Abstract
Memory CD8 T cells are a critical component of protective immunity and inducing effective memory T cell responses is a major goal of vaccines against chronic infections and tumors 1-3. Considerable effort has gone into designing vaccine regimens that will increase the magnitude of the memory response but there has been minimal emphasis on developing strategies to improve the functional qualities of memory T cells 4. In this study we show that mTOR, the mammalian target of rapamycin 5, is a major regulator of memory CD8 T cell differentiation and in contrast to what we expected the mTOR specific inhibitor rapamycin, an immunosuppressive drug, had surprising immunostimulatory effects on the generation of memory CD8 T cells. Treatment of mice with rapamycin following acute lymphocytic choriomeningitis virus (LCMV) infection enhanced not only the quantity but also the quality of virus specific CD8 T cells. Similar effects were seen after immunization of mice with a non-replicating VLP based vaccine. In addition, rapamycin treatment also enhanced memory T cell responses in non-human primates following vaccination with MVA (modified vaccinia virus - Ankara). Rapamycin was effective during both the expansion and contraction phases of the T cell response; during the expansion phase it increased the number of memory precursors and during the contraction phase (effector to memory transition) it accelerated the memory T cell differentiation program. Experiments using RNAi to inhibit mTOR, raptor or FKBP12 expression in antigen specific CD8 T cells showed that mTOR acts intrinsically through the mTORC1 pathway to regulate memory T cell differentiation. Thus, these studies identify a molecular pathway regulating memory formation and provide an effective strategy for improving the functional qualities of vaccine or infection induced memory T cells.
Rapamycin is a commonly used immunosuppressive drug in transplant recipients and specifically inhibits the intracellular kinase mTOR 5. Several recent studies have shown that rapamycin has various effects on the immune system such as inhibiting type I interferon production by plasmacytoid dendritic cells 6, modulating T cell trafficking 7, and regulating Foxp3 expression in regulatory T cells 8, 9. However, the role of the mTOR pathway in regulating CD8 T cell responses is not known. To address this issue we treated B6 mice with rapamycin during the course of an acute LCMV infection and monitored the virus specific CD8 T cell response (Fig. 1a). We made the surprising observation that rapamycin enhanced the LCMV specific CD8 T cell response. Increased numbers of antigen specific CD8 T cells were seen in both lymphoid and non-lymphoid tissues (Fig. 1a and Supplementary Fig. 1). The striking thing about this result was the decreased contraction of the T cell response in the rapamycin treated group. Similar frequencies of virus specific effector CD8 T cells were observed in both groups of mice at the peak of the T cell response on day 8 post-infection but there was minimal contraction of the T cells in the rapamycin treated group (Fig. 1a). To determine whether the decreased T cell contraction seen between days 8 ~ 30 post-infection in the rapamycin treated group was due to increased cell proliferation and/or reduced cell death, mice were infected with LCMV in the presence or absence of rapamycin and then given BrdU during the T cell contraction phase from days 10 ~ 22 (Supplementary Fig. 2). We found that there was minimal incorporation of BrdU by antigen specific CD8 T cells in either group of mice showing that the decreased contraction of T cells in the presence of rapamycin was not due to increased cell proliferation. Thus, it appears that the major effect of rapamycin is to enhance the survival of antigen specific CD8 T cells.
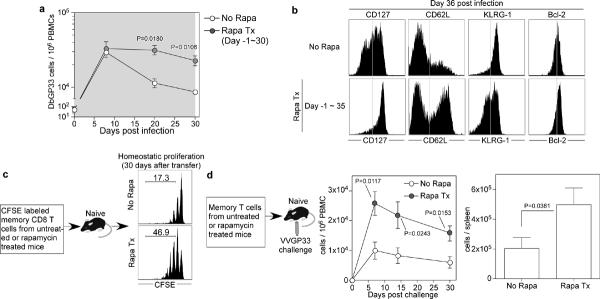
Figure 1. Rapamycin enhances the number and quality of virus specific memory CD8 T cells.
