Abstract
Due to the widespread clinical use of imaging modalities such as ultrasonography, computed tomography and magnetic resonance imaging (MRI), previously unsuspected liver masses are increasingly being found in asymptomatic patients. This review discusses the various characteristics of the most common solid liver lesions and recommends a practical approach for diagnostic workup. Likely diagnoses include hepatocellular carcinoma (the most likely; a solid liver lesion in a cirrhotic liver) and hemangioma (generally presenting as a mass in a non-cirrhotic liver). Focal nodular hyperplasia and hepatic adenoma should be ruled out in young women. In 70% of cases, MRI with gadolinium differentiates between these lesions. Fine needle core biopsy or aspiration, or both, might be required in doubtful cases. If uncertainty persists as to the nature of the lesion, surgical resection is recommended. If the patient is known to have a primary malignancy and the lesion was found at tumor staging or follow up, histology is required only when the nature of the liver lesion is doubtful.
Keywords: Liver mass, Hepatic nodule, Tumor, Lesion, Cirrhosis, Hepatocellular carcinoma, Magnetic resonance imaging, Ultrasonography, Computed tomography, Fine needle aspiration, Biopsy
INTRODUCTION
Liver masses are increasingly being identified due to the widespread use of imaging modalities such as ultrasonography (US), computed tomography (CT), and magnetic resonance imaging. The majority of these lesions are detected incidentally in asymptomatic patients. An accurate history and physical examination are essential to the diagnosis and treatment of solid liver masses. For example, the use of oral contraceptives or anabolic steroids might be related to hepatic adenoma (HA)[1]; alcohol use and occupational exposure are associated with angiosarcoma[2] and primary sclerosing cholangitis, liver fluke, Caroli’s disease, and choledochal cysts are associated with cholangiocarcinoma[3]. Physical examination should look for liver tenderness, stigmata of chronic liver disease, or general deterioration signs (fever, weight loss). High alkaline phosphatase, high lactate dehydrogenase (LDH), low albumin, high prothrombin time, and iron overload are non-specific but might suggest an underlying chronic hepatitis, cirrhosis or an infiltrative process[4]. A history of hepatitis B, C or liver cirrhosis might point to hepatocellular carcinoma (HCC). A previous neoplasm or history of chemotherapy increases the suspicion of metastatic liver disease.
CURRENT KNOWLEDGE
In the majority of patients, a proper diagnosis can be made based on the characteristics on imaging modalities (Lesions size < 1.0 cm are usually benign). For diagnostic purposes, liver masses should be divided into those occurring with and without cirrhosis. A liver mass in a cirrhotic liver should be viewed as HCC until proven otherwise. Multiple liver masses in a cirrhotic liver indicate diffuse HCC, high-grade dysplastic nodules or, on rare occasions, hepatic lymphoma[5]. Benign liver lesions are found in more than 20% of the general population[6], including haemangioma (4%), focal nodular hyperplasia (FNH, 0.4%) and hepatic adenomas (0.004%). Multiple liver lesions in a normal liver usually indicate liver metastasis (most commonly from adenocarcinoma of the colon, stomach, lung or prostate), but could be cysts or hemangiomas[7]. Liver metastasis is a rare finding in a cirrhotic liver[8] (Table 1). Multiple liver lesions of a benign nature such as hemangiomas or focal nodular hyperplasia are not uncommon in a normal liver.
Table 1.
Clinical differential diagnosis of the most common liver masses
| Cirrhotic liver | Common lesions | Non-cirrhotic liver | Common lesions | |
| Malignant mass | Hepatocellular carcinoma | a,d | Metastasis | a,b |
| Cholangiocarcinoma | Well differentiated HCC | |||
| High grade dysplastic nodule | Fibro lamellar HCC | a,b,c,g | ||
| Lymphoma | Cholangiocarcinoma | |||
| Metastasis (exceptional) | Hemangio-Endothelioma | g | ||
| Lymphoma | ||||
| Melanoma | ||||
| Neuroendocrine tumor | a | |||
| Sarcoma (angiosarcoma,leiomyosarcoma) | g | |||
| Benign mass | Low grade dysplasia | d | Hemangioma | b |
| Focal fatty liver | Focal nodular hyperplasia (FNH) | a,b | ||
| Hemangioma | Hepatic adenoma (HA) | a,b | ||
| Hepatic adenoma | g | Nodular regenerative hyperplasia | b,f | |
| Partial nodular transformation | e,f | |||
| Focal fatty infiltration | c,e | |||
| Bile duct adenoma |
a: Hyper vascular liver tumor; b: Tumors that are extremely rare in cirrhosis but relatively frequent in healthy normal liver; c: Tumors frequent in the left lobe; d: Mainly in cirrhosis; e: Equally found in cirrhotic and non cirrhotic; f: Clinically mimics cirrhosis; g: Extremely rare tumors.
Calcifications have no diagnostic utility but might suggest fibro-lamellar carcinoma or colorectal adenocarcinoma, whereas hemorrhage within the lesion suggests adenoma[9]. The initial strategy in cirrhosis should be the measurement of α-fetoprotein (AFP) followed by ultrasound, contrast CT or magnetic resonance imaging (MRI)[10,11]. Fine needle core biopsy (FNCB) might be required, but biopsy of potentially operable lesions should be avoided. This review discusses the various characteristics of the most common solid liver lesions and recommends a practical approach for diagnostic workup.
DIAGNOSTIC VALUE OF TUMOR MARKERS
AFP, PIVKA 2 (< 0.1 U/mL), desgamma-carboxy prothrombin and CA 19-9 (< 37 U/mL) are tumor markers for HCC. AFP is the first choice when diagnosing HCC and 10 ng/mL is the cut-off level. The formula (Ca 19-9 + CEA × 40) provides an index accuracy of 86% in diagnosis of cholangiocarcinoma[12]. AFP values > 400 ng/mL are indicative of HCC. 30% of patients with HCC < 2 cm have normal AFP, 20% of HCCs produce no AFP, and levels from 20-250 are frequently seen in regenerating nodules or viral cirrhosis. An increase in AFP over time is virtually diagnostic of HCC[13]. Globally, the serum level of at least one of the tumor markers was elevated in 88% of patients with proven malignancy. Elevation was marked in 57%. No tumor marker alterations were detected in patients with benign lesions. Early lesions may have elevated tumor markers in fewer than 30% of cases[12].
IMAGING TECHNIQUES (TABLE 2)
Table 2.
Accuracy and key features of imaging techniques in the diagnosis of most common liver masses
| US-US doppler, contrast ultrasound | Triphasic CT | MRI | PET SCAN | CT-angiography | |
| Hemangioma (1-10 cm) | ++ | +++ | ++++ | +++ | |
| Hyperechoic Doppler: low flow, low index, absence of spectral broadening | Peripheral puddles, fill in from periphery, enhancement on delayed scan | Peripheral enhancement centripetal progression Hyperintense on T2, hypo intense on T1 SS > 95%, SP 95% | No uptake | Cotton wool pooling of contrast, normal vessels without AV shunt, persistent enhancement | |
| Focal fatty liver | + | ++ | +++ | Normal finding | |
| Hyper echoic, no mass effect, no vessel displacement | Sharp interface Low density (< 40 u) | No uptake | |||
| FNH (< 3 cm) | + | ++ | ++++ | +++ | |
| Homogenous iso, hypo, or hyper echoic, central hyper echoic area Central arterial signal Doppler: high flow, spectral broadening | Homogeneous enhance strongly with hepatic arterial phase Isodense with liver; Central low density scar | Hyper vascular +Gd Isodense T1 Hyper intense scar T2 SS > 95%; SP > 95% | No uptake | Hyper vascular 70% centrifugal supply | |
| Adenoma (5-10 cm) | + | ++ | ++ | ++ | |
| Heterogeneous Hyper echoic If haemorrhage: anechoic center In doppler: variable flow, spectral broadening | Homogenous > Heterogeneous, Peripheral feeders filling in from periphery | Capsule, Hyper intense in T1 (intra lesional fat) | No uptake uptake if degenera-tion to HCC | Hyper vascular Large peripheral Vessel Central scar if haemorrhage | |
| HCC | + | +++ | +++ | + | ++++ |
| Hypo or hyper echoic Doppler: hyper vascular Doppler: index and flow high, spectral broadening | Hyper vascular, often irregular borders Heterogeneous > Homogeneous abnormal internal vessel Hallmark is venous washout SS 52%-54% | Hyper vascular Poor different: Hypo intense T-1, Hyper intense T2 Well different: Hyper intense T-1, Iso intense T-2 SS 53%-78% | Increased uptake, but many HCCs show no uptake at PET | Hyper vascular Av shunting Angiogenesis | |
| Cholangio-carcinoma | Bile duct dilatation if major ducts are involved. Intra-hepatic CCC: no bile dilatation | Hypo dense lesion. Delayed enhancement | Hypo intense T1 Hyper intense T2 MRCP is useful | Uptake ++ SS 93% | Hypervascular |
| Metastasis | +1 | +++ | +++ | +++++ | ++++ |
| SS 40%-70% hypo to hyper echoic; doppler; low index and flow; presence of spectral broadening | SS 49%-74 % complete ring enhancement | SS 68%-90 % Low intensity T-1 High intensity T-2 | SS 90%-100% | SS 88%-95% hyper vascular |
Intraoperative ultrasound, contrast ultrasound and EUS are highly sensitive to detect liver mass; +: Degree of accuracy; SS: Sensitivity; SP: Specificity; MRI: Magnetic resonance imaging; CT: Computed tomography; HCC: Hepatocellular carcinoma.
