Abstract
Background Highly pathogenic avian influenza (HPAI) outbreaks in domestic poultry bring humans into close contact with new influenza subtypes and represent a threat to human health. In 1999, an HPAI outbreak of H7N1 virus occurred in domestic poultry in Italy, and a wild‐type virus isolate from this outbreak was chosen as a pandemic vaccine candidate.
Objectives We conducted a pilot study to investigate the kinetics of the humoral immune response induced after immunisation with an egg grown whole inactivated H7N1 virus vaccine in BALB/c mice.
Methods Mice were vaccinated with one or two doses of H7N1 vaccine (15 μg total protein) to investigate the influenza specific antibody secreting cell (IS‐ASC) and serum antibody responses.
Results After the first dose of vaccine, only IgM IS‐ASC were detected in the spleen and bone marrow, whereas IgG, IgA and IgM IS‐ASC were found after the second dose. Low antibody titres were detected after the first immunisation, whilst the second dose of vaccine significantly boosted the HI (range 128–512), neutralising and IgG antibody titres. The IgG subclass response was dominated by IgG2a indicating a dominant Th1 response after the first vaccination, whereas a more mixed Th1/Th2 profile was observed after the second dose.
Conclusions This pilot study shows the value of using a number of immunological methods to evaluate the quality of the immune response to potential pandemic candidate vaccines.
Keywords: Influenza H7N1, whole virus vaccine, mice
Introduction
Aquatic birds are the natural reservoir for all known influenza A subtypes, and constitute a risk factor for the introduction of these new subtypes into man. Recently, the urgency for development of pandemic influenza vaccines has been highlighted by a number of avian influenza A subtypes (H5, H7 and H9) crossing the species barrier and infecting humans resulting in illness and death (reviewed in Ref.1). Two of these influenza A subtypes (H5 and H7) exist as low pathogenic (LPAI) and highly pathogenic avian influenza (HPAI) viruses. Highly pathogenic avian influenza outbreaks represent a potential risk to human health, as demonstrated by 403 human cases of H5N1 that have resulted in 254 deaths mainly in South‐East Asia up to January 2009. 2 However, in Europe and North America most HPAI outbreaks are caused by the H7 subtype and this has resulted in conjunctivitis, illness and one known death. 3 , 4 , 5 , 6
Parenterally administered inactivated influenza vaccines are the main method of prophylaxis and current vaccines are conventionally produced in embryonated hens’ eggs. These vaccines have been used for more than half a century, and their quality, safety and efficacy is extensively documented (reviewed in Ref.7). Most pandemic vaccine development has focused on the H5 subtype, and although the World Health Organisation also recognises H7 as a threat 8 there are only a few candidate H7 vaccine strains available for human vaccine production. 9 , 10 , 11 During the course of a LPAI H7N1 virus outbreak in domestic poultry in Italy, a HPAI variant of this virus emerged, which caused widespread disease with mortality close to 100%. 12 In this study, an HPAI H7N1 virus from this poultry outbreak was chosen as a pandemic H7 vaccine candidate and an egg grown inactivated influenza whole virus vaccine produced. The immunogenicity and the kinetics of the humoral immune response elicited to this H7N1 vaccine were investigated in a murine model.
Materials and methods
Vaccine preparation
An egg grown inactivated H7N1 whole virus vaccine was prepared as described below. A HPAI A/chicken/Italy/13474/99 (H7N1) virus, which was lethal to poultry in an Italian outbreak 12 and in a murine model, 11 was chosen as a candidate vaccine virus (kindly supplied by Dr L Campitelli, Italy). Ten day old embryonated hens’ eggs were inoculated into the allantoic cavity and incubated for 30 hours at 37°C. Allantoic fluid was harvested and clarified by centrifugation. The allantoic fluid was treated with β‐ propiolactone (Sigma, UK) to inactivate the virus. A whole virus vaccine was prepared by concentration of virus from allantoic fluid by ultracentrifugation followed by purification on a 10–40% sucrose gradient. 13 The total virus protein was quantified by the method of Lowry and adjusted to 10 mg/ml and stored in aliquots at −70°C. At the present time there are no homologous reagents available for quantifying the amount of haemagglutinin (HA) in the vaccine by single radial immunodiffusion. A total protein concentration of 15 μg was used to immunise the mice in this study, this is approximately 41/(3.75 μg HA) lower than the normal human dose of 15 μg of HA of each of the three seasonal strains in the current trivalent vaccines.
Mice
Six‐week‐old female BALB/c mice (Taconic M&B A/S, Ry, Denmark) were housed according to the Norwegian Animal Welfare Act, at a temperature of 21°C, with 12 hour light/dark cycles and food and water ad libitum.
