Abstract
An essential phase of skeletal myogenesis is the fusion of mononucleated myoblasts to form multinucleated myotubes. Many cell adhesion proteins, including integrins, have been shown to be important for myoblast fusion in vertebrates, but the mechanisms by which these proteins regulate cell fusion remain mostly unknown. Here, we focused on the role of focal adhesion kinase (FAK), an important nonreceptor protein tyrosine kinase involved in integrin signaling, as a potential mediator by which integrins may regulate myoblast fusion. To test this hypothesis in vivo, we generated mice in which the Fak gene was disrupted specifically in muscle stem cells (“satellite cells”) and we found that this resulted in impaired myotube formation during muscle regeneration after injury. To examine the role of FAK in the fusion of myogenic cells, we examined the expression of FAK and the effects of FAK deletion on the differentiation of myoblasts in vitro. Differentiation of mouse primary myoblasts was accompanied by a rapid and transient increase of phosphorylated FAK. To investigate the requirement of FAK in myoblast fusion, we used two loss-of-function approaches (a dominant-negative inhibitor of FAK and FAK small interfering RNA [siRNA]). Inhibition of FAK resulted in markedly impaired fusion but did not inhibit other biochemical measures of myogenic differentiation, suggesting a specific role of FAK in the morphological changes of cell fusion as part of the differentiation program. To examine the mechanisms by which FAK may be regulating fusion, we used microarray analysis to identify the genes that failed to be normally regulated in cells that were fusion defective due to FAK inhibition. Several genes that have been implicated in myoblast fusion were aberrantly regulated during differentiation when FAK was inhibited. Intriguingly, the normal increases in the transcript of caveolin 3 as well as an integrin subunit, the β1D isoform, were suppressed by FAK inhibition. We confirmed this also at the protein level and show that direct inhibition of β1D subunit expression by siRNA inhibited myotube formation with a prominent effect on secondary fusion. These data suggest that FAK regulation of profusion genes, including caveolin 3 and the β1D integrin subunit, is essential for morphological muscle differentiation.
INTRODUCTION
Skeletal muscle terminal differentiation is a temporally ordered process characterized by the expression of the myogenic regulatory factor myogenin, cell cycle withdrawal, the expression of muscle-specific proteins, and myoblast fusion (Andres and Walsh, 1996). Even though the molecular mechanisms regulating myoblast fusion remain largely unknown, many proteins have been shown to be essential for the fusion process to occur normally (Horsley and Pavlath, 2004; Chen et al., 2007). In the Drosophila embryo, the cellular and subcellular events described during fusion include cell recognition, adhesion, alignment, the recruitment of electron-dense vesicles and plaques, and membrane merging (Doberstein et al., 1997; Chen and Olson, 2004). Studies in Drosophila have suggested the importance of pathways involving cytoskeleton remodeling during fusion (Chen et al., 2007). In vertebrates, two stages of fusion have been distinguished: a first phase in which myoblasts fuse to other myoblasts (which we refer to as “primary fusion”) to form nascent myotubes, and a second phase in which fusion of additional myoblasts to the nascent myotubes allows myotube growth (which we refer to as “secondary fusion”). Secondary fusion is regulated by an NFATc2-dependent pathway (Horsley et al., 2003).
Although many proteins have been reported to be essential for myoblast fusion, the link between these molecules and details of the molecular pathways controlling primary fusion remain to be determined. Experimental evidence, obtained primarily by inhibition studies carried out in vitro, implicates several transmembrane proteins such as neural cell adhesion molecule (NCAM), N- and M-cadherins, a disintegrin and metalloprotease 12 (ADAM12), β1 integrins, caveolin 3, and CD9 in the process of myoblast fusion (Galbiati et al., 1999; Abmayr et al., 2003; Gullberg, 2003; Horsley and Pavlath, 2004). However, the mechanisms by which these proteins regulate fusion remain poorly understood, and genetic deletion studies have not confirmed an essential role of several of these molecules for myoblast fusion in vivo. The role of cell adhesion receptors in skeletal muscle has been particularly complex to analyze because they may exhibit redundant functions.
Integrins are heterodimeric proteins consisting of α and β subunits that serve as receptors for many extracellular matrix (ECM) ligands and some cell surface receptors (Van der Flier and Sonnenberg, 2001; Hynes, 2002). Vertebrate skeletal muscle expresses many integrin subunits in developmentally regulated patterns, including the integrin β1 subunit and its partners α1, α3, α4, α5, α6, α7, α9, and αv (Gullberg et al., 1998; Hirsch et al., 1998). The requirement of β1 integrins for myoblast fusion was demonstrated in vivo and in vitro (Schwander et al., 2003). The β1 integrin subunit has five isoforms with alternatively spliced cytoplasmic domains (de Melker and Sonnenberg, 1999). The β1A isoform is present in many tissues, whereas β1D is a muscle-specific variant, the predominant β1 isoform in striated muscle, and highly conserved between species (Van der Flier et al., 1995; Zhidkova et al., 1995; Belkin et al., 1996). β1A is abundantly expressed in proliferating myogenic precursor cells, but during differentiation it is gradually replaced by the β1D isoform (Belkin et al., 1996).
Focal adhesion kinase (FAK) has been identified as the key cytoplasmic tyrosine kinase that transmits integrin-mediated signals at focal adhesions in several cell types (Schlaepfer et al., 2004). Focal adhesions are regions of the plasma membrane in close contact with the ECM, organized around an integrin heterodimer core that connects the plasma membrane both to the ECM and the actin cytoskeleton via adaptor proteins, and enriched in signaling molecules. The localization of FAK at focal adhesions is dependent on its C-terminal focal adhesion targeting (FAT) domain (Hildebrand et al., 1993). The FAT domain of FAK interacts with integrin-associated proteins such as paxillin, which is also a substrate of FAK (Hildebrand et al., 1995; Mitra et al., 2005), and talin (Chen et al., 1995). FAK can be activated by growth factors and G protein-linked stimuli, but the major mode of FAK regulation is integrin-dependent (Burridge et al., 1992; Guan and Shalloway, 1992; Hanks et al., 1992; Kornberg et al., 1992; Schlaepfer et al., 2004). After integrin binding to ECM proteins, FAK undergoes autophosphorylation at Tyr397, creating a docking site for Src-family kinases and other proteins (Schaller, 2001). Activation of FAK initiates intracellular signal transduction cascades that, in turn, regulate cellular processes such as migration, growth, survival, and differentiation (Schaller, 2001; Schlaepfer et al., 2004). In muscle, FAK is phosphorylated during myoblast adhesion to fibronectin via an α5β1 integrin-dependent pathway (Disatnik and Rando, 1999). FAK phosphorylation is biphasic during C2C12 myoblast differentiation, and disruption of this temporal pattern interferes with normal myogenic differentiation (Clemente et al., 2005). The level and phosphorylation of FAK are increased during stretch or load-induced hypertrophy in skeletal muscle (Fluck et al., 1999; Carson and Wei, 2000). We recently reported that FAK is essential for the formation of costameres (Quach and Rando, 2006), protein complexes that in addition to ensuring the mechanical link between myofibrils and the sarcolemma also may transduce hypertrophic signals to regulate gene expression (Carson and Wei, 2000).
In this report, we investigate the requirement of integrin and FAK-mediated signaling for myoblast fusion. Our findings demonstrate that FAK activity is required for normal myoblast fusion in vitro and during muscle regeneration in vivo and that this is mediated at least in part by FAK-dependent expression of caveolin 3 and β1D integrin subunit, essential regulators of the fusion process.
MATERIALS AND METHODS
Animals
The mouse SV129 strain was obtained from Charles River Laboratories (Hollister, CA). We generated mice (FAKSC-knockout [KO] mice) in which the fak gene disruption could be induced in satellite cells by tamoxifen treatment. To obtain these knockouts, Fakflox/flox mice carrying floxed alleles of FAK (Fak-flox) (Beggs et al., 2003) were crossed with mice in which an inducible Cre (CreER) was knocked into the exon encoding the 3′-untranslated region (UTR) of the Pax7 gene (Brack et al., 2007). To induce fak disruption, 2-mo-old Pax7-CreER,Fakflox/flox mice received 150 μl of tamoxifen (20 mg/ml) by intraperitoneal injections for five consecutive days, and injury was performed at least 3 d after the last tamoxifen injection. Mice were genotyped by polymerase chain reaction (PCR) analysis of tail DNA by using the following primers for FAK: P2 (5′-GAATGCTACAGGAACCAAATAAC-3′) and P3 (5′-GAGAATCCAGCTTTGGCTGTTG-3′) (Beggs et al., 2003) and the following primers for Cre: Cre forward (5′-CACCCTGTTACGTATAGCCG-3′) and Cre reverse (5′-GAGTCATCCTTAGCGCCGTA-3′). The presence of the recombined Fak-flox allele was detected with primer P1 (5′-GACCTTCAACTTCTCATTTCTCC-3′) and P2 (5′-GAATGCTACAGGAACCAAATAAC-3′) PCR primers (Beggs et al., 2003). All animals were handled in accordance with guidelines of the Administrative Panel on Laboratory Animal Care of Stanford University (Stanford, CA).