a, Kinetics of endogenous GP33 epitope specific CD8 T cells in PBMCs of LCMV-infected B6 mice treated with rapamycin (from day -1 to 30 post-infection; shaded area) (No Rapa, n=3 mice; Rapa Tx, n=6). b, Phenotypic analysis of endogenous DbGP33 tetramer positive cells in the spleen at day 36 post infection. c, GP33 epitope specific P14 transgenic memory CD8 T cells (day 34 post infection) were generated in the presence or absence of rapamycin, CFSE labeled and then adoptively transferred into naïve mice to monitor their homeostatic proliferation. CFSE dilution of P14 cells at 30 days post transfer is shown and the number represents percentage of memory cells that divided more than two times. d, Memory P14 cells derived from rapamycin treated or untreated mice were adoptively transferred and mice were challenged with vaccinia virus expressing the GP33 epitope (VVGP33). Kinetics of P14 cells in PBMCs after challenge and the total P14 cell numbers in spleen on day 30 post-infection are shown (No Rapa, n=4; Rapa Tx, n=6). Error bars indicate SEM.
We next examined the phenotype of the memory CD8 T cells present in the two groups of mice at day 36 post-infection (Fig. 1b). To investigate this, we performed phenotypic analysis of virus-specific memory CD8 T cells using four markers that are useful in defining memory CD8 T cells: CD127; IL-7 receptor α and essential for memory T cell maintenance 10-13, CD62L; lymph node homing receptor and associated with high proliferative capacity 14, KLRG-1; inversely-correlated with long lived memory cells 15, 16, and Bcl-2; anti-apoptotic and expressed at high levels in memory T cells 12, 17. Memory CD8 T cells generated in the presence of rapamycin expressed higher levels of CD127, CD62L, and Bcl-2, and had a higher frequency of KLRG-1Low cells compared to control mice (Fig. 1b and Supplementary Fig. 3). These data strongly suggested that inhibition of mTOR pathway using rapamycin not only increased the magnitude of the virus specific CD8 T cell response (Fig. 1a) but also improved the functional qualities of the memory CD8 T cells since memory cells with the phenotype (CD127High CD62LHigh Bcl-2High and KLRG-1Low) are associated with long-lived protective immunity 14-16. To directly test this we examined the ability of these memory CD8 T cells to undergo homeostatic proliferation, a property essential for long-term memory maintenance, and to make re-call responses upon re-exposure to antigen. As shown in Figure 1c and 1d, virus specific memory CD8 T cells generated in mice treated with rapamycin were superior to memory cells generated in untreated mice in both of these hallmark memory properties.
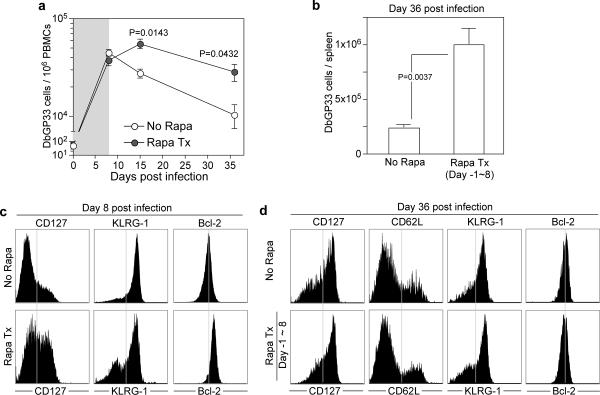
In the experiment shown in Figure 1 mice were continuously treated with rapamycin during the entire course of the T cell response (day -1~35 post-infection). We next examined how rapamycin would effect the CD8 T cell response if it was only given during the T cell expansion phase (days -1~8 post-infection). These results (Fig. 2a and b) were strikingly similar to what we had seen earlier (Fig. 1a); even if the rapamycin treatment was discontinued during the contraction phase (days 8~30) there was only minimal death of the effector CD8 T cells generated in the presence of the drug. Previous studies have shown that the day 8 effector CD8 T cell population consists of two subsets; the terminal effector T cells (CD127Low, KLRG-1High) that mostly die over the ensuing 2~4 weeks and the memory precursor cells (CD127High, KLRG-1Low) that mostly survive and further differentiate to give rise to the pool of long-lived memory cells 10, 15, 16. Our results suggested that rapamycin was enhancing the formation of these memory precursor cells. This was indeed the case and day 8 virus specific effector CD8 T cells generated in rapamycin treated mice contained a higher proportion of CD127High KLRG-1Low cells and these cells also expressed higher levels of Bcl-2 (Fig. 2c). However, we noticed that the phenotype of memory CD8 T cells at day 36 post-infection was similar in the drug treated and control mice (Fig. 2d). This was different from the results obtained upon continuous rapamycin treatment (compare Fig. 1b versus Fig. 2d). Taken together these results clearly show that rapamycin enhances the formation of memory precursors during the naïve to effector T cell differentiation phase but these results suggest that, in addition, rapamycin may also regulate the effector to memory transition phase.