Mice were immunised with 1 or 2 doses, at 3 week intervals, of 15 μg total protein H7N1 whole virus vaccine intramuscularly into the quadricep muscles of both hind legs (50 μl per leg). Groups of four animals were sacrificed at a number of days after each dose of vaccine (0, 3 and 5 days after first dose and 5, 7 and 21 days after the second vaccination), the time points of which were established in a previous study. 14 Mice were exsanguinated by cardiac puncture and the sera separated and stored at −80°C. The spleen, femur and tibia bones of the hind limbs were collected and lymphocytes were harvested from each tissue for use in the enzyme linked immunospot (ELISPOT) assay. Splenic lymphocytes were separated using lymphoprep (Axis‐Shield PoC AS, Oslo, Norway) and density gradient centrifugation, whereas lymphocytes were also isolated from the bone marrow as previously described. 15 , 16
Haemagglutination inhibition test
Sera were treated to remove non‐specific inhibitors with receptor destroying enzyme (Denka Seiken, Tokyo, Japan) overnight at 37°C and then inactivated at 56°C. Haemagglutination inhibition (HI) tests were conducted using the LPAI A/turkey/Italy/3889/99 (H7N1) virus, which is antigenically similar to the HPAI A/chicken/Italy/13474/99 (H7N1), and 0·7% turkey red blood or 1% horse red blood cells. 17 The HI titres are expressed as the reciprocal of the dilution of serum need to inhibit 50% agglutination. Negative titres were assigned an arbitrary value of 4 for calculation of geometric mean titres (GMT). 18
Virus neutralisation assay
Neutralising antibody titres were determined using a previously described neutralisation assay. 14 Briefly, heat‐treated sera were diluted twofold and incubated in duplicate with 100 TCID50 influenza A/turkey/Italy/3889/99 (H7N1) virus for 1 hour at room temperature. Residual virus infectivity was tested on Madin‐Darby canine kidney (MDCK) cell monolayers prepared in 96‐well tissue culture plates at room temperature for 1 hour. After a 72 hour incubation period, the presence of replicative virus was detected by using an HA assay with 0·7% turkey red blood cells. Wells were scored for the presence of virus by 100% haemagglutination. Serum samples were tested in duplicate on at least two occasions from an initial dilution of 1:20. Sera with titres of 20 or greater were considered positive. Antibody titres <20 were assigned an arbitrary value of 10 for calculation purposes. 19 The virus neutralisation titres are expressed as the reciprocal of the titre required to neutralise 50% of infectious virus, calculated by the method of Reed and Muench. 20
The enzyme linked immunospot (ELISPOT) assay
The ELISPOT assay was used to investigate the class and IgG subclass of influenza specific antibody secreting cell (ASC) response in the spleen and bone marrow. 15 Briefly, the ELISPOT plates were coated with 10 μg/ml total protein of A/chicken/Italy/13474/99 (H7N1) whole virus vaccine diluted in phosphate‐buffered saline (PBS) overnight at 4°C. Lymphocytes (400 000/well) from four mice on each day of sacrifice were added in duplicate and incubated a humidified CO2 incubator at 37°C overnight. After incubation, the influenza specific ASC were detected using 2 μg/ml biotinylated class (IgG; 1030‐08, IgA; 1040‐08, IgM; 1020‐08, Southern Biotechnology, Birmingham, AL, USA) and IgG subclass (IgG1; 1070‐08, IgG2a; 1080‐08, IgG2b; 1090‐08, IgG3; 1100‐08, Southern Biotechnology) specific antibodies and then extravidin peroxidase (Sigma, St. Louis, MO, USA). The spots were developed and counted using a dissection microscope. The mean number of class and IgG subclass specific ASC per 500 000 lymphocytes was calculated for each individual mouse.
ELISA
The class and IgG subclass of influenza‐specific serum antibodies were detected in an indirect ELISA assay. 14 Briefly, 96‐well ELISA plates were coated with 5 μg/ml total protein of A/chicken/Italy/13474/99 (H7N1) whole virus vaccine diluted in PBS overnight at 4°C. The influenza specific antibodies were detected using goat anti‐mouse biotinylated class or IgG subclass specific antibodies as described under the ELISPOT assay for 1 hour 45 minutes at room temperature and subsequent detection with 1/1000 dilution of extravidin peroxidase (Sigma, USA) for 45 minutes. The background absorbance was subtracted from the sample absorbance, and the class and IgG subclass antibody concentration of influenza specific antibody (ng/ml) was calculated for each antibody class and each IgG subclass.