A single imaging modality might suffice in cases that show interval development or progression, such as metastasis. Hemangiomas are often diagnosed by a single dynamic contrast enhanced imaging modality (MDCT). When further imaging techniques are necessary, CT angiography, MRI, and contrast enhanced-CT are performed to plan a surgical strategy. If this is not available on site, the patient and physician should decide whether the patient should undergo a biopsy or be referred for additional imaging. The key features of imaging techniques in the diagnosis of liver mass are shown in Table 3. Imaging tools for tumor assessment include: (1) Angiogram, RBC scintigraphy, contrast-enhanced CT, Porto-angiography CT, color Doppler ultrasound, contrast ultrasound, and gadopentetate dimeglumine-enhanced MRI; (2) MD-DPDP enhanced MRI imaging, Gd-Bopta-enhanced MRI imaging, EOB DTPA (to assess hepatocyte function and biliary excretion); (3) Plain film, US, CT scan (to assess tumor calcifications); and (4) US, contrast US, enhanced CT, MRI (utilized to assess capsule formation). US and CT are indicated for diagnosis of biliary obstruction or gallbladder diseases and for differentiation of cysts from solid liver lesions. Intraoperative ultrasound detects small liver lesions (< 5 mm). Endoscopic US assesses the left liver lobe and the gastrohepatic ligament lymph nodes, and can help perform FNA. Doppler US evaluates blood vessels patency and portal hypertension[14].
Table 3.
Immunohistochemical staining in the evaluation of hepatic tumors
| Tumor | Recommended immunostaining |
| HCC | Polyclonal CEA |
| Cytokeratin 8/18 pair (+/+ staining) | |
| Cytokeratin 7/20 pair(-/- staining) | |
| Hep Par 1, AFP | |
| Cholangiocarcinoma | Cytokeratin 7/19 pair (+/+ staining) |
| Cytokeratin 7/20 pair (+/- staining) | |
| B-HCG, CEA, Mucin-1 | |
| Epithelioid hemangioendothelioma | CD34 |
| CD31 | |
| Factor VIII | |
| Angiomyolipoma | HMB-45, smooth muscle actin |
| Metastatic carcinoma | |
| Neuroendocrine | Chromagin, synaptophysin, neural enolase |
| Pancreas | Cytokeratin 7/20 pair (+/+ staining) |
| Colorectal | Cytokeratin 7/20 pair( -/+ staining) |
| Breast | Cytokeratin 7/20 pair (+/- staining) |
| Lung | Cytokeratin 7/20 pair (+/- staining) |
The gold standard for detection and location of focal lesions is MRI or triple phase dynamic spiral CT[15]. Conventionally, a triple phase CT scan includes unenhanced, arterial and venous phases. The fourth phase is a delayed venous scan quadruple phase MDCT[16]. This is required only for small lesions thought to be HCC or cysts and hemangiomas. CT portography is one of the most sensitive imaging modalities for secondary lesions, but it is an examination that is performed in highly selected cases only, in few institutions and not for all types of liver lesions[16,17]. FDG PET CT scan is not very useful for HCC and therefore is not the best imaging modality to distinguish benign from malignant lesions[18]. A nuclear scan with Tc-99m-sulfur colloid shows increased uptake in FNH. MIBG and octreotide scintigraphy detect neuroendocrine tumors[19]. Hepatic Tc-99m red blood cell scan diagnoses hemangiomas > 2.5 cm (most university centers do not use this method and prefer contrast enhanced US, CT, and MRI)[20]. Ultrasound contrast agents and MRI using iron or gadolinium contrast better detect smaller lesions, satellite lesions or distant metastasis[21–23].
Radiographic characteristics favoring HCC include the presence of a capsule bulging beyond the normal hepatic contour or a lesion with different densities. Contrast injection produces an immediate enhancement of most hepatomas.
FINE NEEDLE ASPIRATION AND CORE BIOPSY (FNAB)
FNAB is safe, accurate and cost effective. Its specificity approaches 100% and its sensitivity is 67%-100%[24,16]. FNAB under CT or US in an appropriate location is the method of choice. FNAB is superior to FNCB; however, the methods are complementary, i.e. FNAB and FNCB have an accuracies of 78% separately and 88% when considered in combination[25,26]. However, many pathologists state that core biopsies are much preferred over needle biopsies for diagnosis of hepatic masses, because well differentiated HCCs cannot be separated from normal liver. Complications (mostly hemorrhages) are rare, with 0.5% minor complications and 0.05% major complications[27–30]. Another concern is the possible seeding of a tumor. Blind FNAB is diagnostic in > 50% of cases[31], which increases to 65% when performing a second pass. An additional 5%-10% of tumors will be recognized if a cell block is obtained. Cohn’s cytological criteria help to distinguish HCC from non-neoplastic lesions (81% of HCC show at least two criteria)[32], e.g. increased nuclear/cytoplasmic ratio, trabecular pattern, and atypical naked hepatocyte nuclei. Some key features of immunohistochemical staining in the evaluation of hepatic tumors are shown in Table 3.
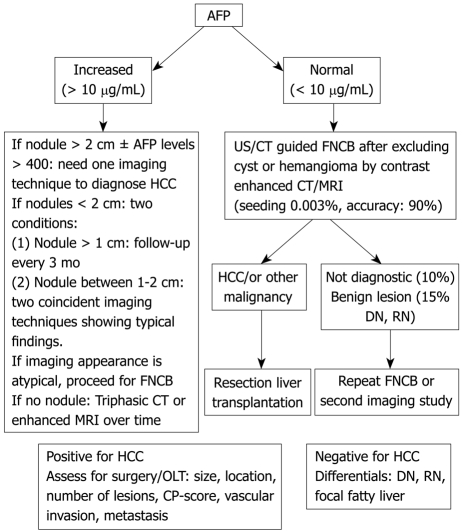
GENERAL APPROACH TO LIVER MASSES IN CIRRHOTIC LIVERS (TABLE 1, Figure 1)
Figure 1.
Algorithm for the investigation of a liver mass in a cirrhotic liver. Some hepatologists consider biopsy to be unnecessary for a mass in a cirrhotic liver even if the α-fetoprotein (AFP) < 10; FNCB: Fine needle core biopsy; MRI: Magnetic resonance imaging.
Mass > 2 cm
Enhancement in the arterial phase and washout in the portal venous phase is essential for the diagnosis of a liver lesion > 2 cm in a cirrhotic liver. More than 80% of masses > 2 cm in a cirrhotic liver are HCC[33,34]. An elevated AFP confirms the diagnosis. If AFP is normal, further imaging will be diagnostic (triphasic CT, MRI)[13]. If there is still doubt, FNCB might be indicated (Figure 1).
Mass < 2 cm
Seventy-five percent of masses < 2 cm in a cirrhotic liver are HCCs[35]. AFP levels and imaging might secure the diagnosis. If still in doubt, repeated imaging that detects enlargement of the lesion, or FNAB/FNCB might be indicated. Due to the risk of tumor seeding, biopsy should be avoided if surgical resection is possible[36]. A small nodule can be preneoplastic or benign. The American Association for the Study of Liver Diseases (AASLD) distinguishes lesions < 1 cm from those > 1 cm but < 2 cm. They suggest performing two imaging techniques from among US, CT, and MRI with IV contrast injection. If two techniques display typical imaging criteria, it is possible to diagnose HCC. Larger nodules should be diagnosed and small lesions should be surveyed every three months[37]. Caturelli showed that 69% of new nodules in a cirrhotic liver are malignant. Moreover, liver cell dysplasia is found in 60% of cirrhotic livers containing HCC and in only 10% of non-cirrhotic livers[38].
AFP is increased without a liver mass
In this case, repeated dynamic CT or MRI every three months is the rule[39]. An elevated AFP does not necessarily diagnose HCC, especially in patients with HCV who commonly have modest elevation of AFP without HCC. A marked AFP is helpful, but modest elevations would certainly not be an indication for OLT in the absence of a liver mass.
HCC: HCC is a common malignancy with an incidence of 1%-6% among cirrhotic patients[13,40–42]. Risk factors include cirrhosis, alcohol, HBV, HCV, metabolic liver diseases, environmental carcinogens, hormonal treatments and smoking[43,44]. Ninety to ninety five percent of HCCs arise in cirrhotic livers. Autopsy studies indicate that 20%-40% of patients with cirrhosis have HCC. Tumor size and severity of liver disease influence the survival rate. Patients with tumors < 5 cm have a survival of 80% at one year and 20% at three years. New abdominal pain, recent hepatomegaly, hemoperitoneum, persistent fever or weight loss in a cirrhotic patient should raise suspicion of HCC. Laboratory results that characterize HCC include a sudden increase in alkaline phosphatases, an increased ratio AST/ALT, an erythrocytosis, persistent leukocytosis, recurrent hypoglycemia, hypercholesterolemia and hypercalcemia. The last four findings are paraneoplastic manifestations[45] together with ectopic hormonal syndrome, hypertrophic osteoarthropathy and porphyria cutana tarda[46]. Complications of HCC include obstructive jaundice[47], and rupture of HCC (60%-90% mortality).