Reagents
For immunoblot and immunostaining analysis, the following antibodies were used: mouse monoclonal anti-sarcomeric α-actin (Sigma-Aldrich, St. Louis, MO), mouse monoclonal anti-sarcomeric α-actinin (Sigma-Aldrich), mouse monoclonal anti-embryonic myosin heavy chain (eMyHC) (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), rabbit polyclonal anti-α5 integrin (Millipore Bioscience Research Reagents, Temecula, CA), rabbit polyclonal anti-β1A integrin subunit (Millipore Bioscience Research Reagents), mouse monoclonal anti-β1D integrin (Novus Biologicals, Littleton, CO), rabbit polyclonal anti-FAK (Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-caveolin 3 (BD Biosciences, San Jose, CA), rabbit polyclonal anti-FAK phosphotyrosine 397 (Santa Cruz Biotechnology), mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Ambion, Austin, TX), mouse monoclonal anti-myogenin (BD Biosciences Pharmingen, San Diego, CA), mouse monoclonal anti-vinculin (Sigma-Aldrich), horseradish peroxidase-linked sheep anti-mouse or donkey anti-rabbit (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), Alexa Fluor 594 donkey anti-mouse (Invitrogen, Carlsbad, CA), Cy3-conjugated goat anti-mouse (GE Healthcare), Cy2-conjugated donkey anti-rabbit (Jackson ImmunoResearch Laboratories, West Grove, PA), and fluorescein isothiocyanate-conjugated goat anti-rabbit (MP Biomedicals, Aurora, OH) antibodies.
Analysis of Muscle Regeneration
For regeneration studies, tibialis anterior muscles of 2- to 4-mo-old mice were injured by injection of 50 μl of BaCl2 (1.2%). Muscles were collected and embedded for cryostat sectioning. Regeneration 3 d after injury was analyzed in eMyHC- and 4,6-diamidino-2-phenylindole (DAPI)-costained muscle sections by measuring the diameter and number of cells expressing eMyHC within the injured area. Regeneration 5 d after injury was analyzed in hematoxylin and eosin (H&E)-stained muscle sections by measuring the diameter and number of centrally nucleated regenerating myofibers within the injured area. Measurements were performed using Volocity analysis software (Improvision, Coventry, England).
Primary Cultures of Myoblasts
Limb muscles from young (14- to 30-d-old) mice previously injured with a 30-gauge needle were dissociated to isolate pure populations of myoblasts as described previously (Quach and Rando, 2006). Primary cultures were plated on 5 μg/ml laminin-1 (Invitrogen)/collagen-coated dishes and amplified in growth medium (GM) consisting of Ham's F-10 (Mediatech, Herndon, VA) supplemented with 20% fetal bovine serum (Mediatech), 2.5 ng/ml basic fibroblast growth factor (Promega, Madison, WI), and penicillin (200 U/ml)/streptomycin (200 μg/ml) (Invitrogen). To induce differentiation, myoblast cultures plated on 10 μg/ml fibronectin (Calbiochem, San Diego, CA)-coated dishes were maintained in differentiation medium (DM) consisting of DMEM supplemented with 2% horse serum and penicillin/streptomycin. Myotubes were defined as cells with three or more nuclei. Cells with two nuclei were not counted as myotubes in order to exclude dividing cells.
Adenoviral Infection
Adenoviruses (Ads) expressing green fluorescent protein (GFP) (University of Iowa) or a FAT-GFP fusion protein (a generous gift from D. Ilic, StemLifeLine, San Carlos, CA) were amplified by infecting human embryonic kidney (HEK) 293 cells. HEK cells and medium were collected, frozen and thawed three times, centrifuged, and the supernatant containing adenoviruses was then aliquoted and stored. The viral titer in the supernatants was determined to be approximately ∼109 infectious units/ml (Adeno-X Rapid Titer kit; Clontech, Mountain View, CA). Myoblasts were plated in GM at a density of 4–4.5 × 105 cells/60-mm dish previously coated with fibronectin and were infected with adenoviral constructs 6 h after plating. The medium was replaced after 24 h, and cells were left for 24 h in GM before switching to DM. More than 80% cells infected with Ad-FAT-GFP expressed GFP 48 h after infection. Negligible cell detachment was observed in cultures infected with FAT-expressing adenovirus.
Small Interfering RNA (siRNA) Transfection
Two pairs of siRNA oligonucleotides targeting different regions of the FAK transcript (sense [GCCCUUGGGUCAAGUUGGAUCAUUU] and antisense [AAAUGAUCCAACUUGACCCAAGGGC] and sense [GAACAAUGAUGUGAUCGGUCGAAUU] and antisense [AAUUCGACCGAUCACAUCAUUGUUC], as well as a negative control siRNA oligonucleotide (Stealth select RNA interference [RNAi] from Invitrogen) were used. Two pairs of siRNA oligonucleotides targeting the β1D integrin subunit transcript (sense [UCCAAACUAUGGACGUAAA] and antisense [UUUACGUCCAUAGUUUGGA] and sense [CCAAACUAUGGACGUAAAG] and antisense [CUUUACGUCCAUAGUUUGG]), as well as negative control siRNA oligonucleotides (sense [GGAAUAACGGUUGCCGUCU] and antisense [AGACGGCAACCGUUAUUCC]; Invitrogen) were used in these studies. Myoblasts were plated at a density of 1.2–1.5 × 105 cells/35-mm dish on the day before the transfection. siRNA oligonucleotides (100 pmol/well) were transfected into myoblasts by using Lipofectamine 2000 (Invitrogen). The transfection medium was removed after 6 h and replaced with DM for β1D siRNA-transfected cells. Cells treated with FAK siRNA were switched to DM only 48 h after transfection, to allow the down-regulation of FAK at the protein level before the induction of differentiation. Negligible cell detachment was observed in FAK siRNA-treated cultures.
Western Blot Analysis
Muscles were homogenized and cells were scraped in lysis buffer (50 mM Tris-HCl, pH 7.5, 0.5% SDS, 20 μg/ml aprotinin, 20 μg/ml leupeptin, 10 μg/ml phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 10 mM sodium fluoride, and 1 mM dithiothreitol). Protein (60 μg) from total extracts was electrophoresed on 10% SDS-polyacrylamide gel electrophoresis gels and transferred to nitrocellulose membranes (GE Osmonics, Minnetonka, MN). The membranes were blocked for 1 h at room temperature with blocking buffer (phosphate-buffered saline [PBS], 0.05% Tween 20, and 5% milk) and were then probed overnight at 4°C with primary antibody diluted in blocking buffer. All primary antibody incubations were followed by incubation with an appropriate horseradish peroxidase-coupled secondary antibody for 1 h at room temperature. An enhanced chemiluminescence system (GE Healthcare) was used to visualize the specific secondary antibody binding. GAPDH was used as loading control.
Immunofluorescence
For immunofluorescence analysis, myoblast cultures were fixed for 10 min in 4% paraformaldehyde. Permeabilization and blocking of nonspecific binding were done for 1 h with 1% normal goat serum or 5% donkey serum in PBS containing 0.1% Triton X-100. Samples were incubated overnight at 4°C with primary antibody diluted in the same solution. Specimens were washed with PBS containing 0.1% Triton X-100 and were then incubated with secondary antibody for 2 h at room temperature. After washes, coverslips were applied with VECTASHIELD (Vector Laboratories, Burlingame, CA). Fluorescence was viewed with an Axioskop microscope (Carl Zeiss, Thornwood, NY).
Fusion Index and Myonuclear Number Determinations
After the induction of differentiation, cultures were analyzed microscopically to determine the fusion indices. Using a 20× objective and phase contrast, random fields were analyzed. Myotubes were defined as cells with three or more nuclei. The fusion index was determined as the percentage of nuclei in myotubes compared with the total number of nuclei in the field. Within a microscope field, the number of myotubes containing three to five nuclei, six to 10 nuclei, or >10 nuclei also was determined to calculate the myonuclear number. Approximately 100 myotubes were counted per dish. The percentage of myotubes containing the number of nuclei in each range was obtained by dividing by the total number of myotubes counted. Measurements were performed in triplicate in at least three independent experiments.
Time-Lapse Imaging
Cells were plated in 60-mm culture dishes coated with fibronectin and transfected with siRNA control or targeting FAK 24 h later. Forty-eight hours after transfection, cells were put in DM and analyzed using time-lapse microscopy. The cells were placed in an incubation chamber (Incubator S; Carl Zeiss) that maintained a constant temperature at 37°C and provided humidified 5% CO2. Images were acquired at 10-min intervals for 48 h by using an Axiovert 200M inverted microscope, Axiocam MRm camera, and Axiovision software (all from Carl Zeiss). The movie was exported and converted into a .mov file displaying images at the speed of 5 frames/s.
Microarray Data Analysis
Total RNA was extracted from primary cultures at 0, 14, and 41 h after differentiation by using TRIzol reagent according to manufacturer's instructions (Invitrogen). The subsequent steps were performed by the protein and nucleic acid biotechnology facility at Stanford University, including mRNA isolation and quality control, transcription to cDNA, reverse transcription to cRNA and biotinylation, fragmentation, hybridization to oligonucleotide Genechip Mouse Genome 430 2.0 array (Affymetrix, Santa Clara, CA), fluorescent labeling, optical scanning of the fluorescent chip, and data review. In total, six arrays, including three time points for each of the two types of cells (GFP or FAT expressing), were analyzed.
Microarray data were downloaded as .CHP files on Genesifter web-based program (http://www.genesifter.net/web/) by using median normalization. Affymetrix annotations for Genechip Mouse Genome 430 2.0 array were downloaded and added to the data set. Genes that received absent calls in the three control arrays were filtered out, which resulted in remaining 22,717 probe sets. We first searched for genes that were at least twofold up-regulated (cut-off >2) during differentiation at 14 h, 41 h, or both in control arrays and found 5831 probe sets. We then searched for genes that were up-regulated in controls but to a lesser extent in FAT-expressing cultures at 14 h, 41 h, or both (cut-off <0.75) and found 3203 probe sets. Uncharacterized probe sets were discarded, and only the probe set with highest intensity signals was retained for probe sets targeting the same gene. Finally, 1919 genes were further analyzed (Supplemental Table 3). Using a similar approach than for up-regulated genes, we found that 6067 probe sets were down-regulated during differentiation in control cultures. At 14 or 41 h after differentiation, 2514 probe sets were less down-regulated in FAT-expressing cells compared with controls. After exclusion of uncharacterized probe sets and probe sets carrying redundant gene title, 1614 genes remained for further analysis (Supplemental Table 4). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and hierarchical clustering analyzes were performed using Genesifter.
