Abstract
Cognitive performance was evaluated in a longitudinal study of APPswe2576 transgenic mice (APP) and a wildtype (WT) comparison group. Subgroups of the APP mice were treated with the ovarian toxicant 4-vinylcyclo-hexene diepoxide (VCD) at 60-75 days of age to induce ovarian atrophy and/or given estrogen (estradiol, 4 μg/day) continuously by pellet from 76 days of age. APP mice had a generally poorer radial maze performance than WT at 4.5, 7.5, 10.5 and 15 months of age. In separate tests, APP mice had a slight motor impairment, higher incidence of homecage stereotypy, hyperactivity in an open field, and reduced object exploration relative to the WT group. Ovarian atrophy led to better maze performance at 7.5 months. The effect of estrogen on maze performance with aging could not be effectively evaluated due to poor survival (30%) of these mice. No effects of ovarian atrophy or estrogen treatment were identified amyloid-beta accumulation or plaque formation at 15 months. Long term longitudinal studies in animal models are needed to explore the consequences of menopause and hormone replacement on Alzheimer's disease, but they are complicated by considerations of survival, pre-aging deficits, testing experience, and selection of appropriate estrogen treatment levels.
1. Introduction
Recently, an added emphasis on research in transgenic mouse models of Alzheimer's disease related to menopause has emerged as a result of the discontinuation of the Women's Health Initiative (WHI) study. This large, randomized, prospective clinical trial, intended to determine the value of hormone replacement therapy in postmenopausal women, was discontinued due to increased incidence of coronary heart disease and breast cancer in the HRT group [28]. The WHI later reported that 4 years of estrogen and progestin treatment in women 65 years of age and older increased the risk of dementia (including Alzheimer's disease, hazard ratio=2.05, 95% CI, 1.21-3.48) [24]. This finding is inconsistent with other studies demonstrating a protective effect of HRT on Alzheimer's disease (6, 25, 31), but the issue is not likely to be explored further in clinical trials because of the health risks demonstrated in the WHI.
One of the difficulties of using transgenic mouse models for this research problem is creating an analogue of menopause. Over the lifetime of women, oocytes are lost through ovulation and atresia, ovarian estrogen production declines, feedback on GnRH is damped, FSH rises, and ovarian cyclicity ceases. The follicle-depleted ovary continues an altered pattern of production of steroid hormones, and compensatory changes occur in the hypothalamus, the adrenal and estrogen responsive tissues including the brain. This situation rarely occurs in the normal lifespan of other animals even including other long-lived primate species. Ovariectomy is often used as a model for menopause. It is unsatisfactory because it does not include a gradual phase of follicular atresia and readjustment of the hypothalamic-pituitary gonadal axis, and because it does not provide residual ovarian tissue that continues to produce steroid hormones, primarily androgens.
This experiment is the first to use chemically induced ovarian atrophy in an animal model of Alzheimer's disease. A procedure for chemically induced ovarian atrophy has been developed by ovarian toxicologists based on 4-vinylcyclohexene diepoxide (VCD), a metabolite of the dimer of the industrial chemical 1,3-butadiene [5, 19, 20]. Butadiene is known to be an ovarian toxicant in women due to occupational exposures. VCD is directly toxic to ovarian follicles increasing the normal rate of atresia of primary and secondary follicles. Mechanisms involve enhancement of cell-death signaling pathways, as well as effects on mitochondria and at the nucleus [11-14]. Using this agent, protocols have been developed for gradual depletion of primordial follicles in rodents that resemble menopause [25].
The present study examined the effects of ovarian atrophy and estrogen administration on the development of cognitive deficits in the APPswe transgenic model of Alzheimer's disease in which beta amyloid is expressed and plaque accumulation resembling that in humans proceeds with aging. Like all transgenic models [10, 26], the APPswe has limitations as a model of Alzheimer's disease, including absence of neuronal degeneration accompanying plaque formation.
In addition to use of VCD-induced ovarian atrophy, this study is notable in using a longitudinal design. Longitudinal life span studies of cognition in transgenic models of Alzheimer's disease are rare. Cross sectional studies of cognitive aging are valuable because they are free of practice effects. However, in cross sectional studies a major determinant of performance is the ability to adapt to the novel environment and demands of the testing situation at different ages. Longitudinal studies are more similar to the human situation in which the ability to perform familiar tasks in familiar environments declines.
2. Methods
2.1 Animal use and care
Transgenic (APP, Tg) and wildtype (WT) mice were received from the supplier (Taconic, Germantown NY) at 34 days of age in 6 cohorts of 10-16 animals. They were housed 4 to a cage in mixed treatment groups and identified individually by ear clips. Transgenic mice were heterozygous female offspring from mating of SJL-TgN (APPSWE)2576 females with C57Bl6 males, while wildtype mice were derived from the same matings. Further specifications were: mice recessive for the RD-1 (retinal degeneration gene) and having pigmented (not red) eyes (Garcia et al. 2004).
Mice were housed in light and temperature controlled rooms in animal housing buildings under supervision of the Center for Laboratory Animal Science, an AAALAC accredited vivarium, in plastic tub type cages with Carefresh bedding, steel tops that held food (5K96, Verified Casein Diet, Lab Diet, Richmond, IN) and water bottles, and microisolaters on racks with solid shelves. Fresh cages and bedding were supplied weekly. The casein diet was employed to avoid phytoestrogens present in soy based diets. Three or four mice of the same cohort were housed together; mice were recaged to maintain social housing when mortality occurred.
2.2 Design
The design resulted in five groups of mice, as outlined in Table 1.
Table 1.
Experimental groups as determined by the design of the experiment
| Ovarian atrophy (day 60 injection series) |
Estrogen (pellet implants) |
|
|---|---|---|
| WT | vehicle | blank |
| APP | ||
| APPC | vehicle | blank |
| APPE | vehicle | estradiol |
| APP-OA | VCD | blank |
| APP-OAE | VCD | estradiol |
2.3 VCD-induced ovarian atrophy
Mice were injected i.p. with 15 daily doses of 160 mg/kg 4-vinylcyclohexene diepoxide (VCD, mixed isomers) at 60-75 days of age. The VCD was obtained as a liquid from Sigma-Aldrich (St. Louis, MO), diluted with a 1:1 DMSO/saline solution (1:16 vol/vol VCD:DMSO/saline mixture), and administered with a 25 gauge needle and Hamilton glass microsyringe (.025-.075 mL volumes) according to previously developed protocols [20]. VCD was discovered to be a highly specific ovotoxicant during the course of exploration of the mechanism of action of butadiene, a human reproductive toxicant. This method for inducing gradual ovarian atrophy is well validated in the scientific literature, and is available commercially through The Jackson Laboratories (Bar Harbor, Maine). The dosing was based on a daily weight at the time of injection. Vaginal lavage for estrous cycles obtained daily for 60 days after termination of the VCD treatment to confirmed absence of ovarian cyclicity. In addition ovaries from a small group of VCD-treated mice were examined in a pilot study after VCD treatment to confirm ovarian atrophy and the absence of ovarian follicles (Table 3).