Screening for HCC includes US + AFP levels every six months. The AASLD guidelines recommend US only. AFP is of little additional value. Lesions > 2 cm need just one imaging technique showing typical findings (arterial hypervascularization) or one imaging technique and AFP levels > 400 in order to make a non-invasive diagnosis of HCC[13]. Lesions < 2 cm are divided into larger and smaller than 1 cm. Nodules > 1 cm but < 2 cm (1-2 cm) need diagnostic workup with two coincident or serial imaging techniques, rather than just proceeding with a biopsy. Nodules < 1 cm need screening follow up every three months. Nine to thirty seven percent of HCC are resectable at diagnosis[48]. Contraindications to resection include decompensated cirrhosis, extra-hepatic metastases, involvement of hepatic nodes or inferior vena cava (IVC), or bilobar extension[45]. The histological variants in cirrhotic livers include trabecular (65%), mixed (15%), compact (12%), pseudo glandular (5%) fibro lamellar (1.5%), and scirrhous 0.5%[49].
Regenerative nodules: Dysplatic nodules often occur within regenerative cirrhotic nodules. They can show low- or high-grade dysplasia. A progression from regenerative nodule with low-grade dysplasia to high-grade dysplasia, in well differentiated and poorly differentiated HCC, is possible[50,51]. MRI best differentiates this iso-or hypo- intense lesion from hyper intense HCC. In difficult cases, histology is required after liver resection or liver transplant. If HCC cannot be confirmed, the investigation must be repeated later. Over time, high-grade dysplastic nodules can become malignant, suggesting preventive ablation by ethanol[7].
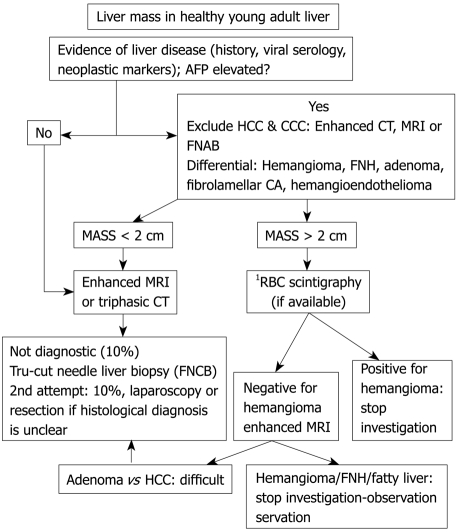
GENERAL APPROACH TO LIVER MASSES IN NON-CIRRHOTIC LIVERS (TABLE 1, FIGURE 2)
Figure 2.
Algorithm for the management of a liver mass in a non-cirrhotic liver. 1Most centers do not use RBC scintigraphy to diagnose hemangioma due to their use of cross sectional imaging such as contrast enhanced ultrasonography (US)/CT/MRI.
Liver masses present with fever, pain, abdominal discomfort, or accidentally without overt symptomology. Benign masses include hemangioma, FNH, nodular regenerative hyperplasia (NRH), and HA. The most frequent malignancy is gastrointestinal, urogenital, lung or breast carcinoma metastasis. Liver primary malignancy includes fibrolamellar carcinoma, cholangiocarcinoma, hepatic lymphoma and angiosarcoma[52,53].
Elevated AFP diagnoses HCC[54], otherwise, further imaging is required. US or CT classifies cysts, metastases and hemangioma. MRI identifies focal fatty liver, FNH, HA and hemangioma[55]. When the diagnosis remains uncertain, FNAB or follow up imaging is considered. Resection is indicated for large (> 5 cm) or growing adenomas.
Benign lesions
Hemangiomas: Hemangiomas are found in 20% of the general population, more commonly in women[56,57]. The majority are asymptomatic. Giant hemangiomas (> 4 cm) are symptomatic in 40% of cases. Symptoms are rare and can include abdominal pain, early satiety, anorexia, and nausea[58]. Tc-99m labelled RBC Spect is the best and least expensive modality (specificity 100%) for lesions > 2.5 cm and MRI for lesions < 2 cm[59,60]. University centers rarely require RBC Spect for diagnosis of hemangiomas due to the use of cross sectional imaging. Histology of liver hemangiomas is blood-filled vascular sinusoids separated by connective tissue septa (peliosis lacks endothelial layer and fibrous trabeculae)[61]. The risk of rupture is minimal and does not justify resection. Other complications include thrombosis, sclerosis, and calcification. Liver hemangiomas can grow during pregnancy or oral contraception. Kasabach-Merritt syndrome (consumption coagulopathy) and Bornman-Terblanche-Blumgart syndrome (fever and abdominal pain) constitute uncommon complications[61].
FNH: Focal nodular hyperplasia is found more often in women 20-50 years of age (80%). The pathogenesis is unknown, but includes vascular injury. The lesion is usually < 3 cm, asymptomatic and discovered accidentally. The main difficulty for the physician is differentiating FNH from adenoma or fibrolamellar carcinoma by imaging techniques. However, fibrolamellar carcinomas enhance heterogeneously, wash out, and have central calcifications and enlarged lymph nodes. These features are very rarely found in FNH. Lack of symptoms, normal liver enzymes and no use of oral contraceptives argue in favour of FNH. Recent literature suggests that MRI has 70% sensitivity and 98% specificity for FNH (homogeneous hypervascular lesion with central scar)[62,63]. If radiology is unequivocal, most hepatologists advocate a “wait and see” attitude. Otherwise, image guided percutaneous biopsies are performed, one in the tumoral tissue and the other in the normal liver tissue. The histology resembles inactive cirrhosis with proliferating hepatocytes around a normal prominent central artery with a central fibrous scar[62]. The natural history of FNH is variable (stable, regressive or progressive over time), but resection is not necessary because it does not progress to malignancy. Complications are rare and include hepatic vein thrombosis or Kasabach-Merritt syndrome[63,64].
HA: Adenoma occurs in women with oral contraception use > 5 years[65] or in diabetic patients. Multiple adenomas are associated with glycogen storage disease type I and type III[66]. Adenomatosis (> 10 adenomas) is observed with anabolic or androgenic steroids consumption[67]. Abdominal discomfort is common[68]. The lesion is hypo- to hyper- echoic on US and hypo- to hyper- dense on CT. MRI is not specific[69,70]. The lesions are often < 8 cm but can be > 15 cm microscopically; they appear as monotonous sheets of normal or small hepatocytes with no bile duct, portal tract or central vein. Five percent of hepatic adenomas transform to HCC[71]. β-catenin immunostaining might be useful for diagnosis. Spontaneous rupture and hemoperitoneum occur in 10% of cases, especially during menstruation, pregnancy or post partum. Most hepatologists advocate resection and discontinuation of oral contraception[72,73].
NRH and partial nodular transformation (PNT): NRH (large regenerative nodule) is associated with Behcet’s disease, rheumatic diseases, myeloproliferative disorders, chronic venous congestion, metastatic neuroendocrine tumors, Budd-Chiari syndrome, and various drugs (steroids, contraceptives, antineoplastics, anticonvulsives, and immunosuppressives)[74]. Some lesions present with portal hypertension and cholestasis. A diagnostic open liver biopsy is rarely required[75]. NRH shows diffuse hyperplastic nodules with thickened liver cell plates without fibrosis. Reticulin changes are characteristic. Portal vein thrombosis could lead to NRH by parenchymal atrophy and compensatory hyperplasia. Portal vein thrombosis has also been invoked in the pathogenesis of PNT[76].
Focal fatty infiltration of the liver: In 10% of patients with fatty liver, fat accumulates focally or shows focal sparing, usually in the anteromedial segment of the left lobe. These patients usually have diabetes, hyperlipidemia, obesity, drink alcohol or take steroids[77]. On US, fat is hyper echoic. On CT, it has low attenuation. Focal fatty liver does not displace intrahepatic vessels. The gold standard imaging technique is MRI with increased signal on T1 sequence[78]. Fat suppression techniques are also very promising.
Other rare benign tumors
Hepatobiliary cyst adenoma: This lesion is rare, occurs predominantly in middle-aged women and causes abdominal pain. It differs from benign cyst by having thick septated wall. Up to 25% become malignant (cystadenocarcinoma), therefore surgical excision is indicated[79].
Bile duct adenoma (cholangioma): Bile duct adenomas are solitary sub capsular nodules measuring 1-20 mm described in patients with α-1-antitrypsin deficiency. The bile ducts are smaller and do not contain bile.
Biliary papillomatosis: Only 50 cases have been described worldwide[80]. It is a tumor of middle age, leading to biliary obstruction by tumor shedding, mucus and lithiasis, cholangitis and hemobilia. Diagnosis is made by endoscopic retrograde cholangiopancreatography (ERCP), which shows typical mucinous discharge from a dilated ampulla, multiple filling defects and stenosis. Hepatectomy can effect a cure.
Mesenchymal and neural tumors: This category includes lipomas, myolipomas, angiomyolipomas, schwannomas, neurofibromas and chondromas.