Quantitative Reverse Transcription-Polymerase Chain Reaction (QRT-PCR)
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen). For each sample, 2 μg of total RNA was reversed transcribed using SuperScript first-strand synthesis system and oligo(dT) primers (Invitrogen). The levels of expression of β1D and total β1 integrin transcripts were individually determined by real-time quantitative PCR using primer sets designed to specifically amplify β1D spliced transcripts or total β1 integrin transcripts in SYBR Green reagent (Applied Biosystems, Foster City, CA). Primer sequences to detect β1D subunit were 5′-CATCCCAATTGTAGCAGGCG-3′ (forward) in exon 6 and 5′-GAGACCAGCTTTACGTCCATAG-3′ (reverse) in exon D; and primer sequences to determine total β1 integrin expression were 5′-CATCCCAATTGTAGCAGGCG-3′ (forward) and 5′-CGTGTCCCACTTGGCATTCAT-3′ (reverse), both in exon 6 common to β1D and β1A isoforms. PCR reactions were run and analyzed using MyIQ Single-Color Real-Time PCR Detection system (Bio-Rad Laboratories, Hercules, CA). Reaction conditions were as follows: 10 min at 95°C and 45 amplification cycles (30 s at 95°C, 2 min at 60°C, and 30 s at 72°C). Mouse β1D subunit expression plasmid was obtained from Dr. Randall Kramer (University of California, San Francisco, San Francisco, CA) and used to determine the standard curve for β1D and total β1 integrin quantification following the recombinant DNA external standard method (Pfaffl, 2001). Determination of caveolin 3 mRNA level was performed using TaqMan probe (Applied Biosystems) following the manufacturer's instructions by using GAPDH for normalization. Each measurement was performed in triplicate in three independent experiments.
Statistical Analysis
Quantitative data are presented as means ± SD of at least three experiments. Statistical analysis to determine significance was performed using paired Student's t tests. Differences were considered to be statistically significant at the p < 0.05 level.
RESULTS
Deletion of FAK In Vivo in Satellite Cells Affects Muscle Regeneration
The requirement of β1 integrins for myoblast fusion has been demonstrated both in vivo and in vitro (Schwander et al., 2003). However, the downstream effectors of integrins that regulate myoblast fusion are unclear. To test whether FAK, as a downstream effector of integrins, is essential for myoblast fusion, we analyzed muscle regeneration in mice in which FAK was specifically disrupted in satellite cells (FAKSC-KO mice). Inducible targeted disruption of Fak in satellite cells was obtained by crossing Fakflox/flox mice (carrying alleles of FAK in which the second exon encoding the kinase domain was flanked by loxP sites with mice in which an inducible Cre (CreER) was knocked into the exon encoding the 3′-UTR of the Pax7 gene (Brack et al., 2007; Nishijo et al., 2009). Pax7 is a transcription factor expressed in adult satellite cells. In this system, CreER is expressed in satellite cells but translocates to the nucleus to catalyze recombination at loxP sites only in the presence of estrogen analogues such as tamoxifen.
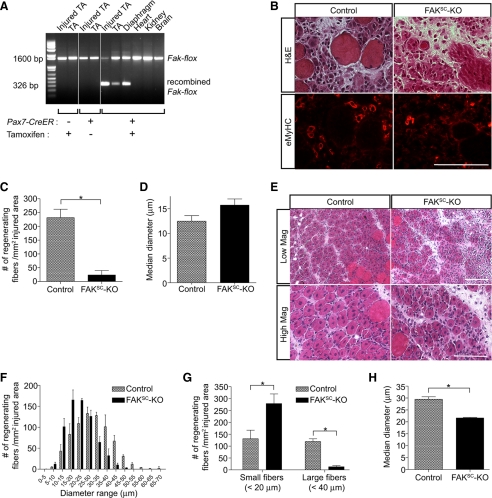
Genotyping of tissues collected from control and FAKSC-KO mice confirmed the specific disruption of Fak in skeletal muscles only after tamoxifen treatment (Figure 1A). Hindlimb muscles were then injured by BaCl2 injection. Regeneration was analyzed 3 d after injury when new myofibers started to form. Early regenerating myofibers expressing eMyHC were observed in injured areas (Figure 1B). Quantitative analysis revealed that the number of eMyHC-expressing myotubes was dramatically decreased in mice with the FAK deletion, although the diameters of the myotubes were comparable to controls (Figure 1, C and D). These results suggest that FAK is essential for normal myotube formation during adult myogenesis. Muscles were also analyzed 5 d after injury when most regenerating myofibers had formed but were still immature. Analysis of muscle sections revealed that muscle from mice in which FAK was deleted in satellite cells displayed the characteristics of delayed regeneration such as smaller regenerating myofibers, heterogeneity in myofiber size, increased number of interstitial cells and increased interstitial space between myofibers (Figure 1, E–H). In keeping with the findings at day 3, the numbers of large fibers (>40 μm) were much greater in the normal muscles (Figure 1G). However, on day 5, the regenerating muscle in FAKSC-KO mice also had a large number of small (<20 μm), newly forming fibers, and as a result the median fiber diameter was significantly less in the FAKSC-KO muscle (Figure 1, G and H), suggesting that FAK is essential for normal satellite cell-mediated myofiber formation. One month after injury, muscle morphology seemed similar in control and KO muscles (diameters of regenerating myofibers, 58.26 ± 7.84 and 59.35 ± 8.37 μm, respectively). Therefore, although the initial phases of myoblast fusion are disrupted in the absence of FAK, there clearly are compensatory mechanisms that ultimately result in effective regeneration even if significantly delayed compared with control muscle.
Figure 1.
Targeted disruption of Fak in satellite cells impairs skeletal muscle regeneration. (A) Tissue genotyping. In 2-mo-old Pax7-CreER,Fakflox/flox mice, recombination at the Fak locus was detected in skeletal muscles only after tamoxifen treatment. (B) Control (Fakflox/flox) and FAKSC-KO (Pax7-CreER,Fakflox/flox) mice treated with tamoxifen were injured by BaCl2 injection in tibialis anterior muscles. Serial sections of 3-d regenerating muscles were stained with H&E (top) and immunostained with an antibody to eMyHC. Bar, 100 μm. (C) The numbers of regenerating myofibers expressing eMyHC were measured in control and FAKSC-KO muscles 3 d after injury (n = 3; *p < 0.05). (D) The diameters of regenerating myofibers expressing eMyHC were measured in control and FAKSC-KO muscles 3 d after injury (n = 3). (E) Muscle regeneration was analyzed 5 d after injury by H&E staining in control and FAKSC-KO muscles. Bar, 100 μm. (F) The numbers of regenerating myofibers (containing centrally located nuclei) were measured in control and FAKSC-KO muscles 5 d after injury and expressed as a histogram plot (n = 3). (G) The average numbers of either small (<20 μm) or large (>40 μm) regenerating fibers in control and FAKSC-KO muscles were determined 5 d after injury (n = 3; *p < 0.05). (H) The median diameters of regenerating myofibers were measured in control and FAKSC-KO muscles 5 d after injury (n = 3; *p < 0.05).
FAK Expression during Myogenic Differentiation of Cultured Primary Myoblasts
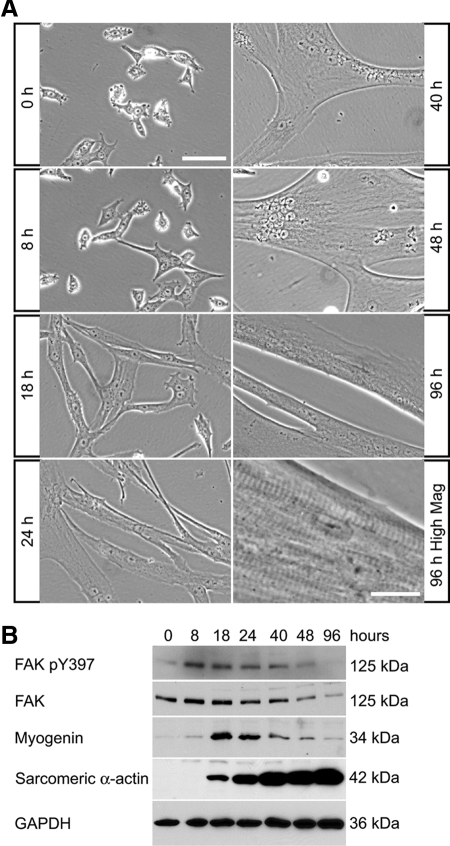
To be able to study the mechanisms by which FAK regulates muscle cell fusion, we isolated primary myoblasts from mice and studied their differentiation in vitro. Skeletal muscle differentiation proceeds by the expression of muscle-specific proteins and the formation of multinucleated myotubes (Andres and Walsh, 1996). When cultured in low serum conditions, myoblasts started to spread, elongate, then fuse to form multinucleated myotubes (Figure 2A). Myoblast fusion was mostly completed within 48 h of the initiation of differentiation. Further maturation of the contractile apparatus resulted in observable sarcomeric striations and myotube contractions (Figure 2A). A transient and rapid increase of phosphorylated FAK (activated form of the protein) was observed at early time points of differentiation, and was accompanied by the transient up-regulation of myogenin, an early marker of terminal differentiation whose expression precedes the induction of muscle-specific structural gene expression such as sarcomeric α-actin (Figure 2B). As myotubes matured, the level of FAK protein declined, occasionally as early as 48 h after the initiation of differentiation but always at later time points (Figure 2B).
Figure 2.
Expression of FAK during myogenic differentiation in primary myoblast cultures. (A) Mouse primary myoblasts were cultured in DM and observed by phase contrast microscopy. Multinucleated myotubes formed within 48 h of initiation of differentiation. Myotube maturation is associated with the appearance of aligned sarcomeric striations and myotube contractions (seen at high magnification at 96 h). Bar, 100 μm (except for 96 h, high-magnification panel, 20 μm). (B) Primary myoblasts were cultured in DM then analyzed by Western blots. The levels of FAK and phosphorylated FAK were high during early differentiation then decreased as myotubes mature. Myogenin was transiently up-regulated during early differentiation, whereas sarcomeric α-actin increased dramatically with myogenic differentiation.
