Abstract
The molybdenum-based monoaryloxide monopyrrolide (MAP) species, Mo(NAd)(CHCMe2Ph)(C4H4N)(HIPTO) (2a), which contains “small” imido (Ad = 1-adamantyl) and “large” aryloxide (HIPTO = O-2,6(2,4,6-i-Pr3C6H2)C6H3) ligands, catalyzes Z-selective metathesis reactions as a consequence of intermediate metallacyclobutane species not being able to have a (anti) substituent pointing toward the HIPTO group. ROMP of dicarbomethoxynorbornadiene (DCMNBD) with 2% 2a in toluene leads to >99% cis and >99% syndiotactic poly(DCMNBD), while ROMP of cyclooctene and 1,5-cyclooctadiene (300 equiv)with initiator 2a leads to poly(cyclooctene) and poly(cyclooctadiene) that have cis contents of >99%; all are previously unknown microstructures. Z-selectivity is also observed in the metathesis of cis-4-octene and cis-3-hexene by initiator 2a to give cis-3-heptene.
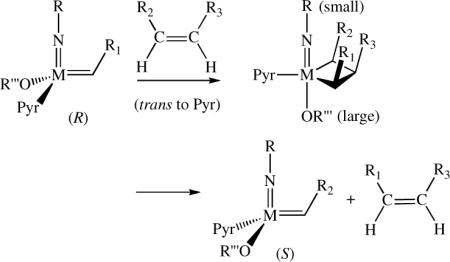
Monoaryloxide-pyrrolide (MAP) olefin metathesis catalysts, which can be prepared through addition of a phenol to a bispyrrolide species,1 can be especially efficient for enantioselective olefin metathesis reactions. For example, mixtures of diastereomers of 1 (R = 1-adamantyl, R' = Me, R” = Br) that are prepared in situ efficiently ring-close an intermediate 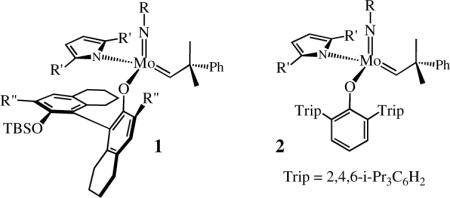 in an enantioselective synthesis of the Aspidosperma alkaloid, quebrachamine,2a,2b and catalyze Z-selective and enantioselective cross-metatheses.2c Z-selectivity is proposed to be possible when olefin attacks at the metal trans to the pyrrolide in a syn complex to yield metallacyclobutane intermediates in which all substituents point toward the “small” axial imido ligand and away from the “large” axial OR'” group (equation 1, Pyr = pyrrolide). Studies involving tungsten3 or molybdenum4 MAP species support the proposals that (i) metallacyclobutanes that contain axial imido and alkoxide ligands are metathesis intermediates, and that (ii) the stereochemistry at the metal inverts as a consequence of each forward metathesis step (equation 1; R1, R2, R3 = alkyl groups).
in an enantioselective synthesis of the Aspidosperma alkaloid, quebrachamine,2a,2b and catalyze Z-selective and enantioselective cross-metatheses.2c Z-selectivity is proposed to be possible when olefin attacks at the metal trans to the pyrrolide in a syn complex to yield metallacyclobutane intermediates in which all substituents point toward the “small” axial imido ligand and away from the “large” axial OR'” group (equation 1, Pyr = pyrrolide). Studies involving tungsten3 or molybdenum4 MAP species support the proposals that (i) metallacyclobutanes that contain axial imido and alkoxide ligands are metathesis intermediates, and that (ii) the stereochemistry at the metal inverts as a consequence of each forward metathesis step (equation 1; R1, R2, R3 = alkyl groups).
 |
(1) |
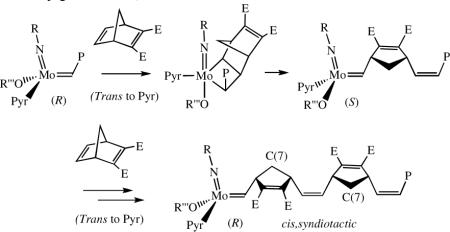
If the mechanism proposed in equation 1 is correct, then ROMP of a substituted norbornadiene initiated by the appropriate MAP species should give rise to a cis,syndiotactic polymer, e.g, that shown in equation 2 (E = ester), a microstructure that is not known in pure form.5 Therefore we became interested in confirming the proposed transformation shown in equation 2, and if successful, in exploring other Z-selective reactions.