Inflammatory pseudotumor: This is a rare, benign inflammatory condition of middle-aged men. Patients might present fever, weight loss, leukocytosis and elevated ESR. If the diagnosis can be made on biopsy, there should be no need for resection; rather the primary source should be treated[81].
Pseudo-lesions: A pseudo-lesion is a non-diseased area of different signal intensity, attributable to focal alteration in hemodynamics or parenchymal metabolism[82]. Pseudo-lesions seen in cirrhotic and non-cirrhotic livers include arterio-portal shunts, regenerating nodules, confluent fibrosis, and abnormal blood inflow.
Malignant lesions
Liver metastasis: The liver is the most common site of metastasis from the gastrointestinal tract, pancreas, breast, and lung[41]. Multiple defects in the liver imaging suggest a metastatic process. Only 20% of liver metastases present as solitary lesions. Generally, both hepatic lobes are involved. On CT-scan, colorectal metastases appear as low attenuation lesions, often with irregular margins and necrotic centres[14]. During the early vascular phase of dynamic CT, metastasis appears with increased enhancement. The sensitivity of CT (85%) can be augmented by CT arterial portography[83]. Intraoperative US has excellent sensitivity and specificity for colorectal adenocarcinoma metastasis[84]. The most promising imaging modality is PET CT with FDG that accumulates in cells with hypermetabolism. Colon, lung and breast cancers can be staged with PET CT with sensitivity of 92%-100% and specificity of 85%-100%[85]. The hypervascularity of neuroendocrine tumors is often evident on dynamic CT[86]. Somatostatin receptor scintigraphy can localize 90% of neuroendocrine tumors (gastrinoma)[87].
In metastatic colorectal carcinoma, the prognosis is improved following surgical resection. Contraindications to resection include: N > 4 liver metastases, extrahepatic spread and involvement of hepatic lymph nodes. Metastatic liver tumors that might calcify include colon, leiomyosarcoma, osteogenic sarcoma, rhabdomyosarcoma, chondrosarcoma, ovarian cystadenocarcinoma, melanoma, pleural mesothelioma, neuroblastoma, and testis tumors. Calcified metastases from stomach, pancreas, lung and breast to the liver are extremely rare. Guided FNA will help identify the primary lesion[88].
HCC (see previous section on HCC): Almost all patients with HCV related HCC have cirrhosis, whereas patients with HCC related to HBV are less likely to have cirrhosis. The absence of cirrhosis makes this tumor more amenable to surgical resection[34].
Fibrolamellar carcinoma: The fibrolamellar variant is a distinctive subtype of HCC but is not associated with classic risk factors for HCC. It occurs at a mean age of 26 years, presenting as a large, solitary painful mass usually located in the left lobe. The AFP level is normal[89]. The term “fibrolamellar” characterizes the microscopic appearance of the lesion: thin layers of fibrosis separate the neoplastic hepatocytes[90]. A fibrous central scar may be seen on imaging studies[91]. 50% of lesions are resectable at the time of diagnosis[90].
Intrahepatic cholangiocarcinoma: Cholangiocarcinoma accounts for 20% of primary liver tumors and arises as adenocarcinoma or papillary or mucinous carcinomas[92]. Risk factors include cirrhosis, primary sclerosing cholangitis (PSC, 10%), bile duct adenoma, choledochal cysts, biliary papillomatosis, Caroli’s disease, and liver fluke[93–95]. Jaundice is the most common clinical presentation[96], and rapidly increasing bilirubin associated with weight loss predicts cholangiocarcinoma. Tumor markers CEA, CA-19-9 or AFP might be elevated. CA 19-9 level > 100 has 89% sensitivity and 86% specificity[97]. There are three anatomic subtypes: Peripheral intrahepatic 15%, perihilar central (Klatskin tumor) 60%, and distal common bile duct 25%. Peripheral cholangiocarcinoma resembles HCC without cirrhosis. The central hilar and distal types are associated to sclerosing cholangitis, inflammatory bowel disease, or other chronic biliary disease. US and CT show marked intrahepatic duct dilatation[98]. An abrupt change in the calibre of the bile duct suggests malignancy[99]. Digital image analysis and fluorescent in situ hybridization are more sensitive than routine standard brush cytology in the diagnosis of cholangiocarcinoma. ERCP, percutaneous transhepatic cholangiography (PTC) and magnetic resonance cholangiopancreatography (MRCP) assess the resectability of the tumor.
PET CT stages these tumors with a sensitivity of 93%. The suggested screening includes US, CEA and CA 19-9 every six months, ERCP and brush cytology if there is biliary stenosis. Combined HCC-cholangiocarcinoma shows features of both hepatocellular and biliary epithelial differentiation[100].
Epithelioid hemangioendothelioma: This low-grade malignancy affects individuals between 20-80 years of age. It is associated with oral contraception and exposure to polyvinyl[101]. Epithelioid hemangioendothelioma presents with abdominal pain, hepatomegaly, low fever and normal liver enzymes. Endothelial cells stain for CD34, CD31 and factor VIII. The prognosis is good with surgical resection or liver transplantation[102,103].
Cystadenocarcinoma: Usually in the right lobe, cystadenocarcinoma is multicystic and contains bile-stained material. It presents as abdominal pain with weight loss[104] and prognosis is good[105].
Lymphomas and leukemia: Liver involvement is common in Hodgkin’s disease including lymphoma infiltration (diffuse small nodules or large masses), drugs, viral hepatitis, and sepsis. Cholestasis is uncommon and vanishing bile duct syndrome has been described[106]. The differential diagnosis includes reactive infiltrate and T-cell lymphomas.
Primary hepatic lymphoma is rare and can present as solitary or multiple masses, as a diffuse hepatic involvement with hepatomegaly, or as hepatic failure with elevated LDH[107]. Peripheral gamma delta T cell lymphomas with massive hepatic sinusoidal infiltration and splenic involvement have also been described[108]. The liver might be diffusely or locally infiltrated by multiple myelomas or leukemia (chronic lymphoid leukemia, hairy cell leukemia)[109–113].
Neuroendocrine tumors: Neuroendocrine tumors originating in the gastrointestinal tract frequently metastasize to the liver[114]. Liver metastases can be resected[115]. Traditional chemotherapy is not effective. α-interferon has been associated with tumor response. The use of somatostatin analogs in the carcinoid syndrome improves symptoms. Liver transplant remains an option[116].
Angiosarcoma: Angiosarcoma is associated with exposure to vinyl chloride[117]. Most patients are not amenable to surgery. Usually both hepatic lobes are involved and rapid tumor growth and tendency to metastasize contribute to its dismal prognosis. Chemotherapy and radiotherapy have no role, and ligation of the hepatic artery might permit palliation.
Undifferentiated sarcoma of the liver: This rare tumor mainly affects children. The clinical features are fever and a liver mass with recurrent hypoglycemia. The median survival is two months. Imaging shows a solid and cystic lesion with multiple loculi[118].
Other mesenchymal cell malignancies: Rhabdomyosarcoma is the most common tumor of the biliary tree in young children. The tumor can mimic a choledocal cyst[119].
Fibrosarcoma presents as a hepatic mass with recurrent hypoglycemia that will resolve after resection. Serum IGF-2 is elevated[120] and Leiomyosarcoma presents with general deterioration and right upper quadrant pain. Angiograms or CT-angiography show a hypervascular tumor. Liver transplantation is possible[121].
LIVER BIOPSY VERSUS LIVER MASS RESECTION
Before hepatic resection, lesions should be measured, counted and localized to the Couinaud segments. Their relationship to major anatomical structures (portal vein, hepatic artery, inferior vena cava, and hepatic vein) should be detailed[122–125]. If malignancy is obvious, biopsy should be avoided because of possible dissemination[30,44,126]. Liver histology by true cut needle biopsy is much more profitable than fine needle aspiration and cytological examination but has several disadvantages. If the tumor is small (< 3 cm), a second attempt should be made in 20% of cases[127], bleeding is mild in 1% and severe in 0.1%. In 10% of cases, a firm diagnosis is not established and resection should be performed.
The Child Pugh score helps select which patients should undergo hepatic resection[128]. Survival depends on the regenerative potential and the presence of cirrhosis[129]. Traditionally, cirrhosis is a contraindication to hepatic resection because of the high mortality rate (20%). A dilemma arises when patients with cirrhosis require a hepatic resection. The problem is that 10%-20% of patients with cirrhosis have primary hepatic malignancy. Moreover, 80%-90% of patients with HCC and 10%-20% of patients with cholangiocarcinoma have cirrhosis. The operative mortality of extensive hepatic resection in patients without cirrhosis is 10%[130].
Treatment modalities include radiofrequency ablation (RFA), percutaneous ethanol injection, cryoablation, hepatic arterial chemoembolisation (TACE), and laparoscopic liver resection. Patients with compensated cirrhosis might benefit from liver resection, RFA or TACE, but patients with decompensated cirrhosis would probably experience no survival benefit[131]. In highly selected patients with incidental, central or multifocal tumors, hepatic transplantation might be more beneficial.