Inhibition of FAK by the Dominant-Negative Form FAT Inhibits Myoblast Fusion
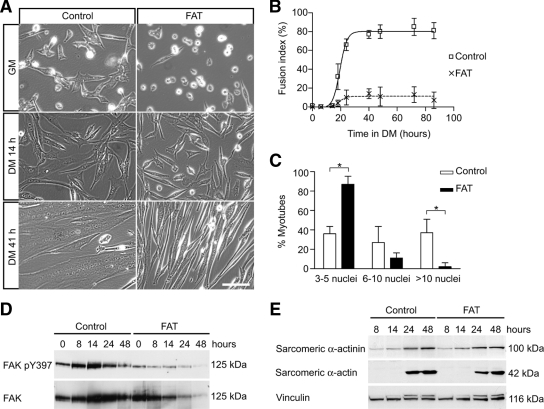
FAK is a known as a major effector of integrin signaling (Mitra et al., 2005), and β1 integrin subunits have been shown to be essential for myoblast fusion in vitro and in vivo (Schwander et al., 2003). To test whether FAK may regulate cell fusion in skeletal muscle, we inhibited FAK in mouse primary myoblast cultures by adenoviral expression of its FAT domain, which acts as a dominant-negative inhibitor of FAK (Gilmore and Romer, 1996; Schaller, 2001). Myoblasts were infected with an FAT-GFP–expressing adenovirus (Ad-FAT) or a GFP-expressing adenovirus (Ad-GFP) as a control and then cultured in DM. After 14 h in DM, most control cells had elongated and begun to fuse to form nascent myotubes, whereas FAT-expressing cells had elongated but remained mostly mononucleated (Figure 3A). After 41 h in DM, control (GFP-expressing) cultures were composed of elongated myotubes containing a large number of nuclei, whereas FAT-expressing cells exhibited minimal fusion (Figure 3A).
Figure 3.
Inhibition of FAK by its dominant-negative FAT inhibits myoblast fusion without blocking the expression of muscle terminal differentiation genes. (A) Myoblasts infected with adenoviruses expressing GFP (“control”) or FAT-GFP (“FAT”) were induced to differentiate to determine the effect of FAK inhibition on myogenesis. The morphology of the cells was analyzed by phase contrast microscopy. Myoblast fusion was impaired in FAT-expressing cultures that were composed mostly of mono- or binucleated cells, even after 3 d in DM. Bar, 100 μm. (B) Quantitative analysis of morphological differentiation by determination of the fusion index. The fusion index in FAT-expressing cultures was dramatically decreased compared with control cultures. (C) Quantitative analysis of myonuclear content after 48 h of differentiation. This analysis demonstrates a decreased number of nuclei in FAT-expressing myotubes, with the proportion of myotubes with three to five nuclei being increased, and the proportion of myotubes with >10 nuclei being decreased compared with control cultures (*p < 0.05). (D) Myoblasts expressing GFP (“control”) and FAT-GFP (“FAT”) were cultured in DM and analyzed by Western blots. A transient up-regulation of phosphorylated FAK and FAK protein was detected during early differentiation in controls but not in FAT-expressing cultures. (E) Expression of myogenic markers. Sarcomeric α-actinin and α-actin were induced normally in FAT-expressing cells, suggesting that the expression of muscle terminal differentiation proteins was not blocked when FAK was inhibited (representative blot of at least 3 independent experiments). Unlike FAK, the expression of focal adhesion protein vinculin did not seem to change appreciably during myogenic differentiation. Note that the muscle-specific isoform, metavinculin, which is of slightly greater molecular weight and thus runs just above vinculin and is recognized by the same antibody, increased during muscle differentiation and is similarly increased in FAT-expressing and control cells.
The morphological observations were quantified by the determination of the fusion index in the cultures. The fusion index of FAT-expressing cultures was much lower than that of control cultures at every time point (Figure 3B), indicating that FAK signaling is essential for myoblast-to-myoblast fusion (“primary” fusion) leading to the formation of nascent myotubes. The fusion index of both cultures reached a plateau about at the same time, but the plateau value of FAT-expressing cells was dramatically lower, suggesting that inhibition of FAK did not simply cause a delay of myoblast fusion. In FAT-expressing myotubes that did form, a lower average myonuclear content was observed compared with control cultures (Figure 3C), indicating that FAK signaling is also essential for myoblast-to-myotube fusion (“secondary” fusion) allowing myotube growth. The number of FAT-expressing myotubes containing three to five nuclei was much higher than control myotubes, whereas FAT-expressing myotubes containing >10 nuclei were rarely observed.
FAK Inhibition Prevents Myoblast Fusion without Blocking the Expression of Muscle-specific Genes
To verify the inhibition of FAK by FAT expression, we analyzed the level of FAK phosphorylation by Western blots (Figure 3D). The transient increase of phosphorylated FAK induced during differentiation was inhibited when FAT was expressed, consistent with the fact that FAT competes with endogenous FAK for the localization at focal adhesions where FAK becomes normally phosphorylated. Surprisingly, expression levels of muscle-specific genes such as sarcomeric α-actin and α-actinin were similar in FAT-expressing and control cells (Figure 3E), even though myoblast fusion was impaired in FAT-expressing cells. The focal adhesion protein vinculin did not change appreciably. Immunostaining of differentiated control and FAT-expressing cultures revealed a similar proportion of cells positive for sarcomeric α-actinin (data not shown). These data suggest that inhibition of FAK selectively affects morphological differentiation without blocking the expression of muscle-specific genes induced during terminal differentiation.
Myoblast Fusion Is Prevented by FAK Inhibition Confirmed by Using siRNA
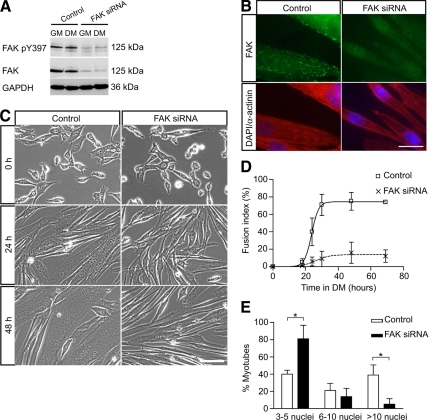
To confirm the requirement of FAK for myoblast fusion using an alternative approach, we inhibited FAK expression by siRNA. Myoblasts transfected with control siRNA or siRNA directed against FAK were cultured in DM. Efficient down-regulation of total and phosphorylated FAK in the cells was observed (Figure 4A). FAK is normally expressed at focal adhesions but could barely be detected in cultures treated with FAK siRNA (Figure 4B), confirming the inhibition of FAK expression.
Figure 4.
Down-regulation of FAK protein expression by siRNA inhibits myoblast fusion. (A) Myoblasts were transfected with control siRNA or FAK siRNA, cultured in DM for 48 h and then analyzed by Western blots to determine the efficacy of FAK knockdown by siRNA. The levels of phosphorylated FAK and FAK protein were dramatically down-regulated in FAK siRNA-treated cells. GAPDH was used as a loading control. (B) Immunofluorescence of siRNA-treated myotubes with an anti-FAK antibody revealed the presence of FAK at focal adhesions in control cells but not in FAK siRNA-treated cells. Coimmunostaining for sarcomeric α-actinin showed expression of this marker of muscle terminal differentiation in FAK-inhibited cells. Nuclei are labeled with DAPI. Bar, 20 μm. (C) Myoblasts transfected with control or FAK siRNA were cultured in DM to determine the effect of FAK down-regulation on myoblast fusion. Myoblast fusion was impaired in FAK siRNA-treated cultures in which most cells were elongated but remained mono- or binucleated. Bar, 100 μm. (D) Determination of the fusion index in control and FAK siRNA-treated cultures confirmed the defect of myoblast fusion when FAK was inhibited. (E) Determination of myonuclear content in control and FAK siRNA-treated cultures. Cells transfected with siRNA oligonucleotides were cultured in DM for 48 h. The proportion of myotubes containing few nuclei was increased whereas the proportion of myotubes containing a large number of nuclei was decreased in FAK siRNA-treated cultures (*p < 0.05).
In DM, control cultures contained many multinucleated myotubes, whereas FAK siRNA-treated cultures were mostly composed of elongated mono- or binucleated cells (Figure 4C). Time-lapse movies of control and FAK siRNA-treated cells undergoing differentiation confirmed the defect of fusion observed when FAK is inhibited (Supplemental Videos 1 and 2). After 48 h in DM, the fusion index and nuclear content of myotubes in control cultures had increased substantially, unlike for FAK siRNA-treated cells (Figure 4, D and E). Together, these data demonstrated that inhibition of FAK, either by FAT expression or by siRNA, resulted in inhibition of myoblast fusion, suggesting an essential role of FAK in that process.
Microarray Analysis to Compare Gene Expression in Normal Versus Fusion-defective Cells
Our observations suggest that FAK signaling is essential for myoblast fusion. To identify FAK-regulated genes that may be involved in myoblast fusion, we used microarray analysis to compare the profile of FAT-expressing cultures, characterized by a fusion-defective phenotype, to the profile of control cells during myogenic differentiation. We investigated the expression profile of these cultures by extracting the total RNA before and at different times after the initiation of differentiation. Analysis was performed using well established Affymetrix arrays to determine which genes were differentially regulated in fusion-defective cells.