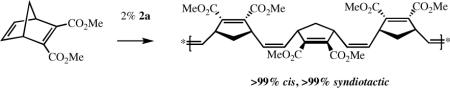
As the OR"' group we chose O-2,6-(2,4,6-i-Pr3C6H2)2C6H3 (hexaisopropylterphenoxide = HIPTO6) (see 2) in order to ensure that OR"' is sufficiently “large,” and adamantyl as the “small” imido substituent (R).2c Addition of HIPTOH to Mo(NAd)(CHCMe2Ph)(C4H4N)21a led to isolable syn-Mo(NAd)(CHCMe2Ph)(C4H4N)(HIPTO) (2a; R' = H) in good yield. Polymerization of dicarbomethoxynorbornadiene (DCMNBD) with 2% 2a in toluene, followed by quenching the
 |
(2) |
reaction with benzaldehyde, yielded a >99% cis, >99% tactic polymer with a C(7) resonance at 38.0 ppm in the 13C NMR spectrum in CDCl3 (cf. 38.7 ppm for cis,isotactic polyDCMNBD5) and an olefinic carbon resonance at 131.5 ppm (the same as in cis,isotactic polyDCMNBD5). A similar highly tactic polymer was formed upon polymerization of 5,6-dicarbomenthoxynorbornadiene (DCMenNBD). Since the inequivalent olefinic protons in poly(DCMenNBD) were not coupled, poly(DCMenNBD) prepared with 2a must be syndiotactic.5 Therefore we conclude that poly(DCMNBD) prepared with 2a as the initiator is also >99% cis and >99% syndiotactic (equation 3, Table 1). Poly(DCMNBD) prepared with an initiator that contains a dimethylpyrrolide (2b, R' = Me, Table 1) was also >99% cis and >99% syndiotactic. Poly(DCMNBD) samples prepared with 2c, 3a, and 3b (Table 1)
 |
(3) |
were found to have lower cis contents than poly(DCMNBD) prepared with 2a or 2b. Clearly the choice of “large” and “small” groups is critical for high Z content, as one might predict if the “all syn” metallacyclobutane intermediate must form (eq 1).
Table 1.
Synthesis of poly(DCMNBD) with various initiators.a
| Initiator | R | R' | or"' | Cis content |
|---|---|---|---|---|
| 2a | Ad | H | HIPTO | >99% |
| 2b | Ad | Me | HIPTO | >99% |
| 2c | Ar | H | HIPTO | 70% |
| 3a | Ad | Me | TPP | 83% |
| 3bb | Ad | H | OSiNaph3 | 44% |
| 1ab | Ar | Me | Bitet; R" = Br | 65% |
| 1bb | Ad | Me | Bitet; R" = Br | 70% |
| 1cb | Ad | Me | Bitet; R" = Me | 90% |
| 1db | Ad | H | Bitet; R" = CHPh2 | 90% |
Ad = 1-adamantyl; Ar = 2,6-i-Pr2C6H3; TPP = 2,3,5,6-Ph4C6H; Naph = 2-naphthyl; Bitet is the aryloxide shown in 1.
Prepared in situ; see Supporting Information.
The only chirality present in racemic initiators of type 2 and 3 is the stereogenic metal center. A stereogenic metal center should exert a powerful electronic control (olefin approach trans to pyrrolide, eqs 1 and 2) in a coordination polymerization reaction that is absent in the vast majority of other types of metal-catalyzed polymerizations. This “stereogenic metal” (SM) control is distinct from enantiomorphic site control and chain-end control, which are both primarily steric in origin and arise from chirality in a ligand or in a polymer chain-end in the last-inserted monomer, respectively.
Poly(DCMNBD) samples prepared with 1a-1d (Table 1), in which OR”' is the large, enantiomerically pure aryloxide in 1,2 do not contain exclusively cis linkages. Evidently one or both of the two diastereomers2-4 (neglecting any chain end chirality) that must be formed sequentially in these circumstances is not (or are not) as Z-selective as 2a or 2b.