CONCLUSION
In the diagnostic strategy of liver masses, two scenarios are examined: (1) incidentally discovered solid lesions or masses in a cirrhotic patient. The most likely diagnosis is HCC, followed by high and low-grade dysplastic nodule. Lesions > 2 cm are diagnosed by imaging techniques, lesions of 1-2 cm require histology if imaging modalities are atypical, and lesions < 1 cm require US follow-up every three months; (2) incidentally discovered solid lesions or masses in a non cirrhotic patient. The most prevalent lesion is hemangioma. FNH and adenoma should be ruled out in young women with contraceptive treatment. If the lesion is found at staging or follow up of a known primary malignancy, histology is required when the lesion is doubtful. The most common liver metastases are from adenocarcinoma of colon, stomach, lung, prostate, or breast.
Footnotes
Peer reviewers: Raffaele Pezzilli, MD, Department of Internal Medicine and Gastroenterology, Sant’Orsola-Malpighi Hospital, Via Massarenti, 9, Bologna 40138, Italy; Paul E Sijens, PhD, Associate Professor, Radiology, UMCG, Hanzeplein 1, 9713GZ Groningen, The Netherlands
S- Editor Tian L L- Editor Stewart GJ E- Editor Lin YP
References
- 1.Giannitrapani L, Soresi M, La Spada E, Cervello M, D’Alessandro N, Montalto G. Sex hormones and risk of liver tumor. Ann N Y Acad Sci. 2006;1089:228–236. doi: 10.1196/annals.1386.044. [DOI] [PubMed] [Google Scholar]
- 2.Dogliotti E. Molecular mechanisms of carcinogenesis by vinyl chloride. Ann Ist Super Sanita. 2006;42:163–169. [PubMed] [Google Scholar]
- 3.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 4.Borentain P, Gérolami R, Dodero F, Chrestian MA, Quillichini F, Ardissone J, Perrimond H, Chamlian A, Gérolami A. [High serum alkaline phosphatase level revealing a liver adenoma] Gastroenterol Clin Biol. 2006;30:304–306. doi: 10.1016/s0399-8320(06)73170-0. [DOI] [PubMed] [Google Scholar]
- 5.Coakley FV, Schwartz LH. Imaging of hepatocellular carcinoma: a practical approach. Semin Oncol. 2001;28:460–473. doi: 10.1016/s0093-7754(01)90139-3. [DOI] [PubMed] [Google Scholar]
- 6.Karhunen PJ, Penttilä A, Liesto K, Männikkö A, Möttönen MM. Occurrence of benign hepatocellular tumors in alcoholic men. Acta Pathol Microbiol Immunol Scand A. 1986;94:141–147. doi: 10.1111/j.1699-0463.1986.tb02976.x. [DOI] [PubMed] [Google Scholar]
- 7.Hussain SM, Semelka RC. Liver masses. Magn Reson Imaging Clin N Am. 2005;13:255–275. doi: 10.1016/j.mric.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Seitz G. [Why are metastases in cirrhotic livers so rare?] Ultraschall Med. 1989;10:123–126. doi: 10.1055/s-2007-1005976. [DOI] [PubMed] [Google Scholar]
- 9.Hussain SM, Terkivatan T, Zondervan PE, Lanjouw E, de Rave S, Ijzermans JN, de Man RA. Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics. 2004;24:3–17; discussion 18-19. doi: 10.1148/rg.241035050. [DOI] [PubMed] [Google Scholar]
- 10.Okuda K. Early recognition of hepatocellular carcinoma. Hepatology. 1986;6:729–738. doi: 10.1002/hep.1840060432. [DOI] [PubMed] [Google Scholar]
- 11.Sherman M. Alphafetoprotein: an obituary. J Hepatol. 2001;34:603–605. doi: 10.1016/s0168-8278(01)00025-3. [DOI] [PubMed] [Google Scholar]
- 12.Ramage JK, Donaghy A, Farrant JM, Iorns R, Williams R. Serum tumor markers for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastroenterology. 1995;108:865–869. doi: 10.1016/0016-5085(95)90462-x. [DOI] [PubMed] [Google Scholar]
- 13.Caturelli E, Solmi L, Anti M, Fusilli S, Roselli P, Andriulli A, Fornari F, Del Vecchio Blanco C, de Sio I. Ultrasound guided fine needle biopsy of early hepatocellular carcinoma complicating liver cirrhosis: a multicentre study. Gut. 2004;53:1356–1362. doi: 10.1136/gut.2003.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsao JI, DeSanctis J, Rossi RL, Oberfield RA. Hepatic malignancies. Surg Clin North Am. 2000;80:603–632. doi: 10.1016/s0039-6109(05)70203-6. [DOI] [PubMed] [Google Scholar]
- 15.Balci NC, Befeler AS, Leiva P, Pilgram TK, Havlioglu N. Imaging of liver disease: comparison between quadruple-phase multidetector computed tomography and magnetic resonance imaging. J Gastroenterol Hepatol. 2008;23:1520–1527. doi: 10.1111/j.1440-1746.2008.05434.x. [DOI] [PubMed] [Google Scholar]
- 16.Murakami T, Oi H, Hori M, Kim T, Takahashi S, Tomoda K, Narumi Y, Nakamura H. Helical CT during arterial portography and hepatic arteriography for detecting hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 1997;169:131–135. doi: 10.2214/ajr.169.1.9207512. [DOI] [PubMed] [Google Scholar]
- 17.Hori M, Murakami T, Kim T, Takahashi S, Oi H, Tomoda K, Narumi Y, Nakamura H. Sensitivity of double-phase helical CT during arterial portography for detection of hypervascular hepatocellular carcinoma. J Comput Assist Tomogr. 1998;22:861–867. doi: 10.1097/00004728-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 18.van Kouwen MC, Oyen WJ, Nagengast FM, Jansen JB, Drenth JP. FDG-PET scanning in the diagnosis of gastrointestinal cancers. Scand J Gastroenterol Suppl. 2004;22:85–92. doi: 10.1080/00855920410014614. [DOI] [PubMed] [Google Scholar]
- 19.Kinnard MF, Alavi A, Rubin RA, Lichtenstein GR. Nuclear imaging of solid hepatic masses. Semin Roentgenol. 1995;30:375–395. doi: 10.1016/s0037-198x(05)80024-0. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Lázaro R, Domínguez P, Pascau J, Bittini A, Lafuente J, Desco M. Usefulness of Tc-99m RBC SPECT/MRI fusion imaging in small suspected hepatic hemangiomas. Clin Nucl Med. 2004;29:844–845. doi: 10.1097/00003072-200412000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Kanematsu M, Hoshi H, Yamada T, Murakami T, Kim T, Kato M, Yokoyama R, Nakamura H. Small hepatic nodules in cirrhosis: ultrasonographic, CT, and MR imaging findings. Abdom Imaging. 1999;24:47–55. doi: 10.1007/s002619900439. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi SN, Brown MA, Wong JG, Aguirre DA, Sirlin CB. MR contrast agents for liver imaging: what, when, how. Radiographics. 2006;26:1621–1636. doi: 10.1148/rg.266065014. [DOI] [PubMed] [Google Scholar]
- 23.Solbiati L, Tonolini M, Cova L, Goldberg SN. The role of contrast-enhanced ultrasound in the detection of focal liver leasions. Eur Radiol. 2001;11 Suppl 3:E15–E26. doi: 10.1007/pl00014125. [DOI] [PubMed] [Google Scholar]
- 24.Torzilli G, Makuuchi M, Ferrero A, Takayama T, Hui AM, Abe H, Inoue K, Nakahara K. Accuracy of the preoperative determination of tumor markers in the differentiation of liver mass lesions in surgical patients. Hepatogastroenterology. 2002;49:740–745. [PubMed] [Google Scholar]
- 25.França AV, Valério HM, Trevisan M, Escanhoela C, Sevá-Pereira T, Zucoloto S, Martinelli A, Soares EC. Fine needle aspiration biopsy for improving the diagnostic accuracy of cut needle biopsy of focal liver lesions. Acta Cytol. 2003;47:332–336. doi: 10.1159/000326529. [DOI] [PubMed] [Google Scholar]
- 26.Borzio M, Borzio F, Macchi R, Croce AM, Bruno S, Ferrari A, Servida E. The evaluation of fine-needle procedures for the diagnosis of focal liver lesions in cirrhosis. J Hepatol. 1994;20:117–121. doi: 10.1016/s0168-8278(05)80477-5. [DOI] [PubMed] [Google Scholar]
- 27.Buscarini L, Fornari F, Bolondi L, Colombo P, Livraghi T, Magnolfi F, Rapaccini GL, Salmi A. Ultrasound-guided fine-needle biopsy of focal liver lesions: techniques, diagnostic accuracy and complications. A retrospective study on 2091 biopsies. J Hepatol. 1990;11:344–348. doi: 10.1016/0168-8278(90)90219-h. [DOI] [PubMed] [Google Scholar]
- 28.Chawla YK, Ramesh GN, Kaur U, Bambery P, Dilawari JB. Percutaneous liver biopsy: a safe outpatient procedure. J Gastroenterol Hepatol. 1990;5:94–95. doi: 10.1111/j.1440-1746.1990.tb01770.x. [DOI] [PubMed] [Google Scholar]
