Abstract
Cell differentiation in developing tissues is controlled by a small set of signaling pathways, which must coordinate the timing and levels of activation to ensure robust and precise outcomes. Highly coordinated activation of signaling pathways can result from cross-regulatory interactions in multi-pathway networks. Here we explore the dynamics and function of pathway coordination between the EGFR and DPP pathways during Drosophila wing-vein differentiation. We show that simultaneous activation of both the EGFR and DPP pathways must be maintained for vein cell differentiation and that above-threshold ectopic activation of either pathway is sufficient to drive vein cell differentiation outside the proveins. The joint activation of the EGFR and DPP signaling systems is ensured by a positive feedback loop, in which the two pathways stimulate each other at the level of ligand production.
Keywords: bistability, DPP, EGFR, vein, wing
Introduction
The idea that switch-like transitions in positive feedback systems can control cell differentiation goes back almost half a century to the seminal paper by Monod and Jacob (1961). In the simplest model for switch-like dynamics, positive feedback generates bistability, a regime where transient stimuli can switch the system between two stable steady states (Ferrell, 2002). Mathematical analysis of bistability is quite advanced, but there have been just a few experimental studies unambiguously connecting bistability to cell differentiation (Xiong and Ferrell, 2003; Angeli et al, 2004; Laslo et al, 2006; Ingolia and Murray, 2007; Paliwal et al, 2007). Studies on positive feedback loops are especially challenging in developing tissues, because of the relative difficulty of implementing the quantitative spatiotemporal perturbations that can probe the possible dynamic regimes (Freeman and Gurdon, 2002). Previous studies of spatial bistability in Drosophila have been limited to special contexts (the syncytial embryo) (Lopes et al, 2008), or, for cellular tissues, inferred indirectly (Wang and Ferguson, 2005; Serpe et al, 2008). Although the connections that form the positive feedback circuit can be deduced from genetic studies and biochemical experiments, information about the feedback architecture alone is not sufficient to determine whether the closed-loop system operates in a bistable regime (Angeli et al, 2004; Li and Carthew, 2005; Mikeladze-Dvali et al, 2005; Ingolia and Murray, 2007). Indeed, an isolated positive feedback module might function either reversibly or as a hysteretic switch, depending on the choice of network parameters, such as the gains of the feedback connections as well as the relative time scales of the pathways (Ferrell and Xiong, 2001; Angeli et al, 2004; Ingolia and Murray, 2007).
Here we show how bistability emerges from the self-sustaining interactions of the highly conserved epidermal growth factor receptor (EGFR) and decapentaplegic (DPP) signaling pathways during the patterning of the Drosophila wing, an established model for studying the connections between signaling and cell differentiation (de Celis, 2003; Blair, 2007). Previous loss-of-function experiments using a vein-specific expression system suggested that the EGFR and DPP pathways form a positive feedback loop, and that DPP signaling has a central role in vein fate induction (Sotillos and De Celis, 2005). Using a combination of genetic and imaging experiments, we show that high levels of EGFR activation are equally essential. Thus, vein differentiation requires sustained activation of both pathways. This high level of activation can be interpreted as the ‘on' state of an inter-pathway positive feedback loop, whereby the EGFR and DPP systems operate on similar time scales and cross-activate each other at the level of ligand production.
Results and discussion
Vein differentiation depends on the joint action of the EGFR and DPP pathways
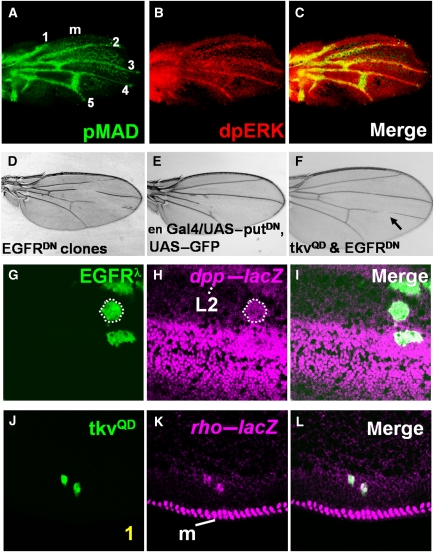
Around 22 h after puparium formation (APF) (Yu et al, 1996; de Celis, 1997; Guichard et al, 1999; Martin-Blanco et al, 1999; Sotillos and De Celis, 2005), phosphorylated MAD (pMAD), which marks DPP activity, and doubly phosphorylated ERK (dpERK), which provides a readout of EGFR activity, co-localize in the provein cells of pupal wing discs (Guichard et al, 1999; Martin-Blanco et al, 1999; Conley et al, 2000) (Figure 1A–C). Both pathways are required for vein differentiation, as inhibition of either of them by expressing a dominant-negative form of the receptors leads to the cell-autonomous loss of vein cell fate (Figure 1D and E) (de Celis, 2003; Blair, 2007). Previous studies led to a model in which EGFR signaling is spatially restricted to the provein cells by the Notch pathway (de Celis et al, 1997), and then interacts with the DPP pathway, which provides a direct input for vein cell differentiation (Sotillos and De Celis, 2005).