Table 3.
Ovarian atrophy after VCD treatment. Four VCD-treated APP mice were compared to 4 vehicle-injected mice 60 days after completion of treatments, at 4.5 months of age, the same age that behavioral evaluations were initiated. Follicle counts were conducted in every 20th section of stained and sectioned right ovaries (13-17 sections/ovary).
| follicle type | control | VCD-treated |
|---|---|---|
| primordial | 252±33.41 | 0.8±0.52 |
| primary | 47.3±9.0 | 1.3±0.53 |
| secondary | 41.0±8.1 | 1.3±0.93 |
| antral | 20.8±2.9 | 0.5±0.52 |
number of follicles, mean±s.e.m.
p<.0001
p<.001
2.4 Estrogen treatments
Slow release estrogen pellets (.36 mg) provided 4 μg 17 ß-estradiol per day for 90 days per manufacturer's specifications (Innovative Research of America, Tampa FL). The estrogen pellets were implanted subcutaneously in the dorsal neck region and the incision closed with wound glue. The pellets were implanted 4 times at 90 day intervals beginning at 76 days of age under 4% avertin or ketamine/xylazine anesthesia.
2.5 Nonbehavioral evaluations
Mice were weighed and measured (body length) at baseline, at estrogen pellet implantation and 30 days after each pellet placement. A ponderal index (weight/height) was computed at each point. In addition the mice were screened for general condition and barbered vibrissae at each weighing.
2.6 Behavioral evaluation schedule
The motor, activity and maze tests were administered 4 times to each mouse at the ages indicated in Table 2. In addition, a baseline test was conducted for the motor and activity tests. The stereotypy screen was conducted once prior to the test series and the novelty preference test and gait tests were administered once at the last timepoint. The tests were scheduled to occur at similar times after estrogen pellet replacement at each repetition, except for the last repetition which was delayed to allow dissipation of any direct effects of the estrogen.
Table 2.
Age (in days) at which behavioral tests were performed
| timepoint (age) |
stereotypy | beam | activity/ metabolism |
RAWM | object novelty |
gait |
|---|---|---|---|---|---|---|
| baseline | 38 | 39 | --- | |||
| 4.5 months | 90 | 121 | 122 | 135 | ||
| 7.5 months | 211 | 212 | 225 | |||
| 10.5 months | 301 | 302 | 315 | |||
| 15 months | 428 | 440 | 450 | 436 | 435 |
2.7 Home cage stereotypy
Mice were videotaped in their home cage under red light during the first two hours of the dark cycle. Videotapes were scored for the frequency of occurrence of common stereotypy patterns including bar mouthing, digging, jumping, route-tracing and twirling.
2.8 Gait
Two training sessions and one testing session were conducted over three days.
For training the mouse was placed in a 42 cm × 4.5 cm brightly illuminated runway and trained to run through a hole (6 × 6 cm) into a small, dark plastic chamber at the end. For testing, the mouse's paws were inked under three conditions, forepaws runway, hindpaws runway and all-paws landing from release 15 cm above the surface. Stride length in the runway condition was measured as the mean of the three longest distance between successive fore/hind paw placements. For the landing footsplay the distance between hind paws and between forepaws was measured.
2.9 Beam traversal
Mice were tested for their ability to traverse a 10 mm diameter, 22 cm long beam 80 cm above the surface. After being placed at one end of the beam, the time required to reach the platform at the other end or fall from the beam was measured to the nearest .01sec to a maximum time of 60 sec per trial in 3 successive trials. In addition foot faults (slipping of the paw) were counted.
2.10 Six-arm radial water maze (RAWM)
The maze was configured in a pool (79 cm diameter) with six arms (20 cm high, 25 cm long, 16.5 cm wide) arranged in the pool radiating from the center. Water temperature was maintained at 20-22°C. This created a central area of 21.6 cm diameter. The escape platform was an inverted clay flower pot (surface 6 cm diameter) 1.0 cm below the water surface. The platform and the inside surface of the pool and arms were the same color rendering the platform invisible. Room cues included a checkered board, room furniture, ceiling mounted cameras and the tester who maintained a position at the same point at the periphery of the pool throughout each session. Two testers were used during the course of the experiment.
There were 4 series of sessions conducted at 4.5, 7.5, 10.5 and 15 months of age. Each series consisted of 9 daily sessions of 5 60-s trials each. The first 4 trials were consecutive with an inter-trial interval of 30 s spent on the platform. After the fourth trial, the mouse was dried off and placed in the warmed home cage for 30 min prior to the fifth (retention) trial.
The task (adapted from previous work [1, 4, 9]) designated one arm as the correct choice for each session. The platform was located in the same arm for all trials in one session, with the correct arm changed from session to session and the start arm varied from trial to trial within sessions, (with the exception that arms adjacent to the correct arm were not used as start arms). The mouse was released at the peripheral end of the start arm facing the central area. If an incorrect arm was entered, the mouse was replaced in the start arm and an error was recorded. The mouse was also replaced in the start position if it failed to leave the start arm within 10 s. The number of errors were counted for the 60 s trial period or until the animal found the platform, along with the time required (latency) to find the platform. If the animal did not find the platform in 60 s, it was guided across the water and placed on the platform for the intertrial interval.
2.11 Activity/metabolism
Activity and oxygen consumption were measured in an integrated system (Integra, Accuscan, Columbus OH) over a 48-h period as previously described [6]. The software produced measures of 11 activity indices based on horizontal and vertical breaks in a grid of photocells. Food intake was also measured during this period. Data were analyzed over the whole period and during day and night segments of the light cycle, as well as during the first 30 min in the apparatus (as a test of adaptation).
2.12 Novelty preference
Mice were placed in a 58 × 39 cm plastic chamber monitored by a videocamera on 2 successive days. On the first day, there were no objects in the chamber during the 5-min monitoring period. On the first trial (5 min) of the second day, identical objects (11 × 6 cm) were placed in each half of the chamber. After the apparatus was cleaned with alcohol, one of the objects was replaced with a novel object of similar size for a second 5-min trial. Automated scoring of the videotapes used Topscan software (CleverSys, Reston, VA) which provided frequency and duration measures for exploration of the novel versus familiar objects in terms of side of the cage, area around the objects, and sniffing of the objects (snout oriented toward object while in proximity).