- 29.Fornari F, Civardi G, Cavanna L, Di Stasi M, Rossi S, Sbolli G, Buscarini L. Complications of ultrasonically guided fine-needle abdominal biopsy. Results of a multicenter Italian study and review of the literature. The Cooperative Italian Study Group. Scand J Gastroenterol. 1989;24:949–955. doi: 10.3109/00365528909089239. [DOI] [PubMed] [Google Scholar]
- 30.Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology. 1991;178:253–258. doi: 10.1148/radiology.178.1.1984314. [DOI] [PubMed] [Google Scholar]
- 31.Edoute Y, Malberger E, Tibon-Fishe O, Assy N. Non-imaging-guided fine-needle aspiration of liver lesions: a retrospective study of 279 patients. World J Gastroenterol. 1999;5:98–102. doi: 10.3748/wjg.v5.i2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen MB, Haber MM, Holly EA, Ahn DK, Bottles K, Stoloff AC. Cytologic criteria to distinguish hepatocellular carcinoma from nonneoplastic liver. Am J Clin Pathol. 1991;95:125–130. doi: 10.1093/ajcp/95.2.125. [DOI] [PubMed] [Google Scholar]
- 33.Durand F, Regimbeau JM, Belghiti J, Sauvanet A, Vilgrain V, Terris B, Moutardier V, Farges O, Valla D. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol. 2001;35:254–258. doi: 10.1016/s0168-8278(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 34.Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52 Suppl 3:iii1–iii8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caturelli E, Biasini E, Bartolucci F, Facciorusso D, Decembrino F, Attino V, Bisceglia M. Diagnosis of hepatocellular carcinoma complicating liver cirrhosis: utility of repeat ultrasound-guided biopsy after unsuccessful first sampling. Cardiovasc Intervent Radiol. 2002;25:295–299. doi: 10.1007/s00270-001-0123-6. [DOI] [PubMed] [Google Scholar]
- 36.Torzilli G, Minagawa M, Takayama T, Inoue K, Hui AM, Kubota K, Ohtomo K, Makuuchi M. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–893. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 37.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 38.Rapaccini GL, Pompili M, Caturelli E, Anti M, Aliotta A, Cedrone A, Amadei E, Grattagliano A, Squillante MM, Rabitti C. Focal ultrasound lesions in liver cirrhosis diagnosed as regenerating nodules by fine-needle biopsy. Follow-up of 12 cases. Dig Dis Sci. 1990;35:422–427. doi: 10.1007/BF01536913. [DOI] [PubMed] [Google Scholar]
- 39.Cedrone A, Covino M, Caturelli E, Pompili M, Lorenzelli G, Villani MR, Valle D, Sperandeo M, Rapaccini GL, Gasbarrini G. Utility of alpha-fetoprotein (AFP) in the screening of patients with virus-related chronic liver disease: does different viral etiology influence AFP levels in HCC? A study in 350 western patients. Hepatogastroenterology. 2000;47:1654–1658. [PubMed] [Google Scholar]
- 40.Sherman M. Screening for hepatocellular carcinoma. Hepatol Res. 2007;37 Suppl 2:S152–S165. doi: 10.1111/j.1872-034X.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- 41.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 42.Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, Rigamonti A, Barbara C, Grigioni W, Mazziotti A. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16:132–137. doi: 10.1002/hep.1840160122. [DOI] [PubMed] [Google Scholar]
- 43.Simonetti RG, Cammà C, Fiorello F, Politi F, D’Amico G, Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci. 1991;36:962–972. doi: 10.1007/BF01297149. [DOI] [PubMed] [Google Scholar]
- 44.Souto E, Gores GJ. When should a liver mass suspected of being a hepatocellular carcinoma be biopsied? Liver Transpl. 2000;6:73–75. doi: 10.1002/lt.500060108. [DOI] [PubMed] [Google Scholar]
- 45.Mor E, Kaspa RT, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med. 1998;129:643–653. doi: 10.7326/0003-4819-129-8-199810150-00013. [DOI] [PubMed] [Google Scholar]
- 46.Reddy KR, Schiff ER. Approach to a liver mass. Semin Liver Dis. 1993;13:423–435. doi: 10.1055/s-2007-1007370. [DOI] [PubMed] [Google Scholar]
- 47.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M, Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251–259. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng YC, Chan CS, Chen GH. The effectiveness of serum alpha-fetoprotein level in anti-HCV positive patients for screening hepatocellular carcinoma. Hepatogastroenterology. 1999;46:3208–3211. [PubMed] [Google Scholar]
- 49.Nakashima T, Kojiro M. Pathologic characteristics of hepatocellular carcinoma. Semin Liver Dis. 1986;6:259–266. doi: 10.1055/s-2008-1040608. [DOI] [PubMed] [Google Scholar]
- 50.Terminology of nodular hepatocellular lesions. International Working Party. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 51.Hussain SM, Zondervan PE, IJzermans JN, Schalm SW, de Man RA, Krestin GP. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002;22:1023–1036; discussion 1037-1039. doi: 10.1148/radiographics.22.5.g02se061023. [DOI] [PubMed] [Google Scholar]
- 52.Craig JR. Fibrolamellar carcinoma: Clinical and pathologic features. In: Okuda K, Tabor E, editors. Liver Cancer. New York: Churchill Livingstone; 1997. pp. 255–262. [Google Scholar]
- 53.Melato M, Laurino L, Mucli E, Valente M, Okuda K. Relationship between cirrhosis, liver cancer, and hepatic metastases. An autopsy study. Cancer. 1989;64:455–459. doi: 10.1002/1097-0142(19890715)64:2<455::aid-cncr2820640219>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 54.Johnson PJ. Role of alpha-fetoprotein in the diagnosis and management of hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14 Suppl:S32–S36. doi: 10.1046/j.1440-1746.1999.01873.x. [DOI] [PubMed] [Google Scholar]
- 55.Wilson SR. Imaging of benign hepatic lesions. In: Freeny PC, editor. Radiology of the liver, biliary tree and pancreas. Reston: American Roentgen Ray Society; 1996. pp. 17–25. [Google Scholar]
- 56.Takagi H. Diagnosis and management of cavernous hemangioma of the liver. Semin Surg Oncol. 1985;1:12–22. doi: 10.1002/ssu.2980010104. [DOI] [PubMed] [Google Scholar]
- 57.Cherqui D, Rahmouni A, Charlotte F, Boulahdour H, Métreau JM, Meignan M, Fagniez PL, Zafrani ES, Mathieu D, Dhumeaux D. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology. 1995;22:1674–1681. [PubMed] [Google Scholar]
- 58.Trastek VF, van Heerden JA, Sheedy PF 2nd, Adson MA. Cavernous hemangiomas of the liver: resect or observe? Am J Surg. 1983;145:49–53. doi: 10.1016/0002-9610(83)90165-4. [DOI] [PubMed] [Google Scholar]
- 59.Stark DD, Felder RC, Wittenberg J, Saini S, Butch RJ, White ME, Edelman RR, Mueller PR, Simeone JF, Cohen AM. Magnetic resonance imaging of cavernous hemangioma of the liver: tissue-specific characterization. AJR Am J Roentgenol. 1985;145:213–222. doi: 10.2214/ajr.145.2.213. [DOI] [PubMed] [Google Scholar]
- 60.Sigal R, Lanir A, Atlan H, Naschitz JE, Simon JS, Enat R, Front D, Israel O, Chisin R, Krausz Y. Nuclear magnetic resonance imaging of liver hemangiomas. J Nucl Med. 1985;26:1117–1122. [PubMed] [Google Scholar]
- 61.Sewell JH, Weiss K. Spontaneous rupture of hemangioma of the liver. A review of the literature and presentation of illustrative case. Arch Surg. 1961;83:729–733. doi: 10.1001/archsurg.1961.01300170085016. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto M, Ariizumi S, Yoshitoshi K, Saito A, Nakano M, Takasaki K. Hepatocellular carcinoma with a central scar and a scalloped tumor margin resembling focal nodular hyperplasia in macroscopic appearance. J Surg Oncol. 2006;94:587–591. doi: 10.1002/jso.20615. [DOI] [PubMed] [Google Scholar]
- 63.Lizardi-Cervera J, Cuéllar-Gamboa L, Motola-Kuba D. Focal nodular hyperplasia and hepatic adenoma: a review. Ann Hepatol. 2006;5:206–211. [PubMed] [Google Scholar]
- 64.Rogers JV, Mack LA, Freeny PC, Johnson ML, Sones PJ. Hepatic focal nodular hyperplasia: angiography, CT, sonography, and scintigraphy. AJR Am J Roentgenol. 1981;137:983–990. doi: 10.2214/ajr.137.5.983. [DOI] [PubMed] [Google Scholar]
