Abstract
Many of the properties of bilayer membranes composed of simple single-chain amphiphiles seem to be well-suited for a potential role as primitive cell membranes. However, the spontaneous formation of membranes from such amphiphiles is a concentration-dependent process in which a significant critical aggregate concentration (cac) must be reached. Since most scenarios for the prebiotic synthesis of fatty acids and related amphiphiles would result in dilute solutions well below the cac, the identification of mechanisms that would lead to increased local amphiphile concentrations is an important aspect of defining reasonable conditions for the origin of cellular life. Narrow, vertically oriented channels within the mineral precipitates of hydrothermal vent towers have previously been proposed to act as natural Clusius−Dickel thermal diffusion columns, in which a strong transverse thermal gradient concentrates dilute molecules through the coupling of thermophoresis and convection. Here we experimentally demonstrate that a microcapillary acting as a thermal diffusion column can concentrate a solution of oleic acid. Upon concentration, self-assembly of large vesicles occurs in regions where the cac is exceeded. We detected vesicle formation by fluorescence microscopy of encapsulated dye cargoes, which simultaneously concentrated in our channels. Our findings suggest a novel means by which simple physical processes could have led to the spontaneous formation of cell-like structures from a dilute prebiotic reservoir.
Lipid membrane encapsulation is ubiquitous in biology and is thought to have been an essential aspect of the earliest cell-like structures.(1) Bilayer membranes composed of simple single-chain amphiphiles, such as fatty acids, seem to be well-suited for a potential role as primitive cell membranes.2,3 However, the spontaneous formation of such membranes can only occur above a critical aggregate concentration (cac) (Table S1 in the Supporting Information). Since most scenarios for the prebiotic synthesis of fatty acids and related amphiphiles would result in dilute solutions well below the cac,(4) the identification of mechanisms that would lead to increased local amphiphile concentrations is an important aspect of defining reasonable conditions for the origin of cellular life.
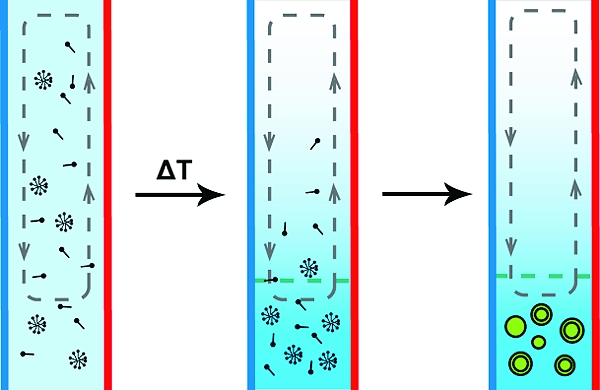
Recently, Baaske et al.(5) proposed that temperature gradients generated in deep-sea alkaline vents could have greatly concentrated diverse molecular species in a prebiotic setting. Vertically oriented microchannels within the carbonate rock precipitates are proximal to both hot vent water and cold ocean water.(6) A lateral thermal gradient across a vertical channel results in convective flow (as heated fluid rises and cooled fluid sinks) and concurrent thermal diffusion (thermophoresis) of molecules along the temperature gradient (Figure 1A). It has been known since the 1930s that coupling of thermophoresis and convective flows can lead to differential concentration effects in both gases and liquid solvents.7,8 Thermal diffusion columns (or Clusius−Dickel separations) were used for industrial purifications during the middle of the 20th century.(9) Using experimentally measured thermal diffusion coefficients, Baaske et al. performed simulations suggesting that nucleotides and nucleic acid oligomers could be locally concentrated in the prebiotic vent context outlined above. Here we experimentally demonstrate that a microcapillary thermal diffusion column can concentrate dilute solutions of nucleotides, oligonucleotides, and fatty acids. Upon concentration, the self-assembly of large vesicles containing encapsulated DNA occurs in regions where the cac of the fatty acid is exceeded. Our findings suggest a novel means by which simple physical processes could have led to the spontaneous formation of cell-like structures from a dilute prebiotic reservoir.
Figure 1.
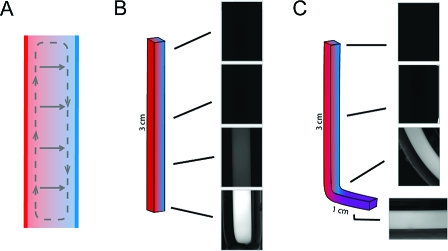
Microcapillary thermal diffusion columns concentrate small molecules. (A) Schematic diagram of a thermal diffusion column showing coupling of convective flow (dashed path) and thermophoresis (solid arrows). (B) Fluorescence microscopy images of a linear capillary (∼3 cm length, 200 μm i.d., initially loaded with 30 μM HPTS) after 24 h at ΔT = 30 K, revealing a strong concentration gradient of HPTS from top to bottom in the capillary. (C) Image of a bent capillary run under identical conditions. The protruding portion (purple) was kept at 4 °C and acted as a reservoir for the accumulation of the concentrated molecules.