Bead immunoassay
The influenza specific IgG antibody response was detected using a bead‐based immunoassay and the Extracellular Protein Buffer Reagent Kit (LMB0001, Invitrogen, Carlsbad, CA, USA). One million beads (Bead set 64, 922541; Qiagen, Hilden, Germany) were coated according to the manufacturers’ instructions with 20 μg total protein of inactivated whole A/chicken/Italy/13474/99 (H7N1) virus. Mouse sera were diluted 1/100 in sample dilution buffer (PBS‐FCS: PBS containing 20% foetal calf sera; 50 μl) and 50 μl of incubation buffer (PBS‐T: PBS with 0·5% Tween‐20) and incubated with 5000 beads/well in a 96 well flat bottom filter plate (MSBVN1210, Millipore, Billerica, MA, USA) for 2 hours at room temperature with agitation (500 rpm). The beads were then washed twice with PBS‐T, and mixed with biotinylated goat anti‐mouse IgG antibody diluted 1:1000 in PBS‐T (1030‐08, Southern Biotechnology) for 1 hour. The beads were washed in PBS‐T and then incubated with the streptavidin‐conjugated to the fluorescent protein, R‐Phycoerythrin for 30 minutes. After washing twice, the beads were resuspended in PBS‐T (100 μl/well) and analysed on a Luminex‐100 instrument (Luminex Corp, TX, USA) using StarStation v.2.0 software (Applied Cytometry, Sheffield, UK). The relative quantities of IgG antibodies are presented as a mean fluorescent intensity (MFI) after subtraction of the background MFI.
Statistical analyses
Statistical analyses were performed using Prism 5 for Mac OSX (Graphpad Software Inc. La Jolla, USA). The HI, ELISA and Luminescent antibody results were analysed using the unpaired Student’s t‐test by comparing groups of mice to the preceding day of sacrifice. For all assays, P‐values ≤ 0·05 were considered significant.
Results
The kinetics of the humoral antibody secreting cell and serum antibody responses after H7N1 vaccination were investigated in the BALB/c mice using a number of different immunological assays.
Influenza specific antibody secreting cell response
The ELISPOT assay was used to enumerate the influenza specific antibody secreting cell (IS‐ASC) response elicited in the spleen and the bone marrow at specific days after the first and second vaccination.
After the first dose, only splenic IgM IS‐ASC were detected (range 0–60 IS‐ASC per 500 000 lymphocytes), with the exception of one mouse that had very low numbers of IgG ASC (Table 1). Higher numbers of IS‐ASC were detected 5 days after the first vaccination than at day 7. After the second immunisation, low number of IS‐ASC secreting all antibody classes (IgG, IgA and IgM) were detected, although IgG IS‐ASC dominated the splenic response at days 3 and 5 (range 8–60 IS‐ASC per 500 000 lymphocytes). This IgG IS‐ASC response consisted mainly of the IgG2a subclass and had the following distribution; IgG2a > IgG2b > IgG1 > IgG3. Higher numbers of splenic IS‐ASC were detected at 3 than 5 days after the second vaccination.
Table 1.
The IS‐ASC response in the spleen and bone marrow after influenza H7N1 vaccination of BALB/c mice
| Organ | Antibody class/IgG subclass | Days post first vaccination | Days post second vaccination | ||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 7 | 3 | 5 | 21 | ||
| Spleen | IgM | 4·4 ± 0·6 | 28·9 ± 10·8 | 9·1 ± 6·5 | 14·1 ± 6·0 | 9·1 ± 2·4 | 8·2 ± 2·5 |
| IgA | < | < | < | 9·2 ± 3·6 | 15·7 ± 4·2 | 0·3 ± 0·3 | |
| IgG | 0·6 ± 0·6 | 0·2 ± 0·2 | < | 33·5 ± 10·9 | 32·4 ± 6·2 | 3·0 ± 1·4 | |
| IgG1 | < | < | < | 5·7 ± 3·2 | 3·8 ± 2·0 | 1·0 ± 0·6 | |
| IgG2a | < | 0·2 ± 0·2 | < | 30·9 ± 8·1 | 19·7 ± 3·7 | 0·4 ± 0·2 | |
| IgG2b | < | 1·1 ± 0·5 | < | 8·3 ± 3·2 | 7·0 ± 1·2 | 0·5 ± 0·5 | |
| IgG3 | < | < | < | 5·0 ± 3·8 | 0·5 ± 0·5 | 0·5 ± 0·3 | |
| Bone marrow | IgM | 0·8 ± 0·6 | 4·4 ± 1·4 | 3·9 ± 1·4 | 3·9 ± 1·4 | 7·4 ± 5·7 | 1·9 ± 0·8 |
| IgA | < | < | < | 0·6 ± 0 | 3·8 ± 1·4 | < | |
| IgG | < | < | < | 1·4 ± 0·4 | 7·8 ± 2·5 | 1·9 ± 0·6 | |
| IgG1 | < | < | < | 0·3 ± 0·3 | 0·3 ± 0·2 | 1·1 ± 0·5 | |
| IgG2a | < | < | < | 3·1 ± 1·2 | 1·6 ± 0·4 | 0·2 ± 0·2 | |
| IgG2b | < | < | < | 1·0 ± 0·4 | 0·8 ± 0·4 | 0·2 ± 0·2 | |
| IgG3 | < | < | < | < | 0·2 ± 0·2 | 0·5 ± 0·5 | |
<no specific IS‐ASC detected per 500 000 lymphocytes.