- 65.Rabe T, Feldmann K, Grunwald K, Runnebaum B. Liver tumours in women on oral contraceptives. Lancet. 1994;344:1568–1569. doi: 10.1016/s0140-6736(94)90374-3. [DOI] [PubMed] [Google Scholar]
- 66.Labrune P, Trioche P, Duvaltier I, Chevalier P, Odièvre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997;24:276–279. doi: 10.1097/00005176-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001;21:877–892; discussion 892-894. doi: 10.1148/radiographics.21.4.g01jl04877. [DOI] [PubMed] [Google Scholar]
- 68.Erdogan D, Busch OR, van Delden OM, Ten Kate FJ, Gouma DJ, van Gulik TM. Management of spontaneous haemorrhage and rupture of hepatocellular adenomas. A single centre experience. Liver Int. 2006;26:433–438. doi: 10.1111/j.1478-3231.2006.01244.x. [DOI] [PubMed] [Google Scholar]
- 69.Mathieu D, Bruneton JN, Drouillard J, Pointreau CC, Vasile N. Hepatic adenomas and focal nodular hyperplasia: dynamic CT study. Radiology. 1986;160:53–58. doi: 10.1148/radiology.160.1.3520655. [DOI] [PubMed] [Google Scholar]
- 70.Kume N, Suga K, Nishigauchi K, Shimizu K, Matsunaga N. Characterization of hepatic adenoma with atypical appearance on CT and MRI by radionuclide imaging. Clin Nucl Med. 1997;22:825–831. doi: 10.1097/00003072-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Colli A, Fraquelli M, Massironi S, Colucci A, Paggi S, Conte D. Elective surgery for benign liver tumours. Cochrane Database Syst Rev. 2007;22:CD005164. doi: 10.1002/14651858.CD005164.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kerlin P, Davis GL, McGill DB, Weiland LH, Adson MA, Sheedy PF 2nd. Hepatic adenoma and focal nodular hyperplasia: clinical, pathologic, and radiologic features. Gastroenterology. 1983;84:994–1002. [PubMed] [Google Scholar]
- 73.Demarco MP, Shen P, Bradley RF, Levine EA. Intraperitoneal hemorrhage in a patient with hepatic focal nodular hyperplasia. Am Surg. 2006;72:555–559. [PubMed] [Google Scholar]
- 74.Al-Mukhaizeem KA, Rosenberg A, Sherker AH. Nodular regenerative hyperplasia of the liver: an under-recognized cause of portal hypertension in hematological disorders. Am J Hematol. 2004;75:225–230. doi: 10.1002/ajh.20024. [DOI] [PubMed] [Google Scholar]
- 75.Trenschel GM, Schubert A, Dries V, Benz-Bohm G. Nodular regenerative hyperplasia of the liver: case report of a 13-year-old girl and review of the literature. Pediatr Radiol. 2000;30:64–68. doi: 10.1007/s002470050016. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka M, Wanless IR. Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology. 1998;27:488–496. doi: 10.1002/hep.510270224. [DOI] [PubMed] [Google Scholar]
- 77.Perlemuter G, Bigorgne A, Cassard-Doulcier AM, Naveau S. Nonalcoholic fatty liver disease: from pathogenesis to patient care. Nat Clin Pract Endocrinol Metab. 2007;3:458–469. doi: 10.1038/ncpendmet0505. [DOI] [PubMed] [Google Scholar]
- 78.Karcaaltincaba M, Akhan O. Imaging of hepatic steatosis and fatty sparing. Eur J Radiol. 2007;61:33–43. doi: 10.1016/j.ejrad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Wheeler DA, Edmondson HA. Cystadenoma with mesenchymal stroma (CMS) in the liver and bile ducts. A clinicopathologic study of 17 cases, 4 with malignant change. Cancer. 1985;56:1434–1445. doi: 10.1002/1097-0142(19850915)56:6<1434::aid-cncr2820560635>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 80.Lam CM, Yuen ST, Yuen WK, Fan ST. Biliary papillomatosis. Br J Surg. 1996;83:1715–1716. doi: 10.1002/bjs.1800831218. [DOI] [PubMed] [Google Scholar]
- 81.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Tamura S, Kihara Y, Yuki Y, Sugimura H, Shimizu T, Adjei ON, Watanabe K. Pseudo lesions on CTAP secondary to arterio-portal shunts. Clin Imaging. 1997;21:359–365. doi: 10.1016/s0899-7071(96)00088-5. [DOI] [PubMed] [Google Scholar]
- 83.Schwartz L, Brody L, Brown K, Covey A, Tuorto S, Mazumdar M, Riedel E, Jarnagin W, Getrajdman G, Fong Y. Prospective, blinded comparison of helical CT and CT arterial portography in the assessment of hepatic metastasis from colorectal carcinoma. World J Surg. 2006;30:1892–1899; discussion 1900-1901. doi: 10.1007/s00268-005-0483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leen E, Ceccotti P, Moug SJ, Glen P, MacQuarrie J, Angerson WJ, Albrecht T, Hohmann J, Oldenburg A, Ritz JP, et al. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection? Ann Surg. 2006;243:236–240. doi: 10.1097/01.sla.0000197708.77063.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagaoka S, Itano S, Ishibashi M, Torimura T, Baba K, Akiyoshi J, Kurogi J, Matsugaki S, Inoue K, Tajiri N, et al. Value of fusing PET plus CT images in hepatocellular carcinoma and combined hepatocellular and cholangiocarcinoma patients with extrahepatic metastases: preliminary findings. Liver Int. 2006;26:781–788. doi: 10.1111/j.1478-3231.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 86.Schillaci O. Somatostatin receptor imaging in patients with neuroendocrine tumors: not only SPECT? J Nucl Med. 2007;48:498–500. doi: 10.2967/jnumed.106.038653. [DOI] [PubMed] [Google Scholar]
- 87.Seemann MD, Meisetschlaeger G, Gaa J, Rummeny EJ. Assessment of the extent of metastases of gastrointestinal carcinoid tumors using whole-body PET, CT, MRI, PET/CT and PET/MRI. Eur J Med Res. 2006;11:58–65. [PubMed] [Google Scholar]
- 88.Wee A. Fine needle aspiration biopsy of the liver: Algorithmic approach and current issues in the diagnosis of hepatocellular carcinoma. Cytojournal. 2005;2:7. doi: 10.1186/1742-6413-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soreide O, Czerniak A, Bradpiece H, Bloom S, Blumgart L. Characteristics of fibrolamellar hepatocellular carcinoma. A study of nine cases and a review of the literature. Am J Surg. 1986;151:518–523. doi: 10.1016/0002-9610(86)90117-0. [DOI] [PubMed] [Google Scholar]
- 90.Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer. 1980;46:372–379. doi: 10.1002/1097-0142(19800715)46:2<372::aid-cncr2820460227>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 91.Brandt DJ, Johnson CD, Stephens DH, Weiland LH. Imaging of fibrolamellar hepatocellular carcinoma. AJR Am J Roentgenol. 1988;151:295–299. doi: 10.2214/ajr.151.2.295. [DOI] [PubMed] [Google Scholar]
- 92.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 93.Callea F, Sergi C, Fabbretti G, Brisigotti M, Cozzutto C, Medicina D. Precancerous lesions of the biliary tree. J Surg Oncol Suppl. 1993;3:131–133. doi: 10.1002/jso.2930530535. [DOI] [PubMed] [Google Scholar]
- 94.Watanapa P. Cholangiocarcinoma in patients with opisthorchiasis. Br J Surg. 1996;83:1062–1064. doi: 10.1002/bjs.1800830809. [DOI] [PubMed] [Google Scholar]
- 95.Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, Ahn YO, Shigemastu T. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25:933–940. doi: 10.1093/ije/25.5.933. [DOI] [PubMed] [Google Scholar]
- 96.Suzuki M, Takahashi T, Ouchi K, Matsuno S. The development and extension of hepatohilar bile duct carcinoma. A three-dimensional tumor mapping in the intrahepatic biliary tree visualized with the aid of a graphics computer system. Cancer. 1989;64:658–666. doi: 10.1002/1097-0142(19890801)64:3<658::aid-cncr2820640316>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 97.Nichols JC, Gores GJ, LaRusso NF, Wiesner RH, Nagorney DM, Ritts RE Jr. Diagnostic role of serum CA 19-9 for cholangiocarcinoma in patients with primary sclerosing cholangitis. Mayo Clin Proc. 1993;68:874–879. doi: 10.1016/s0025-6196(12)60696-x. [DOI] [PubMed] [Google Scholar]
- 98.Nesbit GM, Johnson CD, James EM, MacCarty RL, Nagorney DM, Bender CE. Cholangiocarcinoma: diagnosis and evaluation of resectability by CT and sonography as procedures complementary to cholangiography. AJR Am J Roentgenol. 1988;151:933–938. doi: 10.2214/ajr.151.5.933. [DOI] [PubMed] [Google Scholar]
