David H. Koch Fund provided by the Prostate Cancer Foundation.
Footnotes
DISCLOSURE STATEMENT None of the authors have any competing financial interests.
References
- 1.Meng A, Wang Y, Brown SA, Van Zant G, Zhou D. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp Hematol. 2003;31(12):1348–1356. doi: 10.1016/j.exphem.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63(17):5414–5419. [PubMed] [Google Scholar]
- 3.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63(3):655–666. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 7.Cameron RB, Spiess PJ, Rosenberg SA. Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin 2, and local tumor irradiation. Studies on the mechanism of action. J Exp Med. 1990;171(1):249–263. doi: 10.1084/jem.171.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang CS, Syljuasen RG, Hong JH, Wallis A, Dougherty GJ, McBride WH. Effects of IL-3 gene expression on tumor response to irradiation in vitro and in vivo. Cancer Res. 1997;57(18):3899–3903. [PubMed] [Google Scholar]
- 9.Seetharam S, Staba MJ, Schumm LP, Schreiber K, Schreiber H, Kufe DW, Weichselbaum RR. Enhanced eradication of local and distant tumors by genetically produced interleukin-12 and radiation. Int J Oncol. 1999;15(4):769–773. doi: 10.3892/ijo.15.4.769. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444–2448. [PubMed] [Google Scholar]
- 11.Sumareva R, Ukrainsky G, Kiremidjian-Schumacher L, Roy M, Wishe HI, Steinfeld AD, Cooper JS. Effect of combined adoptive immunotherapy and radiotherapy on tumor growth. Radiat Oncol Investig. 1999;7(1):22–29. doi: 10.1002/(SICI)1520-6823(1999)7:1<22::AID-ROI3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, Camphausen K, Coleman CN, Sullivan F, Steinberg SM, Schlom J, Dahut W. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 13.Kupelian PA, Katcher J, Levin HS, Klein EA. Stage T1-2 prostate cancer: a multivariate analysis of factors affecting biochemical and clinical failures after radical prostatectomy. Int J Radiat Oncol Biol Phys. 1997;37(5):1043–1052. doi: 10.1016/s0360-3016(96)00590-1. [DOI] [PubMed] [Google Scholar]
- 14.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2(10):750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 15.Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, Deeg HJ. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31(5):1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 16.Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108(2):135–144. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92(8):3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56(18):4096–4102. [PubMed] [Google Scholar]
- 20.Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187(10):1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180(1):25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95(3):1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotteveel FT, Kokkelink I, van Lier RA, Kuenen B, Meager A, Miedema F, Lucas CJ. Clonal analysis of functionally distinct human CD4+ T cell subsets. J Exp Med. 1988;168(5):1659–1673. doi: 10.1084/jem.168.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurt-Jones EA, Hamberg S, Ohara J, Paul WE, Abbas AK. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 26.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292(5523):1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 27.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 28.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 30.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, Guha C. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59(24):6028–6032. [PubMed] [Google Scholar]
- 31.Newcomb EW, Demaria S, Lukyanov Y, Shao Y, Schnee T, Kawashima N, Lan L, Dewyngaert JK, Zagzag D, McBride WH, Formenti SC. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12(15):4730–4737. doi: 10.1158/1078-0432.CCR-06-0593. [DOI] [PubMed] [Google Scholar]
- 32.Nishisaka N, Maini A, Kinoshita Y, Yasumoto R, Kishimoto T, Jones RF, Morse P, Hillman GG, Wang CY, Haas GP. Immunotherapy for lung metastases of murine renal cell carcinoma: synergy between radiation and cytokine-producing tumor vaccines. J Immunother (1997) 1999;22(4):308–314. doi: 10.1097/00002371-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Yao Y, Li P, Singh P, Thiele AT, Wilkes DS, Renukaradhya GJ, Brutkiewicz RR, Travers JB, Luker GD, Hong SC, Blum JS, Chang CH. Vaccinia virus infection induces dendritic cell maturation but inhibits antigen presentation by MHC class II. Cell Immunol. 2007 doi: 10.1016/j.cellimm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mihalyo MA, Hagymasi AT, Slaiby AM, Nevius EE, Adler AJ. Dendritic cells program non-immunogenic prostate-specific T cell responses beginning at early stages of prostate tumorigenesis. Prostate. 2007;67(5):536–546. doi: 10.1002/pros.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]