BALB/c mice were immunised with one or two doses of whole virus vaccine (15 μg total protein). Groups of four mice were sacrificed after vaccination or control unimmunised mice (0) and the antibody secreting cell response measured by ELISPOT assay. The mean number of influenza H7N1 specific antibody secreting cells per 500 000 lymphocytes ± standard error of the mean (SEM).
In the bone marrow, only very low numbers of IS‐ASC were detected (range 0–24 IS‐ASC per 500 000 lymphocytes). The response consisted of IgM after the first dose of vaccine, whereas all antibody classes (IgM, IgA and IgG) were detected after the second dose. The highest numbers of IS‐ASC were elicited 5 days after the second vaccination with higher number of IgG ASC mainly secreting the IgG2a subclass. The number of specific IS‐ASC in the bone marrow decreased by 21 days after the second immunisation.
Influenza specific serum antibody responses
HI and neutralising serum antibody response
The HI assay is conventionally used with turkey erythrocytes, but has proved to be less sensitive for detecting antibodies to avian H5 influenza viruses and recently the use of horse erythrocytes in the HI test has been found to greatly increase the sensitivity of the assay. 21 Thus, in this study the HI assays were conducted with both turkey and horse erythrocytes. We found that only one mouse had detectable HI antibody using horse erythrocytes at 5 days after the first immunisation, but by day 7 all animals had low HI titres. In contrast, no HI antibody was detected using turkey erythrocytes after the first vaccination. The second dose of vaccine significantly boosted the HI titres (P < 0·05 t‐test) with antibody titres detected by horse erythrocytes increasing up to day 5 and then remaining stable until day 21 (GMT = 211). In contrast, the HI titres measured using turkey blood cells were much lower and continued to increase up to 21 days post the second dose (GMT = 32) (Figure 1A).
Figure 1.
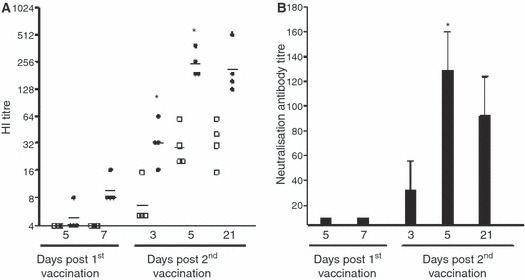
The antibody response induced after vaccination. BALB/c mice were immunised with one or two doses of whole virus vaccine 15 μg total protein. Groups of four mice were sacrificed after vaccination and the serum antibody response analysed by HI and neutralisation assay. (A) The haemagglutination inhibition (HI) antibody response induced after vaccination. The HI titres were measured in an HI assay using turkey (filled symbols) or horse erythrocytes (open symbols). The data are presented as the geometric mean titre ± 95% confidence interval. There was a significant increase (*) in horse HI titres (P < 0·05 t‐test) between 7 days post first and 3–5 days after the second immunisation. (B) The neutralisation antibody response elicited after vaccination. Data are presented as the mean neutralisation titre ± standard error of the mean (SEM). The numbers indicate the day after each dose of vaccine.
The ability of the serum antibodies to neutralise live virus was measured. No neutralising antibody was detected after the first vaccination. Neutralising antibody was found in one mouse at 3 days after the second immunisation (mean titre = 78) and all mice tested at 5 and 21 days (mean titre from 34 to 160) (Figure 1B).
IgG response measured by the bead immunoassay
We have developed a bead‐based immunoassay to detect the influenza specific IgG antibody response after vaccination, which uses low concentrations of virus and small volumes of sera. Only very low levels of influenza specific IgG antibodies were detected at 5 and 7 days post the first immunisation, MFI 14 and 4 on each day respectively (Figure 2A). The antibody titres increased significantly (P < 0·05) after the second dose of vaccine and continued to increase up to day 21 (MFI 131).
Figure 2.