Figure 2. Rapamycin treatment during T cell expansion phase increases the number of memory precursors.
a, Kinetics of endogenous GP33 epitope specific CD8 T cells in PBMCs of LCMV-infected B6 mice treated with rapamycin (from day -1 to 8 post-infection; shaded area) (n=3~6; each time point). b, The average number of DbGP33 tetramer positive cells on day 36 post-infection in spleens of LCMV infected mice treated with rapamycin (No Rapa, n=9; Rapa Tx day -1~8, n=3). c, CD127, KLRG-1, and Bcl-2 expression on endogenous DbGP33 tetramer positive cells in PBMCs at 8 days post LCMV infection in B6 mice. Rapamycin was administered from day -1 to day 8 post infection. d, Phenotypic analysis of DbGP33 tetramer positive cells in spleens of LCMV infected mice (rapamycin treatment from day -1 to 8 post infection). Error bars indicate SEM.
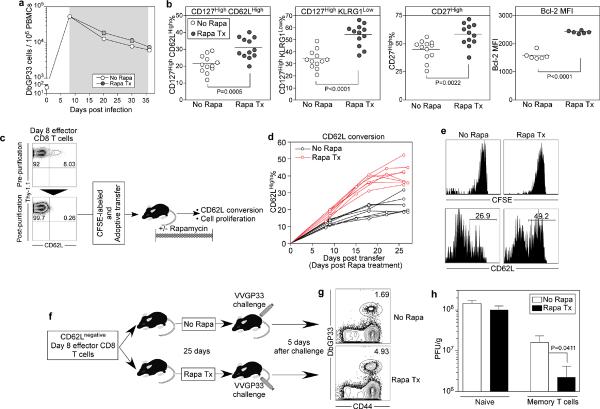
To test this hypothesis we treated mice with rapamycin only during the T cell contraction phase (days 8~35) following acute LCMV infection (Fig. 3). We found that the number of memory cells generated were not affected by the drug (Fig. 3a) but the phenotype of these memory CD8 T cells was strikingly different (Fig. 3b). Thus, rapamycin treatment during the effector to memory transition phase enhanced the memory differentiation program resulting in a significantly higher number of virus specific CD8 T cells with the phenotype characteristic of highly functional memory cells (p value; <0.0001~0.0022) (Fig. 3b). It was important to determine if this represented cell proliferation and outgrowth of a subset of effector CD8 T cells already expressing these memory markers or if rapamycin truly increased the expression of these markers in the surviving effector T cells during this effector to memory differentiation phase. To address this issue, we transferred a highly purified (>99.7%) and CFSE labeled population of day 8 CD62LLow antigen specific effector CD8 T cells into naïve mice and monitored both cell division and memory differentiation of these transferred effector cells in the presence or absence of rapamycin (Fig. 3c). We found that there was no cell division during this effector to memory transition phase (days 1~25 post-transfer) but that the memory T cells that differentiated in the presence of rapamycin re-expressed CD62L much faster (Fig.3d and 3e). More importantly, these memory CD8 T cells were functionally superior and exhibited better re-call responses and protective immunity (viral control) following challenge with vaccinia virus expressing the LCMV GP33 epitope (Fig. 3f, 3g, and 3h). Thus, inhibiting mTOR during the effector to memory transition phase improved the functional qualities of memory T cells.