The global pattern of gene expression changes during differentiation of control cells was similar to changes that have been reported in C2C12 cells undergoing differentiation (Moran et al., 2002). To determine genes that are associated with the fusion defect observed in FAT-expressing cells, we analyzed the expression profile of these cells compared with control cells. We searched for genes that were up-regulated in controls but to a lesser extent in FAT-expressing cultures at 14 h, 41 h, or both. We found 1919 genes as potential candidates regulating myoblast fusion (Supplemental Table 1), including such genes as CD9 and dysferlin (Tachibana and Hemler, 1999; de Luna et al., 2006). Whether or not these candidate genes were expressed in proliferating myoblasts, they were either transiently or persistently up-regulated during normal differentiation (Figure 5A). We analyzed these genes according to the KEGG (Kanehisa et al., 2008) to determine the most represented molecular pathways involved (Supplemental Table 2). Many of these genes are known to be involved in MAPK signaling, the regulation of actin cytoskeleton, focal adhesions, Wnt signaling and insulin signaling, all of which are known to interact with FAK pathways (Schaller, 2001; Cohen et al., 2002; Goel and Dey, 2002; Mitra et al., 2005).
Figure 5.
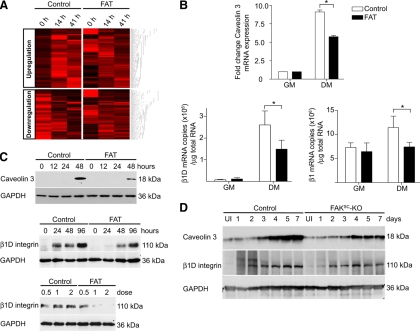
Identification of genes that failed to be normally regulated in FAT-expressing cells by microarray analysis and confirmation for two candidate genes. (A) Hierarchical clustering of the 1919 genes that failed to be up-regulated in cells with inhibited FAK signaling, and 1614 genes that failed to be down-regulated. (B) Quantitative real-time RT-PCR confirmed the inhibition of caveolin 3, β1D integrin subunit, and total β1 integrin transcript up-regulation in FAT-expressing cultures within 48 h after the switch to DM (*p < 0.05). (C) FAT-expressing myoblasts were cultured in DM then analyzed by Western blots to determine the effect of FAK inhibition on caveolin 3 and β1D integrin subunit at the protein level. The induction of caveolin 3 and β1D subunit upon differentiation was delayed in FAT-expressing cultures compared with control cultures. Myoblasts infected with different doses of Ad-GFP (control) or Ad-FAT-GFP (FAT) also were analyzed 48 h after differentiation. Inhibition of expression of the β1D subunit by FAT expression was dose dependent. (D) Protein expression of caveolin 3 and β1D integrins was analyzed in uninjured (UI) and regenerating muscles of control and FAKSC-KO mice. Caveolin 3 and β1D integrin up-regulation failed to occur normally in regenerating FAKSC-KO muscles.
Because myoblast fusion is also dependent on the down-regulation of specific genes (Cerletti et al., 2006), we screened for genes that were down-regulated during normal differentiation but not in FAT-expressing cells. Using a similar approach, we found 1614 genes that failed to be down-regulated in fusion-defective cells expressing FAT (Figure 5A and Supplemental Table 3). Gene ontologies were analyzed using KEGG pathways (Supplemental Table 2).
FAK Inhibition Impairs Caveolin 3 and β1d Integrin Expression during Myogenic Differentiation
Many genes important for myotube formation failed to be normally regulated in fusion-defective cells expressing FAT (Supplemental Table 4). These observations suggest that expression of fusion-related genes is coordinated during myogenic differentiation and that FAK signaling may function upstream in the regulation of the fusion process and in the fusion gene hierarchy. Throughout the process of muscle terminal differentiation that drives the fusion of mononucleated myoblasts into multinucleated contractile myotubes, a switch of expression is observed from ubiquitous to muscle-specific isoforms or members of multigene families (Nadal-Ginard, 1990). This transcriptional transition is associated with structural and functional changes necessary for the formation of differentiated muscle tissue, including the expression of genes necessary for myoblast fusion. Because our primary interest concerns cell surface receptors that may play a role in muscle cell fusion, we searched for transmembrane proteins that have been reported to be involved in muscle development.
Among transmembrane proteins known to be important in myoblast fusion whose expression was dysregulated in fusion-defective cells was caveolin 3 (Galbiati et al., 1999). A ubiquitously expressed member of the caveolin gene family, caveolin 2, failed to be normally down-regulated rapidly during myoblast differentiation whereas the muscle-specific form caveolin 3 failed to be normally up-regulated. We confirmed the aberrant expression of caveolin 3 in fusion-deficient cells by quantitative real-time RT-PCR and Western blot analyses (Figure 5, B and C). During muscle regeneration, caveolin 3 is transiently up-regulated (data not shown). As in vitro, we observed an impaired up-regulation of caveolin 3 protein expression during muscle regeneration in FAKSC-KO mice (Figure 5D). Because caveolin 3 has been shown to be required for myoblast fusion (Galbiati et al., 1999), this provides a mechanism by which FAK may regulate fusion, by controlling the expression of profusion genes.
A muscle-specific gene that failed to be normally up-regulated upon differentiation in our fusion-defective cultures, and of particular interest in terms of the relationship between FAK signaling and muscle cell fusion, was integrin β1D. Interestingly, this gene has not been shown to be regulated by FAK previously. To confirm that integrin β1D failed to be appropriately up-regulated in cells with inhibited FAK signaling, we analyzed β1D subunit mRNA expression by real-time RT-PCR. The expression of the β1D isoform transcripts was highly induced during myogenic differentiation, and that induction was suppressed in cells in which FAK signaling was inhibited by FAT expression (Figure 5B). Interestingly, the level of total β1 integrin transcripts was also decreased in differentiated cells expressing FAT (Figure 5B). The proportion of β1D transcripts compared with the total amount of β1 integrins remained similar in control and FAT-expressing cells (22 and 20%, respectively, after 48 h of differentiation). Therefore, the decline in β1D transcript levels by FAK inhibition was a consequence of reduced expression of total β1 transcript rather than a shift in the proportion of alternative splice variants. We found that the induction of β1D subunit isoform, which had previously been shown to be associated with muscle differentiation (Belkin et al., 1996), also was blocked at the protein level in a dose-dependent manner by the inhibition of FAK (Figure 5C).
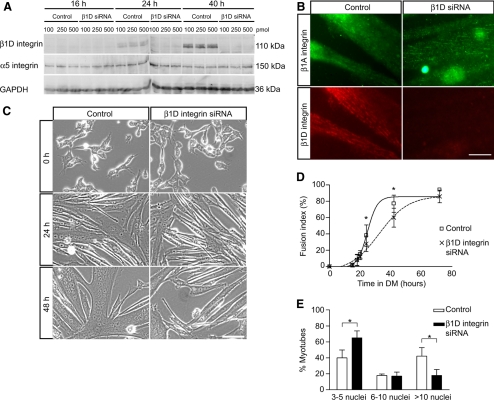
Inhibition of β1D Integrins by siRNA Affects Secondary Fusion
The requirement of β1 integrins for myotube formation was previously shown using function-blocking antibodies (Rosen et al., 1992). The report from Schwander et al. (2003) demonstrated that specific deletion of β1 integrins in skeletal muscle blocked the fusion of myogenic cells in vivo (Schwander et al., 2003). The ubiquitous isoform β1A and the muscle-specific β1D isoform are expressed in mouse skeletal muscle, but β1A is gradually replaced during myogenic differentiation by β1D that becomes the predominant isoform in adult muscle (Belkin et al., 1996). Insofar as β1D subunit expression correlates with myogenic differentiation and is impaired in fusion-deficient cells in which FAK is inhibited, we asked whether this isoform is required for myoblast fusion using an siRNA approach. Myoblasts were transfected with different doses of siRNA specifically directed against β1D subunit (“β1D siRNA”) and then cultured in DM. β1D subunit expression increased during differentiation in control cells but was barely detected in β1D siRNA-treated cultures (Figure 6, A and B), demonstrating an efficient blockade of β1D protein expression. By contrast, the β1A subunit was expressed in both control and β1D siRNA-treated cells (Figure 6B). Multinucleated myotubes did form in β1D siRNA-treated cultures (Figure 6C), and the fusion index of these cultures ultimately reached levels similar to controls with a slight delay (Figure 6D). However, interestingly, the myonuclear content of β1D siRNA-treated cultures was significantly lower than in control cultures after 72 h in DM, reflected by a much higher percentage of myotubes with few nuclei and much lower percentage of myotubes with many nuclei (Figure 6E). These data suggest an important role of β1D subunit in secondary fusion involved in myotube growth. Together, these results support the hypothesis that FAK signaling is essential for myoblast fusion by regulating β1D integrin expression, thereby highlighting the bidirectional signaling involved in integrin–FAK pathway during myogenesis.
Figure 6.
Down-regulation of β1D subunit by siRNA inhibits myoblast fusion. (A) siRNA was used to down-regulate the level of β1D integrins expression during myogenic differentiation to study the effect on cell fusion. Myoblasts were transfected with different amounts of siRNA oligonucleotides (100, 250, or 500 pmol/well) and then cultured in DM. Analysis by Western blots demonstrates an effective inhibition of β1D integrin up-regulation upon differentiation at all doses tested. The α5 integrin subunit, used here as a control, remained unchanged. (B) Immunofluorescence of β1A and β1D integrin subunits in cells treated with siRNA and cultured for 48 h in DM. In control myotubes, both isoforms were localized at focal adhesions. In cultures treated with β1D subunit siRNA, only the β1A isoform was detected. Bar, 20 μm. (C) Myoblasts treated by siRNA were induced to differentiate to determine the effect of β1D subunit inhibition on morphological differentiation. Cells treated with β1D subunit siRNA formed myotubes of a smaller size. Bar, 100 μm. (D) Determination of fusion index in siRNA-treated cultures. Fusion was delayed in β1D siRNA-treated cultures but ultimately reached a similar plateau to that seen in control cultures, suggesting that primary fusion was relatively normal in the absence of the β1D isoform (*p < 0.05). (E) Determination of myonuclear content in siRNA-treated cultures. Cells transfected with siRNA were cultured in DM for 72 h. The proportion of myotubes containing a large number of nuclei was decreased in β1D siRNA-treated cultures suggesting a selective inhibition of secondary fusion (*p < 0.05).