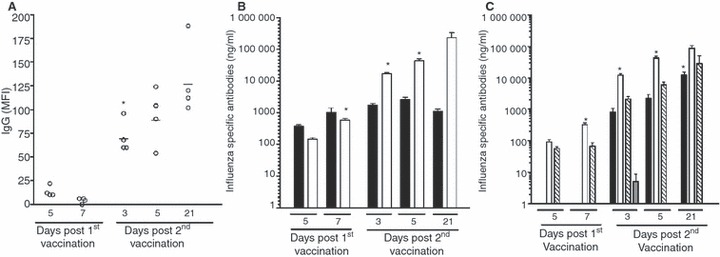
The antibody response induced after vaccination. BALB/c mice were immunised with one or two doses of whole virus vaccine (15 μg total protein). Groups of four mice were sacrificed after vaccination and the serum analysed by luminex bead‐based assay and ELISA. (A) The IgG antibody response induced after vaccination. Beads coated with whole H7N1 virus were used to measure the kinetics of the IgG response in a bead based immunoassay. Data are presented as the mean fluorescent intensity (MFI) of each mouse and the line shows the mean MFI of the group of animals on each day after vaccination. The MFI increased significantly (*, P < 0·05) after the second dose of vaccine. (B) An ELISA assay was used to measure the IgM (black) and IgG (open) serum antibody concentrations. The data are presented as the mean concentration (μg/ml) ± SEM after immunisation. Statistically significant increases (P < 0·05) from the preceding group are indicated by an asterisk (*). (C) The serum IgG subclass distribution after vaccination. The concentration of IgG1 (black) IgG2a (white), IgG2b (striped) and IgG3 (grey) are shown as the mean concentration (μg/ml) ± SEM. Statistically significant increases (P < 0·05) from the preceding group are indicated by an asterisk (*) for the IgG1 and IgG2a subclasses.
The class and IgG subclass serum antibody response
The ELISA was used to measure the class and IgG subclass of influenza specific antibody responses after vaccination (Figure 2B, C). Low concentrations of specific antibody, particularly IgM, were detected 5 days after the first vaccination, however the concentration of IgG and IgM increased by day 7. The IgG antibody response was significantly (P < 0·05) boosted in animals immunised twice, with the highest concentrations detected at day 21. In contrast, the IgM concentration remained constant and then decreased by day 21. Low levels of IgG2a were elicited after the first dose, and the second vaccination significantly boosted this response. IgG1 was only detected after the second vaccination and the concentration of this subclass increased up to day 21, but always remained markedly lower than of IgG2a. The IgG subclass response had the following distribution; IgG2a > IgG2b > IgG1 > IgG3.
Discussion
The influenza H7 subtype has caused zoonoses in Europe and North America, resulting in asymptomatic illness, conjunctivitis and one known death. 3 , 4 , 6 The avian influenza H7 viruses can be subdivided into two geographic and phylogenetic lineages, the Eurasian and North American. 22 For sustained human‐to‐human transmission to occur avian viruses must adapt to humans by changing the HA receptor specificity for α2,6‐ rather than α2,3‐linked sialic acid (SA) receptors. 23 Recently, the North American H7 viruses were shown to have acquired binding to the human α2,6 SA receptor, 24 although for efficient human‐to‐human spread further adaptations are probably required in the viral genes to allow further adaptation to the mammalian cell. 25 H7 viruses thus represent a potential pandemic threat and there is therefore a need to investigate candidate H7 vaccines in pre‐clinical animal models and clinical trials. The Eurasian strains of H7 have been found to elicit more cross reactive antibody responses than the North American viruses. 26 Thus in this pilot study, we have investigated the humoral immune response in a mouse model to an inactivated whole virus vaccine produced from an HPAI Eurasian H7N1 virus.
Candidate pandemic H5 and H9 vaccines have been found to be poorly immunogenic in man 27 , 28 , 29 , 30 and two doses of vaccine are required to produce protective levels of serum antibody. Vaccine candidates containing H2, H5 and H9 have undergone clinical trials and have after two doses fulfilled the conventional licensing criteria 30 , 31 , 32 defined by the Committee for Medicinal Products for Human Use (CHMP) for seasonal influenza vaccines. 33 Most inactivated influenza vaccines are produced in three formulations; whole virus, split virus or subunit vaccines. Whole virus vaccine is more immunogenic in naïve individuals as it elicits systemic antibodies, as well as primes for a cytotoxic lymphocyte response. 34 , 35 In this study, we detected low HI antibody titres after the first immunisation, but the HI and neutralising titres increased rapidly after the second dose. We have previously observed that whole virus vaccine formulation intrinsically induces an earlier and stronger humoral immune response in naïve animals, 14 thus it may also induce earlier and better protection against infection and its associated medical complications in man.