- 99.Tillich M, Mischinger HJ, Preisegger KH, Rabl H, Szolar DH. Multiphasic helical CT in diagnosis and staging of hilar cholangiocarcinoma. AJR Am J Roentgenol. 1998;171:651–658. doi: 10.2214/ajr.171.3.9725291. [DOI] [PubMed] [Google Scholar]
- 100.Dunk AA, Spiliadis H, Sherlock S, Fowler MJ, Monjardino JP, Scheuer PJ, Thomas HC. Hepatocellular carcinoma: clinical, aetiological and pathological features in British patients. Int J Cancer. 1988;41:17–23. doi: 10.1002/ijc.2910410105. [DOI] [PubMed] [Google Scholar]
- 101.Dean PJ, Haggitt RC, O’Hara CJ. Malignant epithelioid hemangioendothelioma of the liver in young women. Relationship to oral contraceptive use. Am J Surg Pathol. 1985;9:695–704. doi: 10.1097/00000478-198510000-00001. [DOI] [PubMed] [Google Scholar]
- 102.Ashraf S, Ashraf HM, Mamoon N, Luqman M. Epithelioid hemangioendothelioma of the liver. J Coll Physicians Surg Pak. 2007;17:280–282. [PubMed] [Google Scholar]
- 103.Bufton S, Haydon G, Neil D. Liver transplantation for hepatic epithelioid hemangioendothelioma: a case series. Prog Transplant. 2007;17:70–72. doi: 10.1177/152692480701700112. [DOI] [PubMed] [Google Scholar]
- 104.Teoh AY, Ng SS, Lee KF, Lai PB. Biliary cystadenoma and other complicated cystic lesions of the liver: diagnostic and therapeutic challenges. World J Surg. 2006;30:1560–1566. doi: 10.1007/s00268-005-0461-7. [DOI] [PubMed] [Google Scholar]
- 105.Hai S, Hirohashi K, Uenishi T, Yamamoto T, Shuto T, Tanaka H, Kubo S, Tanaka S, Kinoshita H. Surgical management of cystic hepatic neoplasms. J Gastroenterol. 2003;38:759–764. doi: 10.1007/s00535-003-1142-7. [DOI] [PubMed] [Google Scholar]
- 106.Schmitt A, Gilden DJ, Saint S, Moseley RH. Clinical problem-solving. Empirically incorrect. N Engl J Med. 2006;354:509–514. doi: 10.1056/NEJMcps051762. [DOI] [PubMed] [Google Scholar]
- 107.Zornoza J, Ginaldi S. Computed tomography in hepatic lymphoma. Radiology. 1981;138:405–410. doi: 10.1148/radiology.138.2.7455122. [DOI] [PubMed] [Google Scholar]
- 108.Belhadj K, Reyes F, Farcet JP, Tilly H, Bastard C, Angonin R, Deconinck E, Charlotte F, Leblond V, Labouyrie E, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102:4261–4269. doi: 10.1182/blood-2003-05-1675. [DOI] [PubMed] [Google Scholar]
- 109.Kuroda N, Mizobuchi M, Shimamura Y, Daibata M, Miyoshi I, Ohara M, Hirouchi T, Mizuno K, Lee GH. Bridging necrosis and reticulin bridging fibrosis induced by intrahepatic involvement of acute biphenotypic leukemia. APMIS. 2006;114:908–911. doi: 10.1111/j.1600-0463.2006.apm_540.x. [DOI] [PubMed] [Google Scholar]
- 110.Dellon ES, Morris SR, Tang W, Dunphy CH, Russo MW. Acute liver failure due to natural killer-like T-cell leukemia/lymphoma: a case report and review of the literature. World J Gastroenterol. 2006;12:4089–4092. doi: 10.3748/wjg.v12.i25.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Powell N, Rusli F, Hubscher SG, Karanth M, Mutimer D. Adult T-cell leukemia presenting with acute liver failure. Leuk Res. 2006;30:1315–1317. doi: 10.1016/j.leukres.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 112.Ando K, Miyazawa K, Kuriyama Y, Kimura Y, Mukai K, Ohyashiki K. Hemophagocytic syndrome associated with CD8 positive T-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2004;45:193–198. doi: 10.1080/1042819031000123537. [DOI] [PubMed] [Google Scholar]
- 113.Kraut EH. Clinical manifestations and infectious complications of hairy-cell leukaemia. Best Pract Res Clin Haematol. 2003;16:33–40. doi: 10.1016/s1521-6926(02)00085-3. [DOI] [PubMed] [Google Scholar]
- 114.Shah NA, Urusova IA, D’Agnolo A, Colquhoun SD, Rosenbloom BE, Vener SL, Geller SA, Younes M, Lechago J, Heaney AP. Primary hepatic carcinoid tumor presenting as Cushing’s syndrome. J Endocrinol Invest. 2007;30:327–333. doi: 10.1007/BF03346308. [DOI] [PubMed] [Google Scholar]
- 115.Garrot C, Stuart K. Liver-directed therapies for metastatic neuroendocrine tumors. Hematol Oncol Clin North Am. 2007;21:545–560; ix-x. doi: 10.1016/j.hoc.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 116.Kulke M. Advances in the treatment of neuroendocrine tumors. Curr Treat Options Oncol. 2005;6:397–409. doi: 10.1007/s11864-005-0043-9. [DOI] [PubMed] [Google Scholar]
- 117.Locker GY, Doroshow JH, Zwelling LA, Chabner BA. The clinical features of hepatic angiosarcoma: a report of four cases and a review of the English literature. Medicine (Baltimore) 1979;58:48–64. doi: 10.1097/00005792-197901000-00003. [DOI] [PubMed] [Google Scholar]
- 118.Lao XM, Chen DY, Zhang YQ, Xiang J, Guo RP, Lin XJ, Li JQ. Primary carcinosarcoma of the liver: clinicopathologic features of 5 cases and a review of the literature. Am J Surg Pathol. 2007;31:817–826. doi: 10.1097/01.pas.0000213431.07116.e0. [DOI] [PubMed] [Google Scholar]
- 119.Zampieri N, Camoglio F, Corroppolo M, Cecchetto M, Ornis S, Ottolenghi A. Botryoid rhabdomyosarcoma of the biliary tract in children: a unique case report. Eur J Cancer Care (Engl) 2006;15:463–466. doi: 10.1111/j.1365-2354.2006.00683.x. [DOI] [PubMed] [Google Scholar]
- 120.Chan G, Horton PJ, Thyssen S, Lamarche M, Nahal A, Hill DJ, Marliss EB, Metrakos P. Malignant transformation of a solitary fibrous tumor of the liver and intractable hypoglycemia. J Hepatobiliary Pancreat Surg. 2007;14:595–599. doi: 10.1007/s00534-007-1210-0. [DOI] [PubMed] [Google Scholar]
- 121.Saint-Paul MC, Gugenheim J, Hofman P, Arpurt JP, Fabiani P, Michiels JF, Fujita N, Goubeaux B, Loubière R, Delmont J. [Leiomyosarcoma of the liver: a case treated by transplantation] Gastroenterol Clin Biol. 1993;17:218–222. [PubMed] [Google Scholar]
- 122.Heiken JP. Liver. In: Lee JKT, Sagel SS, Stanley RJ, Heiken JP, editors. Computed Body Tomography with MRI Correlation. Philadelphia: Lippincott-Raven; 1998. pp. 701–779. [Google Scholar]
- 123.Harned RK 2nd, Chezmar JL, Nelson RC. Imaging of patients with potentially resectable hepatic neoplasms. AJR Am J Roentgenol. 1992;159:1191–1194. doi: 10.2214/ajr.159.6.1442380. [DOI] [PubMed] [Google Scholar]
- 124.Small WC, Mehard WB, Langmo LS, Dagher AP, Fishman EK, Heiken JP, Bernardino ME. Preoperative determination of the resectability of hepatic tumors: efficacy of CT during arterial portography. AJR Am J Roentgenol. 1993;161:319–322. doi: 10.2214/ajr.161.2.8333369. [DOI] [PubMed] [Google Scholar]
- 125.Heyneman LE, Nelson RC. Modality for imaging liver tumors. In: Clavien PA, editor. Malignant Liver Tumors: Current and Emerging Therapies. Malden: Blackwell Science; 1999. pp. 46–62. [Google Scholar]
- 126.Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl. 2000;6:67–72. doi: 10.1002/lt.500060103. [DOI] [PubMed] [Google Scholar]
- 127.Francque SM, De Pauw FF, Van den Steen GH, Van Marck EA, Pelckmans PA, Michielsen PP. Biopsy of focal liver lesions: guidelines, comparison of techniques and cost-analysis. Acta Gastroenterol Belg. 2003;66:160–165. [PubMed] [Google Scholar]
- 128.Assy N, Pruzansky Y, Gaitini D, Shen Orr Z, Hochberg Z, Baruch Y. Growth hormone-stimulated IGF-1 generation in cirrhosis reflects hepatocellular dysfunction. J Hepatol. 2008;49:34–42. doi: 10.1016/j.jhep.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 129.Assy N, Hochberg Z, Amit T, Shen-Orr Z, Enat R, Baruch Y. Growth hormone-stimulated insulin-like growth factor (IGF) I and IGF-binding protein-3 in liver cirrhosis. J Hepatol. 1997;27:796–802. doi: 10.1016/s0168-8278(97)80315-7. [DOI] [PubMed] [Google Scholar]
- 130.Bouwman DL, Walt AJ. Current status of resection for hepatic neoplasms. Semin Liver Dis. 1983;3:193–202. doi: 10.1055/s-2008-1040685. [DOI] [PubMed] [Google Scholar]
- 131.Frezza EE. Therapeutic management algorithm in cirrhotic and noncirrhotic patients in primary or secondary liver masses. Dig Dis Sci. 2004;49:866–871. doi: 10.1023/b:ddas.0000030101.81734.47. [DOI] [PubMed] [Google Scholar]