DISCUSSION
The fusion of myoblasts into myotubes is one of the most critical events during skeletal muscle formation. How mononucleated myoblasts fuse to form multinucleated myotubes that later mature into myofibers is thus a fundamental question in the biology of skeletal muscle. Cellular and molecular events occurring during myoblast fusion have been extensively studied in Drosophila (Abmayr et al., 2008). In vertebrates, many individual proteins required for primary fusion have been identified (Jansen and Pavlath, 2008), but an integrated molecular pathway has yet to be described.
Among transmembrane proteins, several integrins have been shown to be involved in myoblast fusion in vitro and in vivo (Gullberg, 2003). FAK is known as a major effector of integrin signaling, and a prime candidate downstream of integrins to regulate myoblast fusion. In the present article, we show that FAK is transiently up-regulated during terminal differentiation and that inhibition of FAK, whether by the expression of a dominant-negative form of the protein, by siRNA knockdown, or by genetic disruption of Fak, results in impaired myoblast fusion in vitro as well as during muscle regeneration in vivo. Interestingly, expression of terminal differentiation genes was not blocked when FAK was inhibited, suggesting that FAK is important primarily for the morphological aspect of skeletal muscle terminal differentiation.
Evidence of an essential role of FAK for myoblast fusion in vivo is limited. In mice, genetic deletion of FAK results in early embryonic lethality, precluding analysis of the effects on myogenesis (Furuta et al., 1995). In a study reporting the effects of FAK deletion in brain, it was noted (data not shown) that a muscle-specific deletion of FAK resulted in a myopathic phenotype (Beggs et al., 2003), but a detailed characterization of this strain has not been reported. Studies in Drosophila have not demonstrated an essential role of FAK in myoblast fusion in invertebrates. Deletion of the fak56 gene, the Drosophila homologue of Fak sharing ∼33% amino acid identity with human Fak (Schaller, 2001), seems to result in no muscle defect, and the flies are viable and fertile (Grabbe et al., 2004). However, with few exceptions limited to cell–cell adhesion receptors and actin remodeling regulators (Srinivas et al., 2007; Moore et al., 2007; Kim et al., 2007; Pajcini et al., 2008), most molecules reported to be involved in vertebrate myoblast fusion have not been shown to be required for invertebrate myoblast fusion. It remains to be determined whether there is evolutionary divergence of the molecules and pathways involved in myoblast fusion or whether there is evolutionary conservation that has not yet been revealed by functional studies.
What Are FAK Downstream Targets during Myoblast Fusion?
The molecular mechanisms by which FAK and its downstream signaling regulate myoblast fusion need to be further investigated. Our microarray analysis has revealed genes potentially implicated in myoblast fusion and regulated by FAK signaling. The finding that FAK may be an upstream regulator of caveolin 3 expression is novel and requires further investigation, but it is particularly interesting that inhibition of FAK signaling (Figure 3E) and antisense-mediated reduction of caveolin 3 expression (Galbiati et al., 1999) both inhibit fusion without significantly affecting the expression of muscle-specific proteins in myoblasts induced to undergo differentiation. In vivo, down-regulation of caveolin 3 in zebrafish results in defects of myoblast fusion (Nixon et al., 2005), and caveolin 3 knockout mice display a mild muscular dystrophy phenotype (Hagiwara et al., 2000; Galbiati et al., 2001). We also have found that FAK signaling is essential for the normal up-regulation of the β1D integrin isoform and that the expression of this isoform is, in turn, essential for normal myoblast fusion. The regulation of β1D integrin expression by FAK signaling likely represents only one mechanism by which FAK is involved in secondary fusion, because inhibition of FAK results in a more extensive inhibition of fusion than does inhibition of β1D expression. Because FAK is at the intersection of many signaling pathways, it probably regulates myoblast fusion by targeting multiple pathways that remain to be fully determined. In addition to FAK-regulated β1D integrin expression that we observed, it was recently suggested that β1D integrin is required for stretch-activated phosphorylation of FAK during fusion of C2C12 myoblasts (Zhang et al., 2007). Together, these observations suggest complex feedback regulatory loops between integrins and FAK during myoblast fusion.
The involvement of FAK in cytoskeletal remodeling (Mitra et al., 2005) may be another important aspect of FAK function in fusion. In Drosophila, loss of function of the cytoskeleton regulators Mbc and Drac inhibits myoblast fusion (Rushton et al., 1995; Hakeda-Suzuki et al., 2002). Mammalian homologues of Mbc and Drac are DOCK180 and Rac, respectively. By phosphorylating Cas and paxillin, FAK may recruit the Cas–Crk–DOCK180–ELMO complex activator of Rac involved in actin assembly, protrusive activity, and modulation of focal complex stability at the leading edge of cells (Mitra et al., 2005). Intriguingly, many downstream effectors of FAK signaling involved in cytoskeletal rearrangements have been shown to be essential for myoblast fusion, including DOCK180 and Crk in zebrafish (Moore et al., 2007), ELMO (Geisbrecht et al., 2008), Arp2/3 (Richardson et al., 2007), Arf6 (Chen et al., 2003), and members of the WASP family of proteins (Kim et al., 2007; Massarwa et al., 2007; Schafer et al., 2007) in Drosophila. Finally, the Rho-serum response factor pathway involved in the transcription of muscle-specific genes is integrin and FAK dependent (Carson and Wei, 2000). In addition to controlling cell morphology and dynamics, remodeling of actin cytoskeleton is essential for the regulation of muscle gene expression (Formigli et al., 2007). It will be interesting to investigate whether FAK play an essential function in regulating gene expression by controlling cytoskeletal dynamics.
Microarray analysis revealed that many genes failing to be normally up-regulated in fusion-deficient myoblasts expressing FAT belonged to mitogen-activated protein kinase (MAPK) pathway (Supplemental Table 2). The MAPK pathway regulates gene transcription in skeletal muscle and is activated by FAK signaling in many cell types (Schlaepfer et al., 1994, 1999). The role of MAPK signaling in myogenesis is clearly complex, because MAPK activation has been implicated in both positive and negative regulation of myogenic differentiation, depending possibly on the cell lines and growth conditions used. Among MAPK family members, p42 and p38α MAPK activities seem to be essential for myotube formation and both can be activated by integrin signaling in skeletal muscle (Gredinger et al., 1998; Cuenda and Cohen, 1999; Perdiguero et al., 2007). It is not known whether FAK is signaling through MAPK pathway to regulate the expression of profusion genes and myoblast fusion.
Are β1 Integrins Required for Normal Myoblast Fusion in Vivo?
Interest in the role of integrins for myoblast fusion was fostered by reports demonstrating that function blocking antibodies against β1 integrins inhibited myotube formation in vitro (Menko and Boettiger, 1987; Rosen et al., 1992). Since then, the study of integrins in myoblast fusion has lead to conflicting results (Gullberg, 2003). Attempts to confirm the requirement of β1 integrins for myoblast fusion in vivo has been difficult as the knockout embryos die before the effect on muscle can be studied, whereas heterozygous and chimeric mice have a normal phenotype (Fassler and Meyer, 1995; Stephens et al., 1995). The fact that β1-deficient myoblasts isolated from chimeric mice were able to form myotubes in vitro was additional evidence that β1 integrins might not be essential for myoblast fusion (Hirsch et al., 1998). However, it has been proposed that cells maintained in culture for long periods may develop compensatory mechanisms, thus masking an essential process during normal fusion (Gullberg, 2003). In this regard, the development of mice displaying a muscle-specific deletion of the β1 integrin subunit provided an alternate interpretation. These mice die at birth, and analysis of their muscles indicates that β1 integrins are dispensable for myoblast proliferation, migration, and the expression of muscle-specific genes but are required for the fusion of myoblasts into multinucleated myotubes (Schwander et al., 2003).
During myogenic differentiation in vitro and in vivo, it was shown that a switch of β1 isoform occurs, from the ubiquitously expressed β1A isoform to the muscle-specific β1D isoform (Belkin et al., 1996). Because the β1D isoform is normally up-regulated upon differentiation but fails to do so in fusion-defective cells expressing an FAK inhibitor, we hypothesized that it may be involved in myoblast fusion. It was reported that myogenic conversion of NIH3T3 cells by expression of MRF4 activates an incomplete myogenic program characterized by the induction of muscle-specific gene expression such as myogenin, myosin heavy chain, troponin T, and muscle creatine kinase, but without cell fusion (Russo et al., 1998). In this study, the defect of fusion was correlated with the inability of the cells to accumulate the transcripts for muscle-specific isoforms of the β1 integrin subunit and the transcription factor MEF2D (β1D and MEF2D1b2, respectively), which was not the result of a generalized inability to perform muscle-specific differential splicing because other products such as α7A integrin and β-tropomyosin transcripts did undergo muscle-specific alternative splicing. Conversely, myogenic conversion of the C3H10T1/2 cell line by MRF4 expression resulted in biochemical differentiation, cell fusion, and β1D isoform expression. Our data suggest that β1D isoform mainly supports the process of secondary fusion involved in myotube growth, because inhibition of β1D expression by siRNA resulted in the formation of smaller myotubes without dramatically affecting the primary fusion process (Figure 6E).