Once antigenically activated, B cells differentiate into memory B cells and plasmablasts, which secrete antibody before becoming fully differentiated into plasma cells. 36 We found that this H7N1 vaccine elicited an ASC IgM response after the first vaccination, whereas all three classes of antibody were secreted by ASC after the second dose. Whole H7N1 vaccine elicited an IgA ASC response after the second dose of vaccine, although lower than IgG and IgM, and may point to the inherent ability of whole virus vaccine to elicit an IgA response. Locally produced IgA is important in providing the first line of defence against influenza virus, and furthermore reacts with antigenically drifted influenza variant (reviewed in Ref.37). After activation by antigen in the secondary lymphoid organs, B cells differentiate into short‐lived or long‐lived ASC and the latter home to the bone marrow. 38 The number of IgG ASC in the bone marrow decreased 3 weeks after the second vaccination with H7N1 vaccine, which contrasts with the finding of stable bone marrow ASC numbers after immunisation with a seasonal strain 14 suggesting a lower induction of a memory B‐cell response. This A/chicken/Italy/13474/99 (H7N1) virus may have a particularly low intrinsic immunogenicity as we have observed lower antibody response upon infection of ferrets. 11 These findings require further studies as the induction of a good memory B cell response are essential characteristic for an effective vaccine.
Haemagglutination inhibitory antibodies are considered surrogate correlates of protection and an HI titre of ≥40 is considered to indicate 50% protection in humans to seasonal influenza strains. The conventional HI assay using turkey red blood cells is relatively insensitive for detecting antibody responses to avian H5 viruses. 19 , 27 In this study, the use of horse erythrocytes increased the sensitivity of detection of serum H7 HI titres, and two doses of H7N1 vaccine was seen to elicit HI antibody titres considered to be protective. This is in agreement with the findings of others with an avian H7 virus. 39 However, despite the increased sensitivity of the assay, the HI titres were much lower than those observed in other murine studies with H3N2 whole virus vaccine 14 , 40 and this may reflect lack of optimised assays for detecting antibody responses to avian H7 viruses. Other studies have found that the formulation of the vaccine with immune stimulating complex adjuvant (ISCOM) or aluminium adjuvants improved the immunogenicity of candidate H7 vaccines. 9 , 10 , 41 Although, a recent study in man of a pandemic H5N1 vaccine found that whole virus vaccine induced higher antibody titres when administered alone than with alum adjuvant. 31
The IgG subclass profile elicited after vaccination can provide important information on the quality of the immune response. The IgG2a antibody response is a marker of a Th1 cellular response and IgG1 as an indicator of Th2 humoral response. 35 In this study we observed a dominance of IgG2a antibody after the first vaccination indicating a dominant Th1 response, whereas the concentration of IgG1 increased after the second dose representing a mixed Th1/Th2 profile. Similar results have been observed after seasonal whole virus vaccine and the IgG2a antibody is associated with a strong IFN‐γ response after one dose and a more mixed cytokine response including the Th2 cytokine IL‐4, IL‐6 and IL‐10 after the second immunisation. 14 , 40 We have found a mixed Th1/Th2 profile in mice infected with this HPAI H7N1 42 suggesting that the response observed after the second immunisation was more similar to that observed after natural infection. Also, the ratio of IgG2a and IgG1 serum antibodies and IS‐ASC in spleen and bone marrow differed not only after each vaccine dose, but also at the various time points following the second dose. This highlights that the time points selected for analysing the IgG2a/IgG1 antibody ratios are important, as the overall pattern shows a change from a Th1 to a Th2 profile.
There is only one published human clinical trial of wild‐type HPAI H5 vaccine 31 as most vaccine manufactures do not have high containment vaccine production facilities. An H7N1 vaccine strain containing an attenuated HA and the neuraminidase derived from this H7N1 strain has been produced by reverse genetics and a cell‐based inactivated split virion vaccine was manufactured and has entered phase I clinical trial. 11 , 43 In other studies, we have found that a cell‐based split virion vaccine based on this highly pathogenic H7N1 strain induced only low serum antibody titres consisting almost entirely of IgG1, but protected from disease and death in a murine challenge model. 42 In contrast, this H7N1 whole virus vaccine induced a strong Th1 immune response as measured by IgG2a subclass after one dose of vaccine and a more mixed Th1/Th2 response with HI and neutralising antibodies after the second dose. Recently, others have found that the protective efficacy of pandemic candidate H5 vaccines have shown that correlates of resistance to serious illness and death to avian viruses may not be solely reflected by levels of circulating serum antibodies. 44 , 45 , 46 , 47 Thus, the methodology used in this study may aid in future studies defining immunological correlates to pandemic candidate vaccines.
Acknowledgements
We wish to thank Abdullah Madhun, Influenza Centre, University of Bergen, Norway and Maria Zambon Health Protection Agency (HPA), UK for helpful discussion. This work has been supported by the European Union (Flupan QLK2‐CT‐ 2001‐01786) and the Norwegian Ministry of Health and Care Services.