2.13 Necropsy
Mice were killed with Euthosol (0.1 mL/10 g, i.p) and perfused with saline through the aorta. Brain, liver, heart and uterus were removed and weighed. Gross pathology was identified at this time. Brains were then fixed in 4% paraformaldehyde, transferred to Dulbecco's phosphate buffered saline (DPBS), and shipped to Drs. Morgan and Gordon for histological evaluation.
2.14 Histology Methods
Brains were sectioned horizontally at 25 μm on a freezing stage and stored in DPBS pH 7.4 with 100 mM sodium azide at 4°C until staining was performed. Immunohistochemical staining was performed for each marker using eight free-floating sections spaced 200 μm apart encompassing the dorsal to ventral extent of the brain. Sections from each experimental condition were assayed together. Details of this procedure are described elsewhere [7]. Sections were incubated overnight in primary antibody for either Aβ (n-terminal Aβ̤ rabbit polyclonal, 1:10,000) or CD45 (rat monoclonal, 1:10,000; Serotec.com, Raleigh, NC). The following day, sections were washed, and then incubated in appropriate biotinylated secondary antibody (Vector Laboratories, Burlingame, CA). After another cycle of washes, the tissue was incubated with Vectastain® Elite® ABC kit (Vector Laboratories, Burlingame, CA). For CD45, the tissue was then washed and stained with a nickel: diaminobenzidine: peroxide system, followed by final washes, to yield a deep blue reaction product. In the case of Aβ immunostaining, nickel enhancement of the color development was not used, resulting in a brown reaction product. The extent of nonspecific binding was assessed in the absence of primary antibodies.
Separately, slide-mounted sections were stained with Congo red to detect compact amyloid plaques. Slides were briefly hydrated in water, and then incubated in alkaline alcoholic saturated sodium chloride (AASSC) for 20 minutes, followed by 30 minutes in 0.2% Congo red in AASSC. Slides were then quickly dehydrated through a graded ethanol series, cleared in xylene, and cover-slipped.
Stained sections were imaged at 100x final magnification, focusing on the CA1, CA3, and dentate gyrus subregions of the hippocampus, as well as the anterior cerebral cortex. Left and right hemispheres were analyzed separately. Images were then analyzed for area percent positive stain using Image-Pro® Plus software (MediaCybernetics, Silver Spring, MD) as described previously [7]. Positively stained material was segmented from background by thresholding in HSI space, the number of segmented pixels calculated and the number of pixels in the entire field recorded. Area percent stained was calculated (stained pixels / total pixels) for each individual region of the hippocampus of each hemisphere, and these values were averaged to calculate the area percent staining for the hippocampus as a whole. For each brain region, results were averaged for all sections from each animal.
Mice were analyzed histologically in two cohorts. The mean values for these two cohorts differed and the numbers of mice from each experimental group was unbalanced due to uneven attrition amongst the groups. Thus, to accurately portray the results without bias due to cohort differences, we normalized all amyloid load values by the mean value for mice in the OA group. We chose the OA group because it was the only group with a large number of mice in each cohort (5 in one cohort and 6 i the other cohort). Thus all data analyzed and presented are expressed as the fraction of the mean value for the APP-OA group for that analysis.
2.15 Statistical analysis
ANOVAs were conducted on continuous endpoints with appropriate distributions. Independent variables in the ANOVAs depended on the endpoint and were genotype (WT vs. APP), estrogen/OA treatments within the APP genotype, or all five groups (APPC, APP-E, APP-OA, APP-OAE, WT) with post-hoc comparisons. Nonparametric tests were conducted for incidence data. ANOVA were performed with general linear modeling using least squares (SAS Institute, Cary NJ). Screens were conducted for homogeneity of variance prior to analysis. Values in table and figures are sample, rather than least square, means.
3. Results
3.1 Mortality, weight and general condition
The data indicated an adverse effect of the estrogen treatment (APPE and APP-OAE groups) on survival and condition of the mice. As regards mortality, 21 of 30 estrogen treated transgenic mice (11/16 APPE, 9/14 APP-OAE) died prior to completion of the experiment as compared to 10 of 30 nontreated transgenic mice (χ2=8.07 p<.01). Kaplan-Meier analysis of survival also indicated lack of homogeneity in the survival curves of the estrogen-treated and nontreated transgenic mice (χ2=9.92 p<.01). One of 12 WT mice died during the same period. There was no significant difference in mortality between the WT and non-estrogen treated transgenic mice (p=.10).
In addition, testing data gave some evidence of greater disability in the estrogen treated mice. One WT, 1 APPC, 2 APP-OA, 13 APPE and 6 APP-OAE mice had to be discontinued from a RAWM series at least once due to inability to swim or float. Estrogen treated mice also demonstrated a slight increase in fall from the beam during the beam traversal test; one mouse in each of the APPC, APP-OA and WT groups ever fell off the beam, while 2 APP-OAE and 6 APPE mice did so.
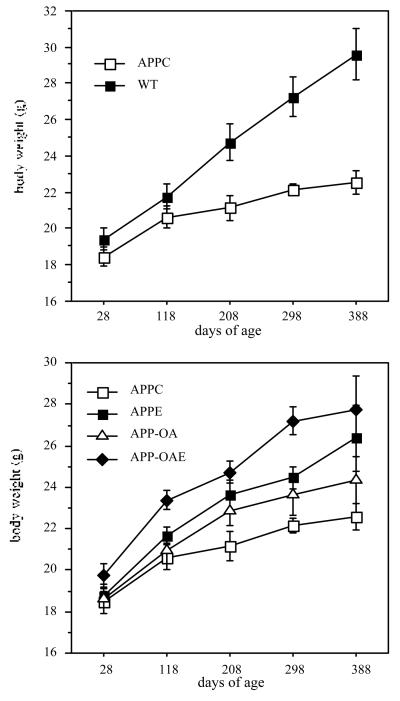
Genotype was the major factor affecting growth in the mice that completed the experiment. RMANOVA for weights were conducted in the animals that survived through the end of the study (Figure 1). Body weights of the WT mice were 16% greater than those of APP mice at the beginning of the experiment (p=.0002), lengths were 8% longer (p<.0001) and body mass index was 10% greater (p=.04). Body weights increased over the experiment, but the weight gain was greater in the WT mice (37% of initial weight) than in the APP mice (14%), with the exception of the APP-OAE group, which gained somewhat more on the average (20%) (F=5.40, p=.007, APPC vs. APP-OAE and WT, p<.01). A similar pattern was seen for length, and body mass index, except for some indication that the APPE group increased their weight-for-length (p=.04).