To mimic the narrow channels found in vent formations, we used square borosilicate microcapillaries. Each capillary was loaded with an initial solution and attached to a reservoir with a volume ∼150 times greater than the capillary volume. We then applied a temperature gradient by clamping the capillary between a heat source and a heat sink kept at constant temperatures. Convective, unicellular flow was confirmed by visualization of the flow pattern of 2 μm polystyrene beads in capillaries under a temperature gradient (Movie S1 in the Supporting Information).
Within 24 h at a thermal gradient (ΔT) of 30 K, linear capillaries exhibited a significant concentrating effect, with a bottom-to-top concentration gradient of ∼800-fold as measured for the nonquenching fluorescent dye 8-hydroxypyrene-1,3,6-trisulfonic acid salt (HPTS) (Figure 1B). On our experimental time scale, concentration was primarily due to depletion of solute from the upper region of the capillary (Text S1 in the Supporting Information). We expect that over longer time periods, concentration would continue via the accumulation of molecules from the reservoir.
We observed a similar concentration effect with fluorescently labeled nucleic acids. This effect was enhanced with increasing molecular size (Table 1). The difference in the accumulations of mono- and oligonucleotides is due to the dependence of the Soret coefficient [the ratio of thermal diffusivity (DT) to molecular diffusivity (D)] on polymer length and was qualitatively predicted by simulation.(5) In addition, there was a large inhibitory effect of salt on the concentration effect, as predicted by measured Soret coefficients(10) (Table 1 and Figure S1 in the Supporting Information). Oceanic salt concentrations are very high (∼0.5 M) and could have been even higher (≥0.7 M) during the late Hadean era.11,12 Salt concentrations in this range would have limited the effectiveness of thermophoresis as a concentration mechanism in marine environments. Analogous fresh-water hydrothermal systems might therefore have provided a much more robust concentrating effect.
Table 1. Bottom-to-Top Accumulation of Solutes in a Linear Microcapillarya.
Indicated solutes were run at 20−40 μM and ΔT = 30 K for 24 h. Concentration was visualized along the capillary by quantitative fluorescence microscopy.
Fluorescently labeled with Cy3.
Fluorescently labeled with fluorescein.
Using 10 mM Tris (pH 7.5).
Using 10 mM Tris (pH 7.5) with 100 mM NaCl.
As it is unlikely that microchannels with such large aspect ratios would be oriented to maintain a uniform thermal gradient throughout, we explored the properties of an alternative capillary geometry. We prepared capillaries bent at 90° near the bottom, with a short (∼1 cm) length extending away from the temperature gradient. This design led to solute accumulation in the protruding portion of the capillary, over which there was no temperature gradient (Figure 1C and Figure S1), as dye first concentrated near the bottom of the vertical segment and then diffused into the adjacent horizontal segment. Such a geometry could therefore result in a low-temperature, high-concentration reservoir of molecules initially concentrated in a thermal diffusion column (e.g., see Figure 4 in ref (5)).
In order to investigate concentration-dependent vesicle self-assembly, we first showed that fatty acids can become highly concentrated within thermal diffusion columns. Linear capillaries were loaded with 60 μM 3H-labeled oleate at pH 11 to prevent vesicle assembly. The capillaries were incubated at ΔT = 30 K for 24 h and then fractionated, after which the oleate concentrations were determined by liquid scintillation. Fractions (0.75 cm or 300 nL) from the bottom of the capillary showed a 5-fold accumulation over those taken from the top (Figure S2). The concentration of fatty acids in a thermal diffusion column leads to the possibility of vesicle formation from a dilute solution. However, the convective flow running along the concentration gradient poses a problem, in that newly formed vesicles would be carried up the capillary, where they would dissolve as a result of the low local fatty acid concentration.