Figure 3. Rapamycin treatment during effector to memory transition phase accelerates memory differentiation.
a, Kinetics of endogenous GP33 epitope specific CD8 T cells in PBMCs of LCMV-infected B6 mice treated with rapamycin (from day 8 to 36 post-infection; shaded area) (No Rapa, n=9 mice; Rapa Tx, n=9). b, Phenotypic changes in endogenous DbGP33 tetramer positive CD8 T cells in spleen on day 36 post LCMV infection (n=12; each group). B6 Mice were treated with rapamycin during the effector to memory T cell transition period (days 8~35 post infection). c, CD62L negative day 8 P14 transgenic effector CD8 T cells were purified, labeled with CFSE, and then adoptively transferred into naïve mice. Half of these mice were treated with rapamycin after transfer and (d) CD62L conversion in the antigen specific CD8 T cells was analyzed longitudinally in the blood. e, CFSE profile and CD62L expression on antigen specific memory CD8 T cells in the spleen at day 27 after transfer of CD62L negative effector T cells. f, CD62L negative day 8 P14 transgenic effector CD8 T cells were adoptively transferred into naïve mice. These mice were treated with rapamycin for 25 days, and were challenged with VVGP33 on day 28 post transfer. At 5 days after challenge, P14 expansion in spleen (g) and viral titers in ovary (h) were analyzed (n=4~6; each group). Flow data were gated on CD8 T cells. Error bars indicate SEM.
The results so far have shown that rapamycin can enhance both the magnitude and quality of the CD8 T cell response following a primary viral infection. We next examined whether similar effects would be seen during a secondary response. As shown in Supplementary Fig. 4 rapamycin also enhanced re-call responses when the drug treatment was only done during secondary LCMV infection. Thus, rapamycin regulates both primary and secondary T cell responses and this would have important implications in designing strategies for improving memory T cell qualities during prime-boost vaccine regimens.
To determine if our findings from the mouse model of LCMV infection could be generalized to other systems we examined the effect of rapamycin following immunization of mice with a non-replicating vaccine. In these experiments mice were vaccinated with VLPs (virus-like particles) derived from hepatitis B core antigen genetically fused to the LCMV GP33 epitope 18. Rapamycin again enhanced both the magnitude and the quality of the VLP-induced memory CD8 T cells (Supplementary Fig. 5). It should be noted that the effects of rapamycin treatment were very long-lasting; memory T cell numbers remained 10-fold higher even 165 days after stopping the drug treatment (Supplementary Fig. 5a). We also tested the applicability of this approach in a non-human primate model. Rhesus macaques previously immunized with vaccinia virus were boosted with MVA in the presence or absence of rapamycin and antigen-specific CD8 T cell responses were analyzed by intracellular IFN-γ staining. We found clear differences in frequencies of antigen specific CD8 T cells between rapamycin treated and untreated monkeys. In the presence of rapamycin, maintenance of higher number of memory CD8 T cells was observed (Supplementary Fig. 6a, b) and slower T cell contraction was evident compared to control animals (Supplementary Fig. 6c). These results demonstrate that rapamycin enhances T cell immunity in both mice and non-human primates following vaccination with either live or inactivated vaccines.
Our results clearly establish that mTOR is a major regulator of memory CD8 T cell differentiation. However, a critical question that needs to be answered is whether mTOR is acting intrinsically in antigen specific CD8 T cells to regulate memory differentiation or if the observed effects of rapamycin on memory formation are mediated by some other cells of the immune system. It is important to resolve this issue since mTOR is ubiquitously expressed by many cells and several recent studies have shown that rapamycin can modulate the functional properties of several other cells of the immune system 6, 8, 9, 19, 20. To address this question, we used a retrovirus based RNA interference (RNAi) system to specifically knock-down various genes of the mTOR pathway (mTOR, raptor, S6K1, eIF4E, and FKBP12) in antigen specific CD8 T cells. Retroviruses marked by GFP and expressing RNAi for a particular gene or a control retrovirus were used to infect LCMV specific transgenic CD8 T cells (P14 cells) and these transduced cells were then adoptively transferred into naïve mice, followed by LCMV infection (Supplementary Fig. 7a). This system allows us to compare the phenotypic changes that occur during memory T cell differentiation in GFP positive retrovirus transduced versus GFP negative non-transduced antigen specific CD8 T cells in the same environment (ie., the same mouse) (Supplementary Fig. 7b). Thus, any differences in memory differentiation that are seen between these two cell populations can be ascribed to the intrinsic effects of that particular gene in antigen specific T cells.