Figure 1.
Body weight change during the experiment in APP compared to WT mice (top panel) and in treatment subgroups of APP mice (bottom panel).
Estrogen treatment had a significant effect on the growth of APP mice (Figure 1). Because of the high rate of mortality in the estrogen treated groups (APPE, APP-OAE), this figure and the analysis include all mice that were alive at the time of weighing at a particular age. Repeated measures analysis of variance using only mice that survived for the duration of the experiment also confirmed a significant effect of estrogen treatment on weight (F=9.65, p=.004). Body length was not affected by estrogen treatment; thus the body mass index (weight over length) was also significantly greater with estrogen treatment.
Food intake was measured at 90 day intervals over a 48 h period when mice were individually housed in the activity chambers. These values, averaging 3 g food per day, did not differ by genotype or treatment group at any of the timepoints for the set of mice which completed the experiment. At P2 timepoint, the APP mice ate more food than WT (F=6.33, p<.01) and also differed in the ratio of food intake to weight change (F=7.70, p=.01), with APP mice eating more food per unit weight gain. These findings suggest a metabolic difference between APP and WT mice.
Organ weights at necropsy are shown in Table 4. Uterine weights were lower in the APP-OA than the APPC group (F=3.51, p=.01, APPC vs. APP-OA p=.01), reflecting the VCD-induced ovarian atrophy, but were maintained in the APP-OAE group. In addition, the two estrogen-treated groups had a higher body mass index than the non-treated APP groups (F=3.98, p=.01,APPC vs. APP-OAE, p=.03). There were no group differences in brain weight.
Table 4.
Body and organ weights at necropsy (460 days of age)
| group | N | Body weight | Uterus weight (mg) | brain weight (mg) |
|---|---|---|---|---|
| APPC | 8 | 22.8±1.4a | 0.199±.026 | 0.448±.008 |
| APPE | 5 | 24.8±1.7 | 0.178±.033 | 0.447±.012 |
| APP-OA | 11 | 23.7±1.2 | 0.108±.023* | 0.449±.007 |
| APP-OAE | 5 | 28.4±1.7* | 0.183±.036 | 0.472±.008 |
| WT | 11 | 29.7±1.2 | 0.227±.024 | 0.454±.009 |
mean ± sem
p<05, vs. APPC, ANOVA post hoc test
3.2 Stereotypy, beam traversal and gait
A higher proportion of APP mice demonstrated stereotypy (APPC 66%, APPE 57%, APP-OA 53%, APP-OAE 60%) than WT mice (30%). Pooling the transgenic groups, about twice as many APP mice performed stereotypies than wild type mice (59% vs. 30%, p=.04, one-tail Fisher exact test). There was no indication of ovarian atrophy or estrogen effects on stereotypy. Jumping was the characteristic stereotypy of the transgenic mice.
In the beam traversal test, the APP mice demonstrated more foot faults than WT mice on 3 of the 4 test series (Table 5). Cohort was a covariate in these analyses. There were no differences due to genotype, estrogen or ovarian atrophy in the proportion of mice who completed the beam traversal or in latencies to complete the beam traversal.
Table 5.
Number of foot faults while performing the beam crossing test.
| timepoint (age) | WT | APP | ANOVA |
|---|---|---|---|
| baseline | 2.4±0.7 a | 4.8±0.7 | F = 8.49, p=.01 |
| 4.5 months | 3.1±1.0 | 3.3±0.6 | ns |
| 7.5 months | 1.7±1.2 | 3.6±0.7 | F = 6.21, p=.02 |
| 10.5 months | 1.1±0.9 | 2.8±0.5 | F = 6.02, p=.02 |
| 15 months | 2.2±1.5 | 3.6±0.9 | ns |
mean ± sem
The gait test conducted in the aged mice demonstrated a significantly longer stride (F=13.02, p=.001) and significantly lower fore limb splay (F=7.54, p=.01) in the APP than in the WT mice. Within the transgenic mice, there was some indication that ovarian atrophy led to greater hindlimb foot splay (F=3.78, p=.06) and longer hindlimb stride length (F=4.11, p=.05). Body length, which was associated with the stride length and footsplay parameters, was included as a covariate in these analyses.
3.3 RAWM Learning and performance
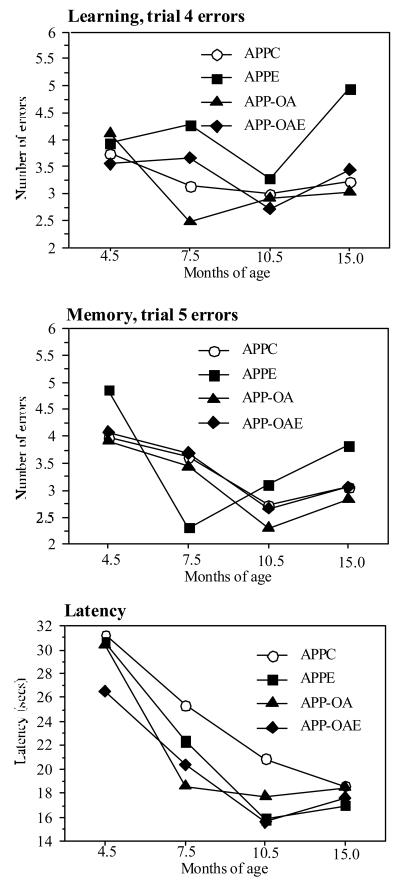
Figure 3 provides a summary of performance across all ages on 3 main performance variables as the mean for each series of sessions: trial 4 errors (learning of the platform location), trial 5 errors (memory for the platform location), and latency (time to reach the platform). This summary includes all animals tested at each timepoint.
APP mice (all groups combined) showed generally poorer maze performance at all ages than WT mice (Table 6). As adults prior to aging, they made more errors than WT in finding each new platform location on the last trial of the 4 trial learning series in each session. They also made more errors on the 5th (memory) trial conducted 30 min after the learning series. Interestingly, average latencies did not differ by genotype. The occurrence of more errors without a difference in latency was supported by a lower index of time per error in the APP mice. This indicated that APP mice entered incorrect arms rapidly, while WT mice were slower in making their choices. Finally, there was less improvement in errors from the first series (4.5 months of age) to the third series (10.5 months of age) in the APP as compared to the WT mice (trial 5 errors, F=8.50, p=.005).