Figure 1.
The DPP and EGFR pathways are jointly required for vein differentiation and reciprocally induce each other's ligands. (A–C) Pupal wings at 22 h APF stained for pMAD (green) and dpERK (red), with anterior (up) and distal (to the right), showing that both pMAD and dpERK are detected in the provein cells. 1–5, longitudinal veins L1–L5, and m, the wing margin. (D) Flip-out clones expressing EGFRDN-interrupted veins L2, L3, and L4. (E) Expressing PutΔI under control of the engrailed–Gal4 (en–Gal4) driver, which is expressed only in the posterior compartment of the wing, caused loss of vein tissues. (F) An adult wing with truncated veins resulting from co-expressing TKVQD and EGFRDN. In contrast to the wing with clones expressing TKVQD alone (Supplementary Figure S1A–C), few, if any, ectopic vein cells are observed (data not shown). (G–I) Ectopic EGFR signaling induces dpp expression. dpp–lacZ expression (magenta in H and I) is detected in the EGFRλ-expressing clones. (J–L) TKVQD activates EGFR by inducing rho expression.
Although the activation of either of the two pathways can induce ectopic veins (Table I; Supplementary Figure S1), it has been proposed that the DPP pathway alone is sufficient for vein differentiation, and that the EGFR pathway is required mainly to maintain the high level of DPP signaling (de Celis, 1997). This model predicts that the DPP pathway should induce vein cell fates even when the EGFR signaling is inhibited. To verify this, we examined the consequence of simultaneously expressing a constitutively active component of DPP signaling (TKVQD) and a dominant-negative variant of EGFR, EGFRDN, thus keeping one pathway (DPP) constitutively active, while repressing the other pathway. In contrast to the results obtained upon expressing TKVQD alone (Supplementary Figure S1A–C), co-expressing TKVQD and EGFRDN not only failed to induce ectopic vein cells, but also inhibited endogenous vein formation (Figure 1F), suggesting that activation of the DPP pathway alone is not sufficient for vein differentiation. A similar effect was observed on co-expressing constitutively active DRAF (DRAFΔN) and the dominant-negative form of the type-II DPP receptor (PUTΔI) (data not shown). These results strongly support a model wherein the vein-differentiation program requires the sustained activation of both pathways (Supplementary Figure S2).
Table 1.
EGFR and DPP signaling are both required for vein differentiation
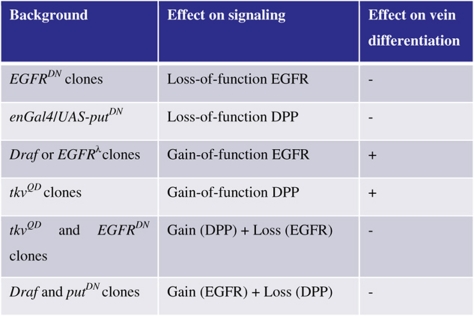 |
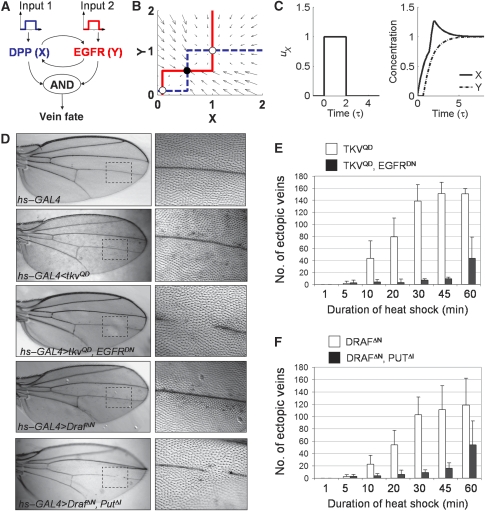
Figure 2.