Despite the evidence of the role of the β1D isoform in myotube formation and growth in vitro, data obtained from genetic deletion studies in mice do not demonstrate a requirement of the β1D isoform in muscle formation in vivo (Baudoin et al., 1998). Such a dichotomy between in vitro inhibition studies and in vivo genetic studies also has been observed for other adhesion proteins implicated in myoblast fusion, including M-cadherin (Zeschnigk et al., 1995; Hollnagel et al., 2002), ADAM12 (Yagami-Hiromasa et al., 1995; Kurisaki et al., 2003), or NCAM (Knudsen et al., 1990; Cremer et al., 1994; Moscoso et al., 1998). Transgenic mice rendered null for the expression of the β1D isoform do form skeletal muscles, suggesting that other integrins or transmembrane receptors may have overlapping functions and compensate for the lack of this isoform (Baudoin et al., 1998). By contrast, mice in which the expression of the β1A is replaced by the expression of the β1D isoform have defective primary myogenesis and reduced skeletal muscle mass in mice that survived until birth, suggesting that the two integrin isoforms are not functionally equivalent (Cachaco et al., 2003). In vitro, overexpression of either β1D or β1A isoform impairs myotube formation in C2C12 myoblasts (Cachaco et al., 2003) and in primary myoblasts (data not shown), suggesting that a precise regulation of either integrin protein levels or the signaling they mediate may be critical for fusion. Our studies both in vitro and in vivo provide evidence in a more regulated system that the β1D isoform is an essential downstream effector of myoblast fusion.
The role of caveolin 3 in myoblast fusion has not been clearly resolved based on gain-of-function and loss-of-function studies, both of which have been shown to disrupt myoblast fusion (Galbiati et al., 1999; Volonte et al., 2003). Likewise, myopathic changes are seen in muscles of mice deficient in caveolin 3 and in mice in which caveolin 3 is overexpressed (Galbiati et al., 2000, 2001). These findings demonstrate that a precise regulation of the levels of caveolin 3, like β1 integrins, is essential for normal muscle development and homeostasis, a regulation perhaps mediated by FAK signaling.
The present study demonstrates that FAK is essential for initial myoblast fusion events but not for the induction of the myogenic terminal differentiation program. Using microarray analysis to compare gene expression in control cultures versus fusion-defective primary myoblast cultures, we found that FAK regulates the expression of a set of muscle-specific genes specifically involved in myoblast fusion during early myogenic differentiation, including β1D integrins. Because FAK is a well known downstream effector of integrin signaling, these findings demonstrate bidirectional regulation between integrins and integrin effectors in the context of muscle cell terminal differentiation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Carmen Bertoni and Stéphane Boutet for helpful advice regarding real-time RT-PCR methodology and Drs. Fabienne Murphy-Seiler and Chris Bjornson for assistance with mice breeding strategies. We are grateful to Dr. Dusko Ilic for generously providing FAT-GFP–expressing adenoviruses and Dr. Randall Kramer for kindly providing the mouse β1D integrin expression plasmid. We thank Drs. Angélica Keller and Dominique Ledoux for helpful discussions on the project. This work was supported by National Institutes of Health grant NS-19090 (to L.F.R.) and NS-40718 (to T.A.R.) and a National Institutes of Health Director's Pioneer Award and grant from the Department of Veterans Affairs (to T.A.R.).
Abbreviations used:
- Ad
adenovirus
- DM
differentiation medium
- ECM
extracellular matrix
- eMyHC
embryonic myosin heavy chain
- FAK
focal adhesion kinase
- FAT
focal adhesion targeting
- GM
growth medium
- H&E
hematoxylin and eosin
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- PCR
polymerase chain reaction
- RT
reverse transcription
- siRNA
small interfering RNA.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-02-0175) on May 20, 2009.
REFERENCES
- Abmayr S. M., Balagopalan L., Galletta B. J., Hong S. J. Cell and molecular biology of myoblast fusion. Int. Rev. Cytol. 2003;225:33–89. doi: 10.1016/s0074-7696(05)25002-7. [DOI] [PubMed] [Google Scholar]
- Abmayr S. M., Zhuang S., Geisbrecht E. R. Myoblast fusion in Drosophila. Methods Mol. Biol. 2008;475:75–97. doi: 10.1007/978-1-59745-250-2_5. [DOI] [PubMed] [Google Scholar]
- Andres V., Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoin C., Goumans M. J., Mummery C., Sonnenberg A. Knockout and knockin of the beta1 exon D define distinct roles for integrin splice variants in heart function and embryonic development. Genes Dev. 1998;12:1202–1216. doi: 10.1101/gad.12.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs H. E., Schahin-Reed D., Zang K., Goebbels S., Nave K. A., Gorski J., Jones K. R., Sretavan D., Reichardt L. F. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin A. M., Zhidkova N. I., Balzac F., Altruda F., Tomatis D., Maier A., Tarone G., Koteliansky V. E., Burridge K. Beta 1D integrin displaces the beta 1A isoform in striated muscles: localization at junctional structures and signaling potential in nonmuscle cells. J. Cell Biol. 1996;132:211–226. doi: 10.1083/jcb.132.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack A. S., Conboy M. J., Roy S., Lee M., Kuo C. J., Keller C., Rando T. A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J. Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachaco A. S., Chuva de Sousa Lopes S. M., Kuikman I., Bajanca F., Abe K., Baudoin C., Sonnenberg A., Mummery C. L., Thorsteinsdottir S. Knock-in of integrin beta 1D affects primary but not secondary myogenesis in mice. Development. 2003;130:1659–1671. doi: 10.1242/dev.00394. [DOI] [PubMed] [Google Scholar]
- Carson J. A., Wei L. Integrin signaling's potential for mediating gene expression in hypertrophying skeletal muscle. J. Appl. Physiol. 2000;88:337–343. doi: 10.1152/jappl.2000.88.1.337. [DOI] [PubMed] [Google Scholar]
- Cerletti M., Molloy M. J., Tomczak K. K., Yoon S., Ramoni M. F., Kho A. T., Beggs A. H., Gussoni E. Melanoma cell adhesion molecule is a novel marker for human fetal myogenic cells and affects myoblast fusion. J. Cell Sci. 2006;119:3117–3127. doi: 10.1242/jcs.03056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. H., Grote E., Mohler W., Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–2193. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Chen E. H., Olson E. N. Towards a molecular pathway for myoblast fusion in Drosophila. Trends Cell Biol. 2004;14:452–460. doi: 10.1016/j.tcb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Chen E. H., Pryce B. A., Tzeng J. A., Gonzalez G. A., Olson E. N. Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell. 2003;114:751–762. doi: 10.1016/s0092-8674(03)00720-7. [DOI] [PubMed] [Google Scholar]
- Chen H. C., Appeddu P. A., Parsons J. T., Hildebrand J. D., Schaller M. D., Guan J. L. Interaction of focal adhesion kinase with cytoskeletal protein talin. J. Biol. Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Clemente C. F., Corat M. A., Saad S. T., Franchini K. G. Differentiation of C2C12 myoblasts is critically regulated by FAK signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R862–R870. doi: 10.1152/ajpregu.00348.2004. [DOI] [PubMed] [Google Scholar]
- Cohen E. D., Mariol M. C., Wallace R. M., Weyers J., Kamberov Y. G., Pradel J., Wilder E. L. DWnt4 regulates cell movement and focal adhesion kinase during Drosophila ovarian morphogenesis. Dev. Cell. 2002;2:437–448. doi: 10.1016/s1534-5807(02)00142-9. [DOI] [PubMed] [Google Scholar]
- Cremer H., Lange R., Christoph A., Plomann M., Vopper G., Roes J., Brown R., Baldwin S., Kraemer P., Scheff S. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Cuenda A., Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- de Luna N., Gallardo E., Soriano M., Dominguez-Perles R., de la Torre C., Rojas-Garcia R., Garcia-Verdugo J. M., Illa I. Absence of dysferlin alters myogenin expression and delays human muscle differentiation “in vitro.” J. Biol. Chem. 2006;281:17092–17098. doi: 10.1074/jbc.M601885200. [DOI] [PubMed] [Google Scholar]
- de Melker A. A., Sonnenberg A. Integrins: alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays. 1999;21:499–509. doi: 10.1002/(SICI)1521-1878(199906)21:6<499::AID-BIES6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Disatnik M. H., Rando T. A. Integrin-mediated muscle cell spreading. The role of protein kinase c in outside-in and inside-out signaling and evidence of integrin cross-talk. J. Biol. Chem. 1999;274:32486–32492. doi: 10.1074/jbc.274.45.32486. [DOI] [PubMed] [Google Scholar]
- Doberstein S. K., Fetter R. D., Mehta A. Y., Goodman C. S. Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J. Cell Biol. 1997;136:1249–1261. doi: 10.1083/jcb.136.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R., Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Fluck M., Carson J. A., Gordon S. E., Ziemiecki A., Booth F. W. Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am. J. Physiol. 1999;277:C152–C162. doi: 10.1152/ajpcell.1999.277.1.C152. [DOI] [PubMed] [Google Scholar]
- Formigli L., Meacci E., Zecchi-Orlandini S., Orlandini G. E. Cytoskeletal reorganization in skeletal muscle differentiation: from cell morphology to gene expression. Eur. J. Histochem. 51 (suppl 1), 21–. 2007;8:21–28. [PubMed] [Google Scholar]
- Furuta Y., Ilic D., Kanazawa S., Takeda N., Yamamoto T., Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- Galbiati F., Engelman J. A., Volonte D., Zhang X. L., Minetti C., Li M., Hou H., Jr, Kneitz B., Edelmann W., Lisanti M. P. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J. Biol. Chem. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- Galbiati F., et al. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc. Natl. Acad. Sci. USA. 2000;97:9689–9694. doi: 10.1073/pnas.160249097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F., Volonte D., Engelman J. A., Scherer P. E., Lisanti M. P. Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2C12 myoblasts. Transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube formation. J. Biol. Chem. 1999;274:30315–30321. doi: 10.1074/jbc.274.42.30315. [DOI] [PubMed] [Google Scholar]