In order to explore the potential generality of Z-selective polymerization with 2a we turned to ROMP of cyclooctene and 1,5-cyclooctadiene (300 equiv). Poly(cyclooctene) was formed with a cis content of >99%. The Tm of cis-poly(cyclooctene) was found to be -10 °C, the temperature predicted by Feast in studies of cyclooctene polymers that contain various lower cis contents.7 We obtained poly(cyclooctene) with a cis content of 20% employing Mo(NAr)(CHCMe2Ph)[OCMe(CF3)2]2 as the initiator and 86% with 1b as the initiator. Poly(cyclooctadiene) was formed with a cis content of >99% (according to 13C NMR) when 2a was employed as an initiator. Poly(cyclooctadiene) prepared employing Mo(NAr)(CHCMe2Ph)[OCMe(CF3)2]2 as the initiator had a cis content of 15%. No Tm could be observed between 50 °C and -75 °C for cis-poly(cyclooctadiene), which is in accord with studies by Feast.7 We are not aware of any report of purecis-poly(cyclooctadiene) or cis-poly(cyclooctene) in the literature.
Z-selectivity is also observed in the metathesis of internal cis olefins with 2a as the initiator. Addition of 1% 2a to a 1:1 mixture of cis-4-octene and cis-3-hexene in diethyl ether leads to an equilibrium mixture that contains 50% cis-3-heptene after 8 hours at 22 °C (equation 4). The slow rate of the Z-selective reaction shown in equation 4 is consistent with the required formation of the highly sterically crowded “all-syn” metallacyclobutane intermediate (eq 1), but reactions that proceed via metallacyclobutane intermediates that lead to trans C=C bonds are even slower. Over a period of three days the cis olefins slowly isomerize to approximately a 1:1 cis/trans mixture.
| (4) |
We prepared the unsubstituted tungstacyclobutane complex, W(NAr)(C3H6)(C4H4N)(HIPTO) (Ar = 2,6-i-Pr2C6H3), from W(NAr)(CHCMe2Ph)(C4H4N)2(dme)8 in a manner analogous to that reported recently for related tungstacyclobutane species.3 (The WNAr species was chosen because molybdacyclobutane species are relatively unstable toward loss of olefin and W=NAd complexes are unknown.) As shown in Figure 1, the imido and phenoxide ligands are located in axial positions, as expected. The plane of central ring of the HIPTO ligand is oriented “perpendicular” to the W-Cβ vector (W-C2) of the WC3 ring so that one set of 2,6 isopropyl groups in the HIPTO ligand are located “under” the WC3H6 ring. A space filling model shows that the three anti protons in the metallacycle are in close contact with isopropyl methyl group protons, making it unlikely that a metallacycle of this type could be formed readily if an anti substituent were present on an α or β carbon. The other set of 2,6-HIPTO isopropyl groups surround the pyrrolide ligand and force it to line up along the N1-W-O1 axis. The W-O-C bond angle is relatively large (W1-O1-C31 = 163.7(4)°), consistent with the significiant steric demands of the HIPTO ligand.
Figure 1.
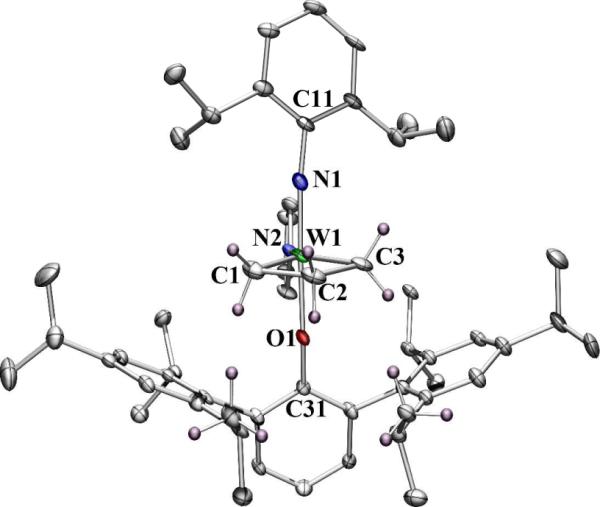
Thermal ellipsoid drawing of W(NAr)(C3H6)(C4H4N)(HIPTO) (50% probability). Hydrogen atoms are removed for clarity except for those on C1, C2, C3, and two of the twelve HIPTO isopropyl methyl carbons.