Transient EGFR or DPP pathway activation leads to vein differentiation. (A) Model of vein differentiation, which depends on the joint activation of the EGFR and DPP systems and is mediated by positive feedback. (B) Phase plot showing three steady states marked by white circles (stable) or a black circle (unstable). The red and blue lines are the nullclines. (C) Depending on network parameters, transient perturbations (left panel) can lead to irreversible switching from an ‘off' to an ‘on' state (right panel). Parameters for (C): ux=1, Δt=2, α=1, ɛ=1, and σ1=σ2=0.5. (D) Pupae were heat-shocked for 20 min at 37°C at 18 h APF. Adult wings (left) of indicated genotypes and a higher magnification of the boxed region (right) are shown. Transient expression of activated DPP signaling (TKVQD) or activated EGFR signaling (DRAFΔN) by heat shock-induced GAL4, resulted in the formation of ectopic vein cells (discrete darker spots). Transient co-expression of a dominant-negative receptor, EGFRDN or PUTΔI, suppressed the ectopic vein formation and disrupted the formation of endogenous veins (gap in L4). (E, F) Quantification of ectopic vein induction as a function of heat-shock duration of 18-h-APF pupae bearing hsp70–GAL4 and the indicated UAS transgenes.
Simultaneous activation of the EGFR and DPP systems could reflect the action of an upstream signal that controls both pathways. Alternatively, the observed co-activation can result from inter-pathway interactions. Our experiments support the latter model. We found that ectopic activation of either of the pathways led to a cell-autonomous increase in the production of the other pathway's ligand. In particular, the expression of dpp–lacZ (dppP10638, a P-element enhancer trap at the dpp locus; Supplementary Figure S3A) was strongly upregulated in clones of cells expressing a constitutively active form of EGFR (Queenan et al, 1997) (Figure 1G–I; Supplementary Figure S3A–H). Furthermore, the DPP pathway similarly triggers EGFR signaling by upregulating rhomboid (rho) expression, a gene encoding a protease responsible for processing of the secreted ligands that bind and activate EGFR (Urban et al, 2002). Consistent with the mutual cross-activation of both pathways, we found ectopic and cell-autonomous rho–lacZ expression in TKVQD-expressing clones (Figures 1J–L and 3I–S). These results identify an inter-pathway positive feedback loop whereby the EGFR and DPP systems cross-activate each other at the level of ligand production. This feedback loop can account for the observed co-localization of EGFR and DPP signaling in the provein cells (Figure 1A–C). Cell-autonomous activation of the EGFR and DPP pathways suggests that the spatial range of signaling is limited by negative feedback. Previous work argues that this is true for DPP signaling (Sotillos and De Celis, 2005) and is likely true for sSPI (Reeves et al, 2005). Although the mechanism restricting the diffusion of Vein has not been shown, negative feedback through Notch signaling has an important role in downregulating EGFR and DPP signaling in adjacent cells (de Celis et al, 1997) (Supplementary Figures S4 and S5).
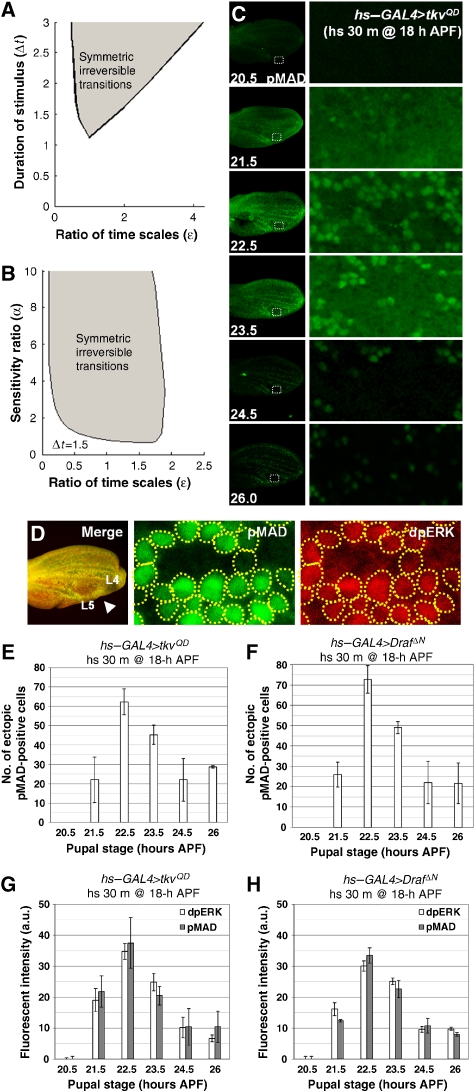
Figure 3.
Symmetry in EGFR and DPP signaling dynamics. (A) Plot of parameter space shows that bounded values for ɛ exist for a measured stimulus of duration, Δt, required for