Re‐use of this article is permitted in accordance with the Creative Commons Deed, Attribution 2.5, which does not permit commercial exploitation.
References
- 1. Stephenson I., Democratis J. Influenza: current threat from avian influenza. Br Med Bull 2005; 75–76:63–80. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. 2008. available at: (http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_01_27/en/index.html) [accessed 27th January 2009].
- 3. HPA Press Statement . Confirmation of avian influenza H7N2 infection. 2007. available at: (http://www.hpa.org.uk/hpa/news/articles/press_releases/2007/070525_avian_flu_H7N2.htm) [accessed 2007; cited 25 May 2007].
- 4. Skowronski DM, Li Y, Tweed SA et al. Protective measures and human antibody response during an avian influenza H7N3 outbreak in poultry in British Columbia, Canada. CMAJ 2007; 176:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koopmans M, Wilbrink B, Conyn M et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in The Netherlands. Lancet 2004; 363:587–593. [DOI] [PubMed] [Google Scholar]
- 6. Fouchier RA, Schneeberger PM, Rozendaal FW et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci USA 2004; 101:1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 2004; 59:1–15. [DOI] [PubMed] [Google Scholar]
- 8. Wood JM, Robertson JS. Reference viruses for seasonal and pandemic influenza vaccine preparation. Influenza Other Respir Viruses 2007; 1:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jadhao SJ, Achenbach J, Swayne DE, Donis R, Cox N, Matsuoka Y. Development of Eurasian H7N7/PR8 high growth reassortant virus for clinical evaluation as an inactivated pandemic influenza vaccine. Vaccine 2008; 26:1742–1750. [DOI] [PubMed] [Google Scholar]
- 10. Pappas C, Matsuoka Y, Swayne DE, Donis RO. Development and evaluation of an influenza virus subtype H7N2 vaccine candidate for pandemic preparedness. Clin Vaccine Immunol 2007; 14:1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whiteley A, Major D, Legastelois I et al. Generation of candidate human influenza vaccine strains in cell culture ‐ rehearsing the European response to an H7N1 pandemic threat. Influenza Other Respir Viruses 2007; 1:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banks J, Speidel ES, Moore E et al. Changes in the haemagglutinin and the neuraminidase genes prior to the emergence of highly pathogenic H7N1 avian influenza viruses in Italy. Arch Virol 2001; 146:963–973. [DOI] [PubMed] [Google Scholar]
- 13. Skehel JJ, Schild GC. The polypeptide composition of influenza A viruses. Virol 1971; 44:396–408. [DOI] [PubMed] [Google Scholar]
- 14. Hovden AO, Cox RJ, Haaheim LR. Whole influenza virus vaccine is more immunogenic than split influenza virus vaccine and induces primarily an IgG2a response in BALB/c mice. Scand J Immunol 2005; 62:36–44. [DOI] [PubMed] [Google Scholar]
- 15. Cox RJ, Mykkeltvedt E, Robertson J, Haaheim LR. Non‐lethal viral challenge of influenza haemagglutinin and nucleoprotein DNA vaccinated mice results in reduced viral replication. Scand J Immunol 2002; 55:14–23. [DOI] [PubMed] [Google Scholar]
- 16. Hunt SV. Preparation of Lymphocytes and Accessory Cells. Lymphocytes a Practical Approach: Oxford, UK: IRL Press, 1987, pp. 1–34. [Google Scholar]
- 17. Stephenson I, Wood JM, Nicholson KG, Zambon MC. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J Med Virol 2003; 70:391–398. [DOI] [PubMed] [Google Scholar]
- 18. Stephenson I, Das RG, Wood JM, Katz JM. Comparison of neutralising antibody assays for detection of antibody to influenza A/H3N2 viruses: an international collaborative study. Vaccine 2007; 25:4056–4063. [DOI] [PubMed] [Google Scholar]
- 19. Rowe T, Abernathy RA, Hu‐Primmer J et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reed LJ, Muench HA. A simple method of estimating fifty percent end points. Am J Hyg 1938; 27:493–497. [Google Scholar]
- 21. Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti‐H5 responses in human sera by HI using horse erythrocytes following MF59‐adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res 2004; 103:91–95. [DOI] [PubMed] [Google Scholar]
- 22. Banks J, Speidel EC, McCauley JW, Alexander DJ. Phylogenetic analysis of H7 haemagglutinin subtype influenza A viruses. Arch Virol 2000; 145:1047–1058. [DOI] [PubMed] [Google Scholar]
- 23. Tumpey TM, Maines TR, Van Hoeven N et al. A two‐amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 2007; 315:655–659. [DOI] [PubMed] [Google Scholar]