Table 6.
Comparison of WT and APP mice on maze performance at four ages. Excludes any mice discontinued for poor swimming during a test series. T4 error is the number of errors during the last learning trial averaged across sessions. T5 error is the number of errors on a retention trial conducted 30 min after original learning averaged across sessions. Sec/error gives a measure of error rate averaged across all trials, since trial length varied.
| Timepoint | ||||
|---|---|---|---|---|
| 4.5 months | 7.5 months | 10.5 months | 15 months | |
| T4 err APP | 3.9±0.2a* | 3.4±0.2* | 3.1±0.2*** | 3.2±0.9 |
| WT | 3.0±0.4 | 2.4±0.4 | 1.9±0.3 | 2.3±0.4 |
| T5 err APP | 4.3±0.2** | 3.6±0.2*** | 2.9±0.2*** | 2.9±0.2 |
| WT | 3.0±0.5 | 2.3±0.4 | 1.4±0.3 | 1.3±0.3 |
| sec/error APP | 6.4±0.2* | 6.1±0.2*** | 6.2±0.2** | 6.2 0.2**** |
| Wt | 7.3±0.4 | 7.6±0.3 | 7.0±0.3 | 7.9 0.2 |
mean ± SEM
p<.05
p<.02
p<.01
p<.0001 ANOVA
An additional repeated measures analysis (RMANOVA) was conducted for the APPC (n=10) and WT (n=11) mice that completed all 4 series. APPC mice had more trial 4 errors (F=12.46, p=.003) and trial 5 errors (F=18.98 p=.0005) across the 4 series. The number of trial 4 errors (F=4.25, p=.03) and trial 5 errors (4.09 p=.03) decreased across the 4 series for all the mice with no interaction between age and genotype.
At the aged timepoint (15months), performance of the APP mice continued to be impaired, but group differences on average trial 4 and trial 5 errors were not significant (Table 7). One explanation may be lower power associated with the smaller sample size. Another possibility is the very low mean error rates achieved by the WT mice in the last test series. On the last trial of the last series, the WT mice had an average of <1 error, indicating an asymptote of performance and an inability to show further improvement relative the APP mice on the average error/trial measure. However, aged APP and WT mice could be distinguished on other error measures. WT mice also had more trials with no errors in this series (WT 14±1, APP 10±1, F=10.3, p=.003). No WT mice (0/11) had platform failures in the last 2 sessions of the last maze series as compared to 9/19 of the APP mice (p=.01, Fisher's Exact Test). WT mice also had fewer trials with more than 6 errors, indicating at least one working memory error (WT 2±1, APP 5±0.5, F=12.7, p=.001).
Table 7.
Performance of aged (15 month old) APP mice in the water maze. The APPE group is omitted since only two mice survived to this age and were able to be tested. T4 error is the number of errors during the last learning trial averaged across sessions. T5 error is the number of errors on a retention trial conducted 30 min after original learning averaged across sessions. Sec/error gives a measure of error rate averaged across all trials, since trial length varied. “No platform >2” gives the number of mice who failed to reach the platform more than twice during the nine session series (45 trials).
| APPC | APP-OA | APP-OAE | WT | |
|---|---|---|---|---|
| T5 | 2.9±0.4 a* | 2.8±0.3* | 3.1±0.5* | 1.3±0.3 |
| T4 | 3.2 0.5 | 2.9 0.4 | 3.4 0.6 | 2.3 0.4 |
| T1-4 | 3.2±0.2* | 2.8±0.2 | 3.0±0.4 | 2.3±0.2 |
| sec/err | 5.7±0.3* | 6.6±0.3* | 6.1±0.4* | 7.9±0.3 |
| no platform>2 | 5/8(62%) | 7/10(70%) | 2/4(50%) | 3/10(30%) |
mean ± SEM
different from WT, p<.05 ANOVA post-hoc test. There were no statistically significant differences among the three treatment groups of APP mice.
Floating (remaining motionless in the water) was scored as present or not present on each trial by observation during the water maze testing of the aged mice (15 months). Floating was seen only in the WT group; 5 of the 10 WT mice demonstrated floating on 1 or 2 of the 45 trials, 1 demonstrated floating on 5 trials and 1 demonstrated floating on 8 of the trials.
Effects of VCD-induced ovarian atrophy (APP-OA and APP-OAE groups) were seen primarily at 7.5 months of age. These mice had shorter average latencies in the last two sessions of the series (F=4.72. p=.04) and across all trials of the series (F=3.86, p=.06) than the other two groups (APPC and APPE). Further they showed a larger decrease in errors from trial 1-4 of the series (F=5.12, p=.03). In the last test series (15 months of age), the mice with ovarian atrophy had higher error rate (sec/error) than the APPC group. Also APP-OA and APP-OAE groups had fewer error free trials than APPC mice (F=4.88, p=.02, APPC vs. APP-OA, p=.01, APPC vs. APP-OAE, p=.02). APP groups did not differ in the number of trials with more than 6 errors.
The effect of estrogen on maze performance with aging could not be effectively evaluated due to poor survival in the estrogen treated groups (APPE, APP-OAE). The intact estrogen treated group generally demonstrated more errors and fewer completed trials as treatment progressed. Only two APPE mice survived to the final test series at 15 months of age and were able to swim; they demonstrated erratic performance failing to improve across the series.
3.4 Activity/metabolism
Activity was greater in the APP than WT groups throughout the experiment as demonstrated particularly in horizontal activity, and especially nonlocomotor activity (termed stereotypy by the software program) (Table 8). Individual data on activity in APP mice showed a subgroup of 3-5 mice per timepoint with very high activity counts (>800 horizontal beam breaks/3 min as compared to an average of 200/ 3min in WT mice). A total of nine APP mice (3 APPC, 2 APPE, 2 APP-OA and 2 APP-OAE) demonstrated this high activity level for at least one timepoint and this continued to subsequent timepoints in individual mice once it was initiated. Mortality in this subgroup was 5/9. These high individual activity counts were reflected in lack of homogeneity of variance for most activity measures and resulted in use of the Welch ANOVA (JMP, Cary NC). There were no differences across the four groups of APP mice in activity endpoints. There were no effects of genotype or treatment on the respiratory exchange ratio (RER) as measured in the chambers during activity monitoring.
Table 8.