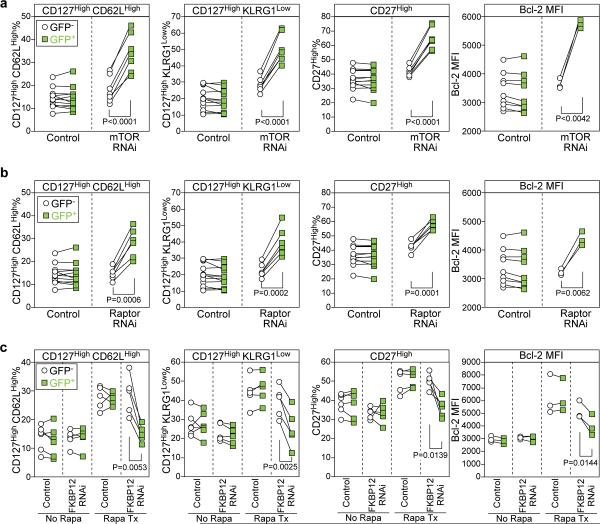
First, we knocked down mTOR itself in antigen specific CD8 T cells. We found that mTOR RNAi retrovirus-transduced GFP+ P14 cells showed significantly higher expression of the canonical memory T cell markers (CD127, CD62L, Bcl-2, CD27) and lower expression of KLRG-1 compared to non-transduced or control vector transduced P14 cells (Fig. 4a). These data show that mTOR acts intrinsically in antigen specific CD8 T cells to regulate memory differentiation. However, since mTOR forms two distinct complexes, the rapamycin-sensitive mTOR complex 1 (mTORC1) and the rapamycin - insensitive mTORC2 5, mTOR knockdown does not completely mimic rapamycin treatment. To distinguish between these two pathways, we knocked down the gene, raptor, that is an essential component of the mTORC1 complex 21, 22. As shown in Fig. 4b, inhibition of raptor in antigen specific T cells gave results identical to what was seen upon knock down of mTOR identifying the mTORC1 complex as the regulator of memory differentiation. To gain more insight into mechanisms by which mTOR regulates memory formation we examined the role of S6K1 and eIF4E 5. We found that knockdown of these mTORC1 downstream effectors significantly enhanced memory CD8 T cell differentiation (Supplementary Fig. 8). Thus, our results show that mTOR is exerting its effect through these two downstream molecules.
Figure 4. mTOR acts intrinsically in antigen specific CD8 T cells through the mTORC1 pathway to regulate memory T cell differentiation.
Specific genes (mTOR or raptor) were knocked down using a retrovirus based RNAi system. Retrovirus transduced LCMV specific P14 transgenic CD8 T cells (marked by GFP expression) were adoptively transferred into naïve mice, followed by LCMV infection. Phenotypic analysis of retrovirus transduced cells (GFP+) and nontransduced (GFP-) P14 cells in the PBMCs was performed on days 14~16 post infection. a, and b, Each line shows expression of the indicated phenotypic markers on transduced and nontransduced antigen specific CD8 T cells in individual animals. a, mTOR RNAi. b, raptor RNAi. Same control data are shown in panels (a) and (b). c, FKBP12 RNAi expressing retrovirus- or control retrovirus-transduced P14 transgenic CD8 T cells (marked by GFP expression) were adoptively transferred into naïve mice, followed by LCMV infection. Half of the mice were treated with rapamycin throughout infection. Phenotypic analysis of retrovirus transduced cells (GFP+) and nontransduced (GFP-) P14 cells in the PBMCs was performed on days 14~16 post infection.
To further explore the role of mTOR in T cell intrinsic versus external effects on memory differentiation we made rapamycin-insensitive antigen specific CD8 T cells by knockdown of the FKBP12 protein. This intracellular protein binds rapamycin and it is this FKBP12 - rapamycin complex that inhibits the mTORC1 pathway. Thus, by knocking down FKBP12 in P14 CD8 cells we made these cells insensitive to any intrinsic effects of rapamycin but the drug could still act effectively on all the other cells in the mouse. This system allows us to examine if inhibition of mTOR in other cells can effect memory CD8 T cell differentiation. As shown in Fig. 4c inhibiting mTOR in other cells when the antigen specific cells themselves were rapamycin insensitive did not effect memory differentiation. The effect of rapamycin on memory differentiation almost disappeared upon knock down of FKBP12 from the P14 cells and these cells did not show increased expression of the characteristic memory markers (CD127, CD62L, Bcl2, etc.) (see last column of the figures in Fig. 4c). Thus, taken together the results shown in Fig. 4a, b, c establish that mTOR not only acts intrinsically in antigen specific CD8 T cells but that inhibiting mTOR in other cells has minimal to no effect on memory T cell differentiation.