“Mistakes” that yield trans C=C bonds can arise either when a cis olefin reacts with an (unseen) anti alkylidene9 to yield a syn(α)/syn(β)/anti(α) metallacyclobutane, or when a cis olefin attacks a syn alkylidene to yield an anti(α)/anti(β)/syn(α) metallacyclobutane. Anti alkylidenes in rare cases have been observed in the solid state or in solution.10 Previous ROMP studies suggest that anti species may be orders of magnitude more reactive than syn species, and that trans C=C bonds can form even though no anti alkylidene can be observed.5c Preventing formation of any significant amount of product derived from a reaction that involves an anti alkylidene is likely to be a key aspect of Z-selectivity in MAP catalysts in which the imido R group is “small” and the OR"' ligand is “large.”
Supplementary Material
Acknowledgment
We are grateful to the U.S. Army (Institute for Soldier Nanotechnology, under Contract DAAD-19-02-D-0002 with the U.S. Army Research Office to R.R.S.), the NIH (Grant GM-59426 to R.R.S. and A.H.H.), and the National Science Foundation (CHE-0554734 to R.R.S.) for financial support. We thank Dr. Brad Bailey for the sample of Naph3SiOH.
Footnotes
Supporting Information Available: Experimental details for the synthesis of all compounds and metathesis reactions, and details of the X-ray study. Supporting Information is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).(a) Hock A, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2006;128:16373. doi: 10.1021/ja0665904. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Singh R, Schrock RR, Müller P, Hoveyda AH. J. Am. Chem. Soc. 2007;129:12654. doi: 10.1021/ja075569f. [DOI] [PubMed] [Google Scholar]
- (2).(a) Malcolmson SJ, Meek SJ, Sattely ES, Schrock RR, Hoveyda AH. Nature. 2008;456:933. doi: 10.1038/nature07594. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sattely ES, Meek SJ, Malcolmson SJ, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:943. doi: 10.1021/ja8084934. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ibrahem I, Yu M, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:3844. doi: 10.1021/ja900097n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jiang AJ, Simpson JH, Müller P, Schrock RR. J. Am. Chem. Soc. 2009;131 doi: 10.1021/ja9012694. in press. [DOI] [PubMed] [Google Scholar]
- (4).Marinescu SC, Schrock RR, Li B, Hoveyda AH. J. Am. Chem. Soc. 2009;131:58. doi: 10.1021/ja808308e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).(a) McConville DH, Wolf JR, Schrock RR. J. Am. Chem. Soc. 1993;115:4413. [Google Scholar]; (b) O'Dell R, McConville DH, Hofmeister GE, Schrock RR. J. Am. Chem. Soc. 1994;116:3414. [Google Scholar]; (c) Oskam JH, Schrock RR. J. Am. Chem. Soc. 1993;115:11831. [Google Scholar]; (d) Bazan G, Khosravi E, Schrock RR, Feast WJ, Gibson VC, O'Regan MB, Thomas JK, Davis WM. J. Am. Chem. Soc. 1990;112:8378. [Google Scholar]
- (6).Stanciu C, Olmstead MM, Phillips AD, Stender M, Power PP. Eur. J. Inorg. Chem. 2003:3495. [Google Scholar]
- (7).Dounis P, Feast WJ, Kenwright AM. Polymer. 1995;36:2787. [Google Scholar]
- (8).Kreickmann T, Arndt S, Schrock RR, Müller P. Organometallics. 2007;26:5702. [Google Scholar]
- (9).Recent studies of tungsten methylene MAP complexes3 show that the methylidene ligand rotates readily about the W=C bond (10-100 s-1), making it likely that syn and (unseen) anti isomers of monosubstituted methylidenes interconvert relatively rapidly.
- (10).(a) Schrock RR. Chem. Rev. 2002;102:145. doi: 10.1021/cr0103726. [DOI] [PubMed] [Google Scholar]; (b) Schrock RR. Chem. Rev. 2009;109 doi: 10.1021/cr800502p. online 3/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