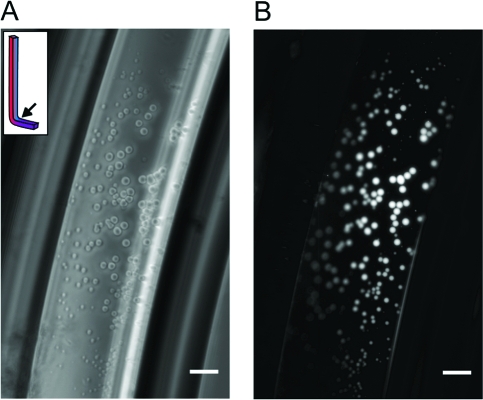
To circumvent the destructive effects of convective flow, we utilized the bent capillary geometry described above. We were able to visualize the formation of multilamellar vesicles after incubation for 48 h at ΔT = 30 and pH ∼8.5 (Figure 2). These vesicles adhered to the glass wall and were unusually large and monodisperse (Text S2). We confirmed that these structures were indeed vesicles by the encapsulation of fluorescent dye, which was included in the starting solution. After incubation, the capillaries were gently purged with dye-free buffer, leaving only the vesicle-encapsulated cargo to be visualized (Figure 2B). We used a similar protocol to demonstrate that such vesicles can encapsulate fluorescently tagged DNA oligomers (Figure S3).
Figure 2.
Locally concentrated oleate forms vesicles in bent capillaries incubated at ΔT = 30 for 48 h. (A) Phase-contrast image of a bent capillary loaded with 70 μM buffered oleate and 40 μM HPTS. The oleate concentrated in the capillary and formed large vesicles. (B) Fluorescence image of the same frame. HPTS in the solution was washed away with dye-free buffer, leaving only encapsulated cargo to be visualized. Scale bars = 50 μm.
Recent protocell models are based on fatty acid vesicles containing encapsulated nucleic acid polymers capable of self-replication.(13) Our experiments demonstrate that thermal gradients across narrow channels can provide the energy necessary to concentrate dilute molecular solutions and thus allow the self-assembly of cell-like structures from an initially homogeneous dilute solution. They also highlight the potential hazards (and advantages) of self-assembly in environments where building blocks are concentrated only locally. While the dynamic properties of fatty acids are necessary for protocell growth, division, and solute permeability,3,13 the same dynamic properties dictate that fatty acid bilayer membranes are not kinetically trapped structures. Any prolonged disruption of the temperature gradient (and thus the concentration gradient) would cause the system to equilibrate to an isotropic state with consequent dissolution of the vesicles. In such a variable environment, early cellular life might have been discontinuous, with the genetic information alternately residing within protocells and free in solution. Occasional disruption of the cellular state followed by re-encapsulation of genetic molecules could have allowed genetic exchange (recombination) or provided a means for genetic molecules to migrate to new niches. Such a variable environment would also impose a strong selective pressure for the evolution of a more stable cellular state, perhaps based on the evolution of the catalytic machinery for the synthesis of more complex chemical components, such as diacyl lipids. In view of the chasm between the complexity of fundamental cellular processes and the simple systems from which they must have once arisen, further study of relevant physical processes will lead to important clues as to how life first began.
Acknowledgments
R.J.B. was supported by a fellowship from the Harvard Origins Initiative. This work was supported in part by Grant EXB02-0031-0018 from the NASA Exobiology Program. J.W.S. is an Investigator of the Howard Hughes Medical Institute. The authors thank Dr. H. Stone, Dr. J. Higgins, and S. Tobé for helpful discussions and Dr. A. Ricardo, M. Elenko, T. Zhu, and Q. Dufton for comments on the manuscript.
Supporting Information Available
Materials and methods, cac values, analysis of capillary solute transport, additional characterization of vesicles, and visualization of convective flow (AVI). This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Szostak J. W.; Bartel D. P.; Luisi P. L. Nature 2001, 409, 387. [DOI] [PubMed] [Google Scholar]
- Hanczyc M. M.; Fujikawa S. M.; Szostak J. W. Science 2003, 302, 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T. F.; Szostak J. W. J. Am. Chem. Soc. 2009, 131, 5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushdi A. I.; Simoneit B. R. T. Origins Life Evol. Biosphere 2001, 31, 103. [DOI] [PubMed] [Google Scholar]
- Baaske P.; Weinert F. M.; Duhr S.; Lemke K. H.; Russell M. J.; Braun D. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. S. Nature 2001, 412, 145. [DOI] [PubMed] [Google Scholar]
- Clusius K.; Dickel G. Naturewissenschaften 1938, 26, 546. [Google Scholar]
- Furry W. H.; Onsager L. Phys. Rev. Lett. 1939, 55, 1083. [Google Scholar]
- Jones A. C.; Milberger E. C. Ind. Eng. Chem. 1953, 45, 2689. [Google Scholar]
- Duhr S.; Braun D. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 19678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland H. D.The Chemical Evolution of the Atmosphere and Oceans; Princeton University Press: Princeton, NJ, 1984; pp 110−111. [Google Scholar]
- Knauth L. P. Paleo 2005, 219, 53. [Google Scholar]
- Mansy S. S.; Schrum J. P.; Krishnamurthy M.; Tobé S.; Treco D. A.; Szostak J. W. Nature 2008, 454, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.