Activity of APP and WT mice over a 48-h period.
| timepoint (months of age) |
horizontal activitye |
vertical activitye |
stereotypy numberf |
stereotypy counte |
stereotypy timeg |
|---|---|---|---|---|---|
| 4.5a APP | 322±43h,** | 17±4 h | 13.0±0.2 | 162±15 h,** | 19.6±0.9 h,** |
| WT | 209±15 | 11±3 | 12.0±0.5 | 110±8 | 16.1±0.8 |
| 7.5b APP | 335±43 h,** | 20±5 h,** | 13.4±0.4 | 172±18 | 19.6±0.9 h,*** |
| WT | 194±15 | 7±1 | 11.7±0.5 | 102±8 | 15.3±0.9 |
| 10.5c APP | 385±54 h,** | 17±3 h,** | 13.3±0.3* | 175±19 h,** | 20.1±1.0 h,** |
| WT | 198±14 | 7±1 | 11.7±0.5 | 106±8 | 15.5±0.9 |
| 15.0d APP | 386±100 h,* | 12±3 h | 19.3±0.4*** | 157±27 h,** | 18.7±1.1 h,*** |
| WT | 156±8 | 7±1 | 16.1±0.3 | 81±4 | 13.2±0.5 |
n=58,12
n=46.11
n=38,11
n=19,11
counts/3 min
episodes/3 min
seconds/3min
lack of homogeneity of variance, Welch ANOVA used
p<.05
p<.01
p<.001, ANOVA post-hoc test
3.5 Novelty preference
In the novelty preference test conducted in the aged mice, the APP mice showed a lower percent of the time sniffing the objects than the WT group during the familiarization period (WT 11.38±1.46 %, APP 6.9±0.9 %, F=6.79, p=.01) and also during the preference period, (WT 12.5±1.6 %, APP 7.8±0.9 % F=6.59 p=.01). Six APP mice (1APPC, 1 APPE, 3 APP-OA and 1 APP-OAE) were excluded from calculations because they spent more than 90% of their time on one side of the box, usually the side in which they were placed. There was no genotype (APP vs. WT) effect on novelty preference measured as the frequency and duration of time spent in the area around the novel vs. familiar object, or the time spent sniffing the novel vs. familiar object. Similarly, APP treatment groups did not differ in novelty preference.
3.6 Brain histology/histochemistry
As anticipated, WT mice did not demonstrate staining for amyloid beta or for amyloid plaques. For APP mice, averages were obtained for three hippocampal areas (CA1, CA3, dentate) and then for the two hemispheres (Table 9). There was a trend for OA groups (APP-OA, APP-OAE) to have a smaller area of staining for amyloid beta in the hippocampus and a greater area in the cortex. The effect of OA in a two factor ANOVA (estrogen, OA) was marginally significant for the hippocampal average amyloid beta measure (p=.037). However, no clear pattern was seen in individual hippocampal regions, nor was the effect also present in the cerebral cortex. No group differences or trends were seen for the Congo red staining. No group differences were seen for the inflammation marker (CD45).
Table 9.
Amyloid beta and Congo red staining of hippocampal and cortical areas of brains from APP mice.
| ANOVAa factors | |||||
|---|---|---|---|---|---|
| APPC n=8 |
APPE n=5 |
APP-OA n=11 |
APP-OAE n=6b |
||
| Amyloid beta | |||||
| hippocampusc | 1.3±0.3d | 1.6±0.4 | 1.0±0.1 | 0.8±0.2 | ovarian atrophy* estrogen |
| cortex | 0.9±0.2 | 0.8±0.1 | 1.0±0.1 | 1.1±0.2 | ovarian atrophy estrogen |
| Congo red | |||||
| hippocampus | 1.3±0.3 | 1.1±0.4 | 1.0±0.2 | 1.3±0.4 | ovarian atrophy estrogen |
| cortex | 0.9±0.2 | 1.3±0.3 | 1.0±0.1 | 1.0±0.2 | ovarian atrophy estrogen |
Two factor ANOVA, ovarian atrophy, estrogen.
n=5, amyloid beta, cortex
average of CA1, CA3, dentate gyrus
Data are expressed as a fraction of the APP-OA group values
p<.05
4. Discussion
4.1 Comparison of APP and WT mice
Our study illustrates that APP mice exhibit maze performance impairment as adults and this does not change appreciably at aging (15 months). In agreement with previous work [22] no cognitive decline was seen in the WT (SJL/C57 F1) mice at the oldest age tested (15 months). Similarly King and Arendash (2002) failed to find a distinctive age-related deficit in performance of cognitive tasks in APP vs. WT mice in a cross-sectional study. Although behavioral impairment has been shown to correlate with amyloid deposition in aged APP mice [3], separation of cognitive and noncognitive impairment and age-dependent and –independent impairment has continued to be a challenge in transgenic mouse models of Alzheimer's disease.
The behavioral phenotype of APP mice in this experiment included increased incidence of stereotypy, greater spontaneous activity, decreased object exploration (when aged) and mild motor impairment. Impairment of beam walking and greater activity were also recorded in work of Arendash and collaborators [2, 15, 16, 23] using somewhat different protocols. Westerman et al. reported a higher percent of “performance impaired” mice based on Morris water maze testing in APPswe than WT mice [27]. Our experiment additionally documented a higher incidence of stereotypy patterns in the home cage in younger mice, and in the open field activity apparatus as the mice aged. In addition we found differences in gait and stride length in the aged mice. The experiment was not appropriately structured to determine an association between mortality and maze performance on the one hand, and motor impairment on the other, but this may deserve further attention. Sensory capabilities important to maze testing, such as vision, also were not tested in the mice. We did not find reduced survival of APP compared to WT mice, but group sizes and discontinuation of the study at 16 months limited this conclusion. APP mice were found to demonstrate lower weights and weight gains than the WT group.
The conclusion that APP mice demonstrate behavioral impairment prior to aging is consistent with the literature. Westerman et al. [27] also documented an onset of spatial learning impairment at 6-12 months of age, which they attributed to the onset of aggregation of soluble amyloid in brain. However, we found deficits at an even earlier age (4.5 months) in the radial arm water maze. In our study, the very low error scores of the WT mice in the last test series may have created a “basement” effect that limited the opportunity to compare performance levels of the APP mice.
4.2 Effects of ovarian atrophy and estrogen on survival in APP mice
This is the first paper examining cognition after chemically induced ovarian atrophy and also the first paper documenting the aging process after VCD-induced ovarian atrophy. The VCD procedure was not apparently associated with increased mortality or any adverse health effects over the lifespan of the mice.