- Geisbrecht E. R., Haralalka S., Swanson S. K., Florens L., Washburn M. P., Abmayr S. M. Drosophila ELMO/CED-12 interacts with Myoblast city to direct myoblast fusion and ommatidial organization. Dev. Biol. 2008;314:137–149. doi: 10.1016/j.ydbio.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A. P., Romer L. H. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol. Biol. Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel H. L., Dey C. S. Focal adhesion kinase tyrosine phosphorylation is associated with myogenesis and modulated by insulin. Cell Prolif. 2002;35:131–142. doi: 10.1046/j.1365-2184.2002.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe C., Zervas C. G., Hunter T., Brown N. H., Palmer R. H. Focal adhesion kinase is not required for integrin function or viability in Drosophila. Development. 2004;131:5795–5805. doi: 10.1242/dev.01462. [DOI] [PubMed] [Google Scholar]
- Gredinger E., Gerber A. N., Tamir Y., Tapscott S. J., Bengal E. Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J. Biol. Chem. 1998;273:10436–10444. doi: 10.1074/jbc.273.17.10436. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Gullberg D. Cell biology: the molecules that make muscle. Nature. 2003;424:138–140. doi: 10.1038/424138a. [DOI] [PubMed] [Google Scholar]
- Gullberg D., Velling T., Lohikangas L., Tiger C. F. Integrins during muscle development and in muscular dystrophies. Front. Biosci. 1998;3:D1039–D1050. doi: 10.2741/a344. [DOI] [PubMed] [Google Scholar]
- Hagiwara Y., Sasaoka T., Araishi K., Imamura M., Yorifuji H., Nonaka I., Ozawa E., Kikuchi T. Caveolin-3 deficiency causes muscle degeneration in mice. Hum. Mol. Genet. 2000;9:3047–3054. doi: 10.1093/hmg/9.20.3047. [DOI] [PubMed] [Google Scholar]
- Hakeda-Suzuki S., Ng J., Tzu J., Dietzl G., Sun Y., Harms M., Nardine T., Luo L., Dickson B. J. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl. Acad. Sci. USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J. D., Schaller M. D., Parsons J. T. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J. Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J. D., Schaller M. D., Parsons J. T. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol. Biol. Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E., Lohikangas L., Gullberg D., Johansson S., Fassler R. Mouse myoblasts can fuse and form a normal sarcomere in the absence of beta1 integrin expression. J. Cell Sci. 1998;111:2397–2409. doi: 10.1242/jcs.111.16.2397. [DOI] [PubMed] [Google Scholar]
- Hollnagel A., Grund C., Franke W. W., Arnold H. H. The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Mol. Cell. Biol. 2002;22:4760–4770. doi: 10.1128/MCB.22.13.4760-4770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V., Jansen K. M., Mills S. T., Pavlath G. K. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- Horsley V., Pavlath G. K. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jansen K. M., Pavlath G. K. Molecular control of mammalian myoblast fusion. Methods Mol. Biol. 2008;475:115–133. doi: 10.1007/978-1-59745-250-2_7. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–D484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Shilagardi K., Zhang S., Hong S. N., Sens K. L., Bo J., Gonzalez G. A., Chen E. H. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev. Cell. 2007;12:571–586. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Knudsen K. A., McElwee S. A., Myers L. A role for the neural cell adhesion molecule, NCAM, in myoblast interaction during myogenesis. Dev. Biol. 1990;138:159–168. doi: 10.1016/0012-1606(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J. Biol. Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- Kurisaki T., et al. Phenotypic analysis of meltrin alpha (ADAM12)-deficient mice: involvement of meltrin alpha in adipogenesis and myogenesis. Mol. Cell. Biol. 2003;23:55–61. doi: 10.1128/MCB.23.1.55-61.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarwa R., Carmon S., Shilo B. Z., Schejter E. D. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev. Cell. 2007;12:557–569. doi: 10.1016/j.devcel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Menko A. S., Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987;51:51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Mitra S. K., Hanson D. A., Schlaepfer D. D. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Moore C. A., Parkin C. A., Bidet Y., Ingham P. W. A role for the myoblast city homologues Dock1 and Dock5 and the adaptor proteins Crk and Crk-like in zebrafish myoblast fusion. Development. 2007;134:3145–3153. doi: 10.1242/dev.001214. [DOI] [PubMed] [Google Scholar]
- Moran J. L., Li Y., Hill A. A., Mounts W. M., Miller C. P. Gene expression changes during mouse skeletal myoblast differentiation revealed by transcriptional profiling. Physiol. Genomics. 2002;10:103–111. doi: 10.1152/physiolgenomics.00011.2002. [DOI] [PubMed] [Google Scholar]
- Moscoso L. M., Cremer H., Sanes J. R. Organization and reorganization of neuromuscular junctions in mice lacking neural cell adhesion molecule, tenascin-C, or fibroblast growth factor-5. J. Neurosci. 1998;18:1465–1477. doi: 10.1523/JNEUROSCI.18-04-01465.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Ginard B. Muscle cell differentiation and alternative splicing. Curr. Opin. Cell Biol. 1990;2:1058–1064. doi: 10.1016/0955-0674(90)90156-9. [DOI] [PubMed] [Google Scholar]
- Nishijo K., et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 2009 doi: 10.1096/fj.08-128116. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon S. J., Wegner J., Ferguson C., Mery P. F., Hancock J. F., Currie P. D., Key B., Westerfield M., Parton R. G. Zebrafish as a model for caveolin-associated muscle disease; caveolin-3 is required for myofibril organization and muscle cell patterning. Hum. Mol. Genet. 2005;14:1727–1743. doi: 10.1093/hmg/ddi179. [DOI] [PubMed] [Google Scholar]
- Pajcini K. V., Pomerantz J. H., Alkan O., Doyonnas R., Blau H. M. Myoblasts and macrophages share molecular components that contribute to cell-cell fusion. J. Cell Biol. 2008;180:1005–1019. doi: 10.1083/jcb.200707191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero E., et al. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation. EMBO J. 2007;26:1245–1256. doi: 10.1038/sj.emboj.7601587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach N. L., Rando T. A. Focal adhesion kinase is essential for costamerogenesis in cultured skeletal muscle cells. Dev. Biol. 2006;293:38–52. doi: 10.1016/j.ydbio.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Richardson B. E., Beckett K., Nowak S. J., Baylies M. K. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development. 2007;134:4357–4367. doi: 10.1242/dev.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G. D., Sanes J. R., LaChance R., Cunningham J. M., Roman J., Dean D. C. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992;69:1107–1119. doi: 10.1016/0092-8674(92)90633-n. [DOI] [PubMed] [Google Scholar]
- Rushton E., Drysdale R., Abmayr S. M., Michelson A. M., Bate M. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development. 1995;121:1979–1988. doi: 10.1242/dev.121.7.1979. [DOI] [PubMed] [Google Scholar]
- Russo S., Tomatis D., Collo G., Tarone G., Tato F. Myogenic conversion of NIH3T3 cells by exogenous MyoD family members: dissociation of terminal differentiation from myotube formation. J. Cell Sci. 1998;111:691–700. doi: 10.1242/jcs.111.6.691. [DOI] [PubMed] [Google Scholar]
- Schafer G., Weber S., Holz A., Bogdan S., Schumacher S., Muller A., Renkawitz-Pohl R., Onel S. F. The Wiskott-Aldrich syndrome protein (WASP) is essential for myoblast fusion in Drosophila. Dev. Biol. 2007;304:664–674. doi: 10.1016/j.ydbio.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Schaller M. D. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Hanks S. K., Hunter T., van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Hauck C. R., Sieg D. J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Mitra S. K., Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim. Biophys. Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Schwander M., Leu M., Stumm M., Dorchies O. M., Ruegg U. T., Schittny J., Muller U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev. Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Srinivas B. P., Woo J., Leong W. Y., Roy S. A conserved molecular pathway mediates myoblast fusion in insects and vertebrates. Nat. Genet. 2007;39:781–786. doi: 10.1038/ng2055. [DOI] [PubMed] [Google Scholar]
- Stephens L. E., Sutherland A. E., Klimanskaya I. V., Andrieux A., Meneses J., Pedersen R. A., Damsky C. H. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Tachibana I., Hemler M. E. Role of transmembrane 4 superfamily (TM4SF) proteins CD9 and CD81 in muscle cell fusion and myotube maintenance. J. Cell Biol. 1999;146:893–904. doi: 10.1083/jcb.146.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Flier A., Kuikman I., Baudoin C., van der Neut R., Sonnenberg A. A novel beta 1 integrin isoform produced by alternative splicing: unique expression in cardiac and skeletal muscle. FEBS Lett. 1995;369:340–344. doi: 10.1016/0014-5793(95)00814-p. [DOI] [PubMed] [Google Scholar]
- Van der Flier A., Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- Volonte D., Peoples A. J., Galbiati F. Modulation of myoblast fusion by caveolin-3 in dystrophic skeletal muscle cells: implications for Duchenne muscular dystrophy and limb-girdle muscular dystrophy-1C. Mol. Biol. Cell. 2003;14:4075–4088. doi: 10.1091/mbc.E03-03-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagami-Hiromasa T., Sato T., Kurisaki T., Kamijo K., Nabeshima Y., Fujisawa-Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377:652–656. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- Zeschnigk M., Kozian D., Kuch C., Schmoll M., Starzinski-Powitz A. Involvement of M-cadherin in terminal differentiation of skeletal muscle cells. J. Cell Sci. 1995;108:2973–2981. doi: 10.1242/jcs.108.9.2973. [DOI] [PubMed] [Google Scholar]
- Zhang S. J., Truskey G. A., Kraus W. E. Effect of cyclic stretch on beta1D-integrin expression and activation of FAK and RhoA. Am. J. Physiol. Cell Physiol. 2007;292:C2057–C2069. doi: 10.1152/ajpcell.00493.2006. [DOI] [PubMed] [Google Scholar]
- Zhidkova N. I., Belkin A. M., Mayne R. Novel isoform of beta 1 integrin expressed in skeletal and cardiac muscle. Biochem. Biophys. Res. Commun. 1995;214:279–285. doi: 10.1006/bbrc.1995.2285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.