- 24. Belser JA, Blixt O, Chen LM et al. Contemporary North American influenza H7 viruses possess human receptor specificity: Implications for virus transmissibility. Proc Natl Acad Sci USA 2008; 105:7558–7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yen HL, Lipatov AS, Ilyushina NA et al. Inefficient transmission of H5N1 influenza viruses in a ferret contact model. J Virol 2007; 81:6890–6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joseph T, McAuliffe J, Lu B, Jin H, Kemble G, Subbarao K. Evaluation of replication and pathogenicity of avian influenza a H7 subtype viruses in a mouse model. J Virol 2007; 81:10558–10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nicholson KG, Colegate AE, Podda A et al. Safety and antigenicity of non‐adjuvanted and MF59‐adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 2001; 357:1937–1943. [DOI] [PubMed] [Google Scholar]
- 28. Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354:1343–1351. [DOI] [PubMed] [Google Scholar]
- 29. Treanor JJ, Wilkinson BE, Masseoud F et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 2001; 19:1732–1737. [DOI] [PubMed] [Google Scholar]
- 30. Stephenson I, Nicholson KG, Gluck R et al. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet 2003; 362:1959–1966. [DOI] [PubMed] [Google Scholar]
- 31. Ehrlich HJ, Muller M, Oh HM et al. A clinical trial of a whole‐virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584. [DOI] [PubMed] [Google Scholar]
- 32. Hehme N, Engelmann H, Kunzel W, Neumeier E, Sanger R. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med Microbiol Immunol (Berl) 2002; 191:203–208. [DOI] [PubMed] [Google Scholar]
- 33. Products ECfPM . Note for Guidance on Harmonization of Requirements for Influenza Vaccines, March 1997 (CPMP/BWP/214/96). London, UK: European Agency for the Evaluation of Medicinal Products, 1997. [Google Scholar]
- 34. Keitel WA, Cate TR, Nino D et al. Immunization against influenza: comparison of various topical and parenteral regimens containing inactivated and/or live attenuated vaccines in healthy adults. J Infect Dis 2001; 183:329–332. [DOI] [PubMed] [Google Scholar]
- 35. Renegar KB, Small PA Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 2004; 173:1978–1986. [DOI] [PubMed] [Google Scholar]
- 36. Hasbold J, Corcoran LM, Tarlinton DM, Tangye SG, Hodgkin PD. Evidence from the generation of immunoglobulin G‐secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat Immunol 2004; 5:55–63. [DOI] [PubMed] [Google Scholar]
- 37. Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis 2004; 57:236–247. [PubMed] [Google Scholar]
- 38. O’Connor BP, Cascalho M, Noelle RJ. Short‐lived and long‐lived bone marrow plasma cells are derived from a novel precursor population. J Exp Med 2002; 195:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meijer A, Bosman A, Van De Kamp EE, Wilbrink B, Van Beest Holle Mdu R, Koopmans M. Measurement of antibodies to avian influenza virus A(H7N7) in humans by hemagglutination inhibition test. J Virol Methods 2006; 132:113–120. [DOI] [PubMed] [Google Scholar]
- 40. Szyszko E, Brokstad K, Cox RJ, Hovden AO, Madhun A, Haaheim LR. Impact of influenza vaccine formulation with a detailed analysis of the cytokine response. Scand J Immunol 2006; 64:467–475. [DOI] [PubMed] [Google Scholar]
- 41. De Wit E, Munster VJ, Spronken MI et al. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low‐pathogenicity vaccine strain. J Virol 2005; 79:12401–12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Major DL, Cox RJ, Smith J, Vogel F, Haaheim LR, Wood JM. Evaluation of a PER.C6 Cell Grown Influenza H7N1 Virus Vaccine in BALB/c Mice. Options for the Control of Influenza VI; 2007; Toronto, Canada: Abstract P1438, 2007. [Google Scholar]
- 43. Cox RJ, Madhun AS, Hauge , et al A phase I clinical trial of a PER‐C6® cell grown influenza H7 virus Vaccine, 2009; in press. [DOI] [PubMed]
- 44. Ninomiya A, Imai M, Tashiro M, Odagiri T. Inactivated influenza H5N1 whole‐virus vaccine with aluminum adjuvant induces homologous and heterologous protective immunities against lethal challenge with highly pathogenic H5N1 avian influenza viruses in a mouse model. Vaccine 2007; 25:3554–3560. [DOI] [PubMed] [Google Scholar]
- 45. Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross‐protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J Infect Dis 2006; 194:1040–1043. [DOI] [PubMed] [Google Scholar]
- 46. Suguitan AL Jr, McAuliffe J, Mills KL et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross‐protection in mice and ferrets. PLoS Med 2006; 3:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mahmood K, Bright RA, Mytle N et al. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine 2008; 26:5393–5399. [DOI] [PubMed] [Google Scholar]