Estrogen toxicity was seen in this study; a higher proportion of estrogen-treated APP mice died during the experiment than non-estrogen treated APP mice. We selected a dose of estrogen (0.36 mg/pellet) considerably lower than doses in studies that demonstrated effects on cerebral amyloid concentrations in transgenic mice (1.7 mg pellet) [17, 30], but higher than a dose that did not influence beta amyloid concentrations (.18 mg pellet) in APP/PS1 mice [9]. In the present longitudinal experiment, deaths related to estrogen treatment began to occur after the implantation of the second pellet, or after more than 90 days of treatment. The underlying pathology was not investigated but deaths were preceded by indications of morbidity, rather than appearing suddenly. We could not measure plasma estradiol during this longitudinal study, but Mayer et al. [21] reported supraphysiological levels of estradiol in VCD-treated LDL receptor knock out mice using the same pellet dose as we did.
Very little information is available on estrogen toxicity leading to mortality in mice. Estradiol administered in diet at a number of doses up to 10 mg/kg/d in standard one and two generation toxicity studies was not associated with increased mortality relative to controls (Tyl, BDR one gen, SOT two gen). Levin-Allerhand and Smith [18] described urinary retention in 2 of 9 APPswe mice treated with estrogen at doses of 1.7 mg/pellet in a study with a high mortality rate (9/9 died as compared to 1/14 controls). Two of 5 mice implanted with an 0.72 mg pellet developed urinary retention but survival was not assessed. Heikkinen et al. (2004b) also reported a low rate of deaths (8/225) associated with urinary retention detected by swollen abdomens in aged, estrogen-treated (0.18 mg pellet) APP/PS1mice. No systematic data on cause of death was gathered in our study. Swollen abdomens were not reported in scheduled health checks in our study; necropsies conducted in connection with tissue collection at the end of the study reported extended bladders in 3 mice, all in estrogen treated groups. Most studies with exogenous estrogen treatment in rats and mice use short term exposures and do not report mortality data.
4.3 Effects of ovarian atrophy and estrogen on spatial learning and memory in APP mice
We found improvement in spatial maze learning associated with VCD-induced ovarian atrophy during the second test series at 7.5 months of age. This apparent age-selective effect may have been due to the performance level at this point in the training, the state of the hypothalamic-pituitary-gonadal (HPG) system at this age, or an adaptation/sensitization to the extended estrogen treatment over time. There are a number of studies of ovariectomy in rats and mice, many of which use spatial learning and memory tests. All these studies have used surgical ovariectomy, but the age at ovariectomy and the dose and duration of estrogen used as “replacement” varied widely, as did effects on cognitive function. Results of these studies are most relevant to an ongoing role of estrogen in supporting brain function, to brain function changes at menopause, or to a role of estrogen in maintaining brain function with aging, but not necessarily to Alzheimer's disease induced cognitive pathology. Ovariectomy has not been found to influence amyloid production or deposition in the few studies available in transgenic mice expressing beta-amyloid [9, 17, 29].
Direct effects of VCD on brain need to be considered. The toxicology of VCD and associated agents VCH (dimer of butadiene and metabolic precursor of VCD) and butadiene have been extensively studied in the National Toxicology Program as well as in the investigator-initiated literature. These studies demonstrate that VCD, as an epoxide, is highly reactive at the point of contact with tissues, leading to skin lesions and tumors when applied dermallly and gastrointestinal lesions when ingested. By both routes of application VCD also induces ovarian atrophy. Clinical signs, organ weight, and histopathology data from these studies (including durations of exposure 14 days, 13 weeks and two years) did not identify any effects on brain. In our experiment VCD did not adversely effect mortality, or morbidity, or amyloid deposition after the brief 10 day treatment. The improved performance of the radial arm maze at 7.5 months of age are more likely attributable to loss of estrogen than in a direct, delayed effect of VCD on brain or other tissues.
Like ovariectomy, estrogen was also found to influence spatial performance only at 7.5 months of age, when errors made during a memory trial 30 min after training were lower in estrogen treated than other APP groups. Estrogen effects on trial five errors at 7.5 months may have to do with previously demonstrated facilitative effect of estrogen on consolidation of spatial memory in C57 mice [8]. However, single estrogen treatments were involved in those studies as contrasted to chronic estrogen treatment in the present study.
We were able to locate only one other paper looking at the effects of loss of ovarian function and estrogen replacement on behavior in a transgenic mouse model of Alzheimer's disease. Heikkinen and colleagues [9] performed surgical ovariectomy (3 months of age) in transgenic mice (APP/PS1) that develop brain amyloid plaque pathology with aging. Estrogen replacement (90 days, 0.18 mg by subcutaneous pellet) was initiated at 3, 6 or 9 months after ovariectomy. No effect of ovariectomy or estrogen was found on plaque formation (Beilchowsky stain) or brain beta amyloid concentrations at 9 or 17 months of age. This finding is consistent with the histopathology results of the current experiment. Heikkinen et al. (2004) study used an 8-arm radial maze with one baited arm, similar to the present study, but with food reward, rather than water escape as the motivation. Separate groups of mice were tested at 6, 9 and 12 months of age. Working memory and reference memory errors were tallied separately. In the RAM test, the APP/PSI mice treated with estrogen beginning 2 months before testing had fewer reference memory errors than WT controls, with a similar trend for working memory errors. The authors attributed the difference primarily to the performance of the ovariectomized, estrogen-treated APP/PS1 mice at the age of 6 mos. This result is potentially similar to our finding of better performance of ovariectomized and estrogen treated APP mice at 7.5 months of age, and to the finding of Westerman et al. [27] that cognitive deficit begin to appear in APP mice at 6-11 months of age when insoluble beta amyloid aggregates begin to be formed. If this is the case, a relatively young adult age would be indicated as an appropriate time for evaluation of therapeutic hormone treatments in the APP model.
4.5 Conclusion
Hormone replacement therapy cannot be adequately tested for its effects on development of Alzheimer's disease in animal models without the coincidence of amyloid deposition and loss of ovarian cyclicity. Since all women undergo menopause prior to aging, estrogen treatment must be given on a background of absent or altered ovarian hormone production and hypothalamic-pituitary gonadotropin release. In designing such a study, we were successful in inducing ovarian atrophy in a transgenic mouse model for Alzheimer's disease by a mechanism similar to mid-aged women (depletion of primordial ova). However we encountered additional barriers to adequate modeling of the human situation: (1) deficits in spatial learning and memory were apparent in adult female transgenic mice prior to aging and did not progress at the time of aging; (2) continuous estrogen treatment by pellet (.36 mg) over the remainder of the lifespan was toxic, leading to disability and enhanced mortality. A more satisfactory model for evaluating this issue maybe achieved by adjustments of the spatial learning protocol to be more challenging at the aged timepoint, and by focusing on earlier adult ages and reduction of estrogen to physiological replacement levels.