During the past few years considerable progress has been made in understanding the lineage relationships between naïve, effector and memory T cells and in defining the phenotypic and functional changes that underlie memory CD8 T cell differentiation 1, 23. However, much less is known about the intracellular molecules and pathways that regulate the generation of memory T cells. In this study we now identify a molecular pathway that regulates memory T cell differentiation and also provide a strategy for modulating the formation of memory cells. In particular, the ability to increase the functional qualities of memory T cells provides a new approach for enhancing the efficacy of vaccines against infectious diseases and cancer.
Methods Summary
Mice, viral infection, and VLP
C57BL/6j mice and Thy-1.1 P14 transgenic mice bearing the DbGP33 specific TCR were used. Mice were intraperitoneally (IP) infected with LCMV Armstrong or recombinant vaccinia virus GP33 (VVGP33), which expresses the LCMV GP33 epitope. For VLP immunization, mice were subcutaneously given VLP genetically fused to LCMV GP33 epitope 18.
Rhesus macaques and vaccination
Six rhesus macaques were inoculated with Dryvax (Wyeth, Madison, NJ) by scarification. At 105 days post Dryvax vaccination animals were then vaccinated intramuscularly (IM) with MVA.
Administration of rapamycin
For the murine experiments, rapamycin (Wyeth, Madison, NJ) was administered IP daily. Relatively low-dose of rapamycin was used during T cell expansion phase because the higher dose inhibited T cell responses (Supplementary Fig.9). For the rhesus macaque experiments rapamycin was administered daily intramuscularly to Dryvax immunized animals prior to MVA vaccination.
Generation and isolation of effector and memory T cell subsets
Naïve P14 transgenic T cells were adoptively transferred into B6 recipients (P14 chimeric mice). Effector and memory P14 T cells were isolated from LCMV-infected P14 chimeric mice. CD62L negative effector CD8 T cells were purified using anti-CD62L magnetic beads and a CD8 T cell isolation kit (Miltenyi Biotec, Auburn, CA).
Retrovirus based RNAi
The pMKO.1 GFP retroviral vector (Addgene plasmid 10676, Cambridge, MA) was kindly provided by Dr. William Hahn. Double strand oligonucleotides for short hairpin RNA (shRNA) were cloned into pMKO.1 GFP. Recombinant retrovirus was made by transfection in 293T cells. Retrovirus transduced P14 cells were adoptively transferred into naïve mice, then the mice were infected with LCMV Armstrong. The GFP+ P14 CD8 T cells were purified by FACS on day 7 ~ 8 post infection, and then protein expression levels were analyzed by western blotting (Supplementary Fig. 10).
Methods
Mice, viral infection, VLP, and virus titrations
Twelve- to sixteen-week old C57BL/6j mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Thy-1.1 P14 transgenic mice bearing the DbGP33 specific TCR were fully backcrossed to C57BL/6 mice in our animal colony. LCMV Armstrong (2 × 105 PFU, IP) and recombinant vaccinia virus GP33 (VVGP33, 5 × 106 PFU, IP), which expresses the LCMV GP33 epitope, were used for infection. VVGP33 titers were determined in the ovary by plaque assay as described previously 14. For VLP immunization, mice were subcutaneously given 50 μg of VLP, which was derived from the Hepatitis B core antigen (HBcAg) genetically fused to the LCMV gp33-41 epitope (KAVYNFATM) and packaged with CpG-ODN.
Administration of rapamycin in mice
Rapamycin (Wyeth, Madison, NJ) was administered to mice IP daily during the treatment period. Three different treatment periods were used: 1) throughout LCMV infection (day -1 prior to infection to the memory phase, day 35 post-infection); 2) the T cell expansion phase (day -1 prior to infection to day 8 post-infection); or 3) T cell contraction phase (day 8 to the memory phase, day 35 post-infection). The daily dose of rapamycin was 75 μg/kg (blood levels; 5~20 ng/ml) for treatments 1) and 2) and 600 μg/kg (blood levels; 40~100 ng/ml) for treatment 3) (the contraction phase treatment) because the higher dose (600 μg/kg) inhibits T cell responses during the expansion phase of the CD8 T cell response (Supplementary Fig.9). Control mice received sham treatment during the same time periods described above (daily of injection of the buffer without rapamycin).