Figure 2.
Performance in the radial arm maze across the lifespan in four groups of APP mice and in WT mice. All mice tested at each timepoint are included. Data are group means. Statistical analyses are presented in the text.
Acknowledgments
Supported by UC Davis Health Research Award Program. The authors appreciate the conscientious work of undergraduate laboratory assistants Carlos Montoya and John David.
Footnotes
FEDEX: California National Primate Research Center Hutchison and Road 98, Davis, CA 95616
Authors' disclosure statement
None of the authors have conflict of interest. All protocols were approved by the University of California, Davis IACUC committee prior to implementation.
References
- 1.Arendash GW, Garcia MF, Costa DA, Cracchiolo JR, Wefes IM, Potter H. Environmental enrichment improves cognition in aged Alzheimer's transgenic mice despite stable beta-amyloid deposition. Neuroreport. 2004;15(11):1751–4. doi: 10.1097/01.wnr.0000137183.68847.4e. [DOI] [PubMed] [Google Scholar]
- 2.Arendash GW, King DL. Intra- and intertask relationships in a behavioral test battery given to Tg2576 transgenic mice and controls. Physiol Behav. 2002;75(5):643–52. doi: 10.1016/s0031-9384(02)00640-6. [DOI] [PubMed] [Google Scholar]
- 3.Ashe KH. Learning and memory in transgenic mice modeling Alzheimer's disease. Learn Mem. 2001;8(6):301–8. doi: 10.1101/lm.43701. [DOI] [PubMed] [Google Scholar]
- 4.Austin L, Arendash GW, Gordon MN, Diamond DM, DiCarlo G, Dickey C, Ugen K, Morgan D. Short-term beta-amyloid vaccinations do not improve cognitive performance in cognitively impaired APP + PS1 mice. Behav Neurosci. 2003;117(3):478–84. doi: 10.1037/0735-7044.117.3.478. [DOI] [PubMed] [Google Scholar]
- 5.Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female Fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Appl Pharmacol. 2000;167(3):191–8. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- 6.Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav Brain Res. 2004;153(1):159–70. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Gordon MN, Holcomb LA, Jantzen PT, DiCarlo G, Wilcock D, Boyett KW, Connor K, Melachrino J, O'Callaghan JP, Morgan D. Time course of the development of Alzheimer-like pathology in the doubly transgenic PS1+APP mouse. Exp Neurol. 2002;173(2):183–95. doi: 10.1006/exnr.2001.7754. [DOI] [PubMed] [Google Scholar]
- 8.Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.02.019. epub( [DOI] [PubMed] [Google Scholar]
- 9.Heikkinen T, Kalesnykas G, Rissanen A, Tapiola T, Iivonen S, Wang J, Chaudhuri J, Tanila H, Miettinen R, Puolivali J. Estrogen treatment improves spatial learning in APP + PS1 mice but does not affect beta amyloid accumulation and plaque formation. Exp Neurol. 2004;187(1):105–17. doi: 10.1016/j.expneurol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Higgins GA, Jacobsen H. Transgenic mouse models of Alzheimer's disease: phenotype and application. Behav Pharmacol. 2003;14(56):419–38. doi: 10.1097/01.fbp.0000088420.18414.ff. [DOI] [PubMed] [Google Scholar]
- 11.Hoyer PB, Devine PJ, Hu X, Thompson KE, Sipes IG. Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanistic model. Toxicol Pathol. 2001;29(1):91–9. doi: 10.1080/019262301301418892. [DOI] [PubMed] [Google Scholar]
- 12.Hu X, Christian P, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001;65(5):1489–95. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Christian PJ, Thompson KE, Sipes IG, Hoyer PB. Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol Reprod. 2001;65(1):87–93. doi: 10.1095/biolreprod65.1.87. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, Flaws JA, Sipes IG, Hoyer PB. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2002;67(3):718–24. doi: 10.1095/biolreprod.102.004259. [DOI] [PubMed] [Google Scholar]
- 15.King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer's disease through 19 months. Physiol Behav. 2002;75(5):627–42. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- 16.King DL, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ. Progressive and gender-dependent cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer's disease. Behav Brain Res. 1999;103(2):145–62. doi: 10.1016/s0166-4328(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 17.Levin-Allerhand JA, Lominska CE, Wang J, Smith JD. 17Alpha-estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in AbetaPPSWE transgenic mice. J Alzheimers Dis. 2002;4(6):449–57. doi: 10.3233/jad-2002-4601. [DOI] [PubMed] [Google Scholar]
- 18.Levin-Allerhand JA, Sokol K, Smith JD. Safe and effective method for chronic 17beta-estradiol administration to mice. Contemp Top Lab Anim Sci. 2003;42(6):33–5. [PubMed] [Google Scholar]
- 19.Lohff JC, Christian PJ, Marion SL, Arrandale A, Hoyer PB. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comp Med. 2005;55(6):523–7. [PubMed] [Google Scholar]
- 20.Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71(1):130–8. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 21.Mayer LP, Dyer CA, Eastgard RL, Hoyer PB, Banka CL. Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arterioscler Thromb Vasc Biol. 2005;25(9):1910–6. doi: 10.1161/01.ATV.0000175767.46520.6a. [DOI] [PubMed] [Google Scholar]
- 22.Nicolle MM, Prescott S, Bizon JL. Emergence of a cue strategy preference on the water maze task in aged C57B6 × SJL F1 hybrid mice. Learn Mem. 2003;10(6):520–4. doi: 10.1101/lm.64803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pompl PN, Mullan MJ, Bjugstad K, Arendash GW. Adaptation of the circular platform spatial memory task for mice: use in detecting cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer's disease. J Neurosci Methods. 1999;87(1):87–95. doi: 10.1016/s0165-0270(98)00169-1. [DOI] [PubMed] [Google Scholar]
- 24.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 25.Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, Hoyer PB. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol Appl Pharmacol. 2005;203(2):114–23. doi: 10.1016/j.taap.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 26.van Leuven F. Single and multiple transgenic mice as models for Alzheimer's disease. Prog Neurobiol. 2000;61(3):305–12. doi: 10.1016/s0301-0082(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22(5):1858–67. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progesin in healthy postmenopausal women. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 29.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proc Natl Acad Sci U S A. 2005;102(52):19198–203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng H, Xu H, Uljon SN, Gross R, Hardy K, Gaynor J, Lafrancois J, Simpkins J, Refolo LM, Petanceska S, Wang R, Duff K. Modulation of A(beta) peptides by estrogen in mouse models. J Neurochem. 2002;80(1):191–6. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]