Rhesus macaques and vaccination
Six colony-bred, SPF Rhesus macaques (Macaca mulatta) were inoculated with Dryvax (Wyeth, Madison, NJ) by scarification in accordance with the US Food and Drug Administration guidelines. Briefly, a bifurcated needle was immersed in the vaccine suspension and used to poke the skin 15 consecutive times. At 105 days post Dryvax vaccination, animals were vaccinated with 108 PFU MVA intramuscularly.
Administration of rapamycin in rhesus macaques
Daily administration of rapamycin (10 ~ 50 μg/kg/day) was given intramuscularly to three of the six Dryvax immunized rhesus macaques around 5 days before MVA vaccination. Blood levels of rapamycin were maintained within a range of 5~15 ng/ml. The other three macaques were left untreated as controls.
Generation and isolation of effector and memory T cell subsets
To generate LCMV specific P14 effector T cells, mice into which 1 × 105 P14 naïve T cells were adoptively transferred were infected with LCMV. On day 8 post-infection, effector P14 cells were isolated from the spleen, and CD62L negative CD8 T cells were purified using anti-CD62L magnetic beads and a CD8 T cell isolation kit (Miltenyi Biotec, Auburn, CA). These cells were then used for a CD62L conversion experiment and a protective immune response experiment. For the CD62L conversion experiment, CFSE labeled CD62L negative P14 effector cells (7~10 × 106 cells) were adoptively transferred into naïve mice. For the protective immune response experiment, 3 × 105 CD62L negative P14 effector cells were adoptively transferred into naïve mice, and rapamycin was administered for 25 days. On day 28 post-transfer, mice were challenged with VVGP33 to examine protective immune responses. To obtain memory P14 cells generated in rapamycin treated mice, B6 mice into which 1 × 105 P14 naïve T cells were adoptively transferred were infected with LCMV, and these mice were then treated with rapamycin from day -1 to day 33 post-infection. On day 34 post-infection, memory P14 T cells generated in the presence of rapamycin were isolated from the spleen. Control memory P14 cells were obtained using the same method without the rapamycin treatment. For the homeostatic experiment, CFSE labeled memory P14 cells (1 × 106 cells) obtained from either rapamycin treated or untreated mice were adoptively transferred into separate naïve recipients. For the recall response experiment, 1 × 104 P14 memory T cells derived from either rapamycin treated or untreated mice were adoptively transferred into separate naïve mice, and the day after transfer these mice were then infected with VVGP33. To investigate effects of rapamycin during secondary T cell responses, 2.5 × 104 P14 memory T cells (> 60 days post infection) were adoptively transferred naïve mice, and rapamycin treatment was started. The day after transfer, these mice were infected LCMV.
Flow cytometry
Flow cytometric analysis was performed on a LSRII, a CantoII, or a FACSCalibur (BD biosciences). MHC class I tetramers were made as described previously 24. All antibodies for flow cytometry were purchased from BD biosciences except for CD127, KLRG-1, and CD27. Antibodies to CD127 and CD27 were purchased from eBiosciences (San Diego, CA) and anti-KLRG-1 was purchased from Southern biotech (Birmingham, AL). Single cell suspensions of spleen cells, lymph nodes, livers, or PBMCs from mice were prepared and direct ex-vivo staining was carried out as described previously 14. For in vivo BrdU incorporation, LCMV-infected mice were fed 0.8 mg/ml BrdU in their drinking water every day. BrdU in virus-specific CD8 T cells was measured by BrdU flow kit (BD Biosciences), according to the manufacturer's instructions. To detect vaccinia virus specific CD8 T cells generated in rhesus macaques, 1.5 × 106 PBMCs isolated by density gradient centrifugation were incubated at 37 °C for 15 hours with vaccinia virus at a multiplicity of infection of 1 in a volume of 300 μl RPMI containing 10% heat inactivated FBS. Brefeldin A (5ug/mL) was added for the final 5 hours of incubation. IFN-γ producing vaccinia virus specific CD8 T cells were detected by intracellular cytokine staining.
Retrovirus based RNAi
The pMKO.1 GFP retroviral vector (Addgene plasmid 10676, Cambridge, MA) was kindly provided by Dr. William Hahn. Double strand oligonucleotides for short hairpin RNA (shRNA) against mTOR, raptor, FKBP12, S6K1, and eIF4E were cloned into pMKO.1 GFP between AgeI and EcoRI sites. The sequences for mTOR shRNA are: 5'-CCGGGCCAGAATCCATCCATTCATTCTCGAGAATGAATGGATGGATTCTGGCTTTTTG-3' (sense strand) and 5'-AATTCAAAAAGCCAGAATCCATCCATTCATTCTCGAGAATGAATGGATGGAT TCTGGC-3' (antisense strand). The sequences for raptor shRNA are: 5'-CCGGGCCCGAGTCTGTGAATGTAATCTCGAGATTACATTCACAGACTCGGGC TTTTTG-3' (sense strand) and 5'-AATTCAAAAAGCCCGAGTCTGTGAATGTAATCTCGAGATTACATTCACAGAC TCGGGC-3' (antisense strand). The sequences for FKBP12 shRNA are: 5'-CCGGGCCAAACTGATAATCTCCTCACTCGAGTGAGGAGATTATCAGTTTGGCT TTTTG-3' (sense strand) and 5'-AATTCAAAAAGCCAAACTGATAATCTCCTCACTCGAGTGAGGAGATTATCAG TTTGGC-3' (antisense strand). The sequences for S6K1 shRNA are: 5'-CCGGGCATGGAACATTGTGAGAAATCTCGAGATTTCTCACAATGTTCCATGCT TTTTG-3' (sense strand) and 5'-AATTCAAAAAGCATGGAACATTGTGAGAAATCTCGAGATTTCTCACAATGTT CCATGC-3' (antisense strand). The sequences for eIF4E shRNA are: 5'-CCGGCCGAAGATAGTGATTGGTTATCTCGAGATAACCAATCACTATCTTCGGT TTTTG-3' (sense strand) and 5'-AATTCAAAAACCGAAGATAGTGATTGGTTATCTCGAGATAACCAATCACTAT CTTCGG-3' (antisense strand). Recombinant retrovirus was made by co-transfection with pMKO.1 GFP and pCL-Eco (Imgenex, San Diego, CA) in 293T cells using TransIT-293 (Mirus, Madison, WI). 48 hours after transfection culture supernatants were collected. To transduce P14 cells with the recombinant retrovirus, P14 transgenic mice were infected with 1 × 106 PFU of LCMV intravenously and 24-hours later, P14 transgenic spleen cells were isolated and then spin-transduced at 37 °C for 90 minutes with fleshly collected retrovirus containing 8 μg/ml of polybrene (Sigma, St. Louis, MO). 5 × 105 retroviral transduced P14 spleen cells were adoptively transferred into naïve mice, followed by LCMV infection (2 × 105 PFU, IP). The GFP+ P14 CD8 T cells were purified by FACS on day 7 ~ 8 post infection, and then protein expression levels were analyzed by western blotting (Supplementary Fig. 10).
Statistical analysis
For the statistical analysis of the RNAi experiments a two-tailed paired Student's t test was used. To determine the statistical significance of the viral titers we used an nonparametric Mann-Whitney test. All other statistical analysis was performed using a two-tailed unpaired Student's t test.
Acknowledgements
We thank Bogumila T. Konieczny for technical assistance; David Garber for providing us with the Modified Vaccinia Ankara; Rama Amara for help developing rhesus macaque assays; William Hahn for providing pMKO.1 GFP vector; Elizabeth Strobert and Pamela L. Turner for technical assistance. This work was supported by NIH grant AI030048 (to R.A.) and N01-AI-50025 (to C.P.L).
References
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–92. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–24. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 5.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Cao W, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTORp70S6K pathway. Nat Immunol. 2008 doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008 doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–74. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 11.Huster KM, et al. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101:5610–5. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 13.Tan JT, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–32. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008 doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–4. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 18.Storni T, et al. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J Immunol. 2004;172:1777–85. doi: 10.4049/jimmunol.172.3.1777. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani M, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–43. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weichhart T, et al. The TSC-mTOR Signaling Pathway Regulates the Innate Inflammatory Response. Immunity. 2008 doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Hara K, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim DH, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 23.Kaech SM, Wherry EJ. Heterogeneity and Cell-Fate Decisions in Effector and Memory CD8(+) T Cell Differentiation during Viral Infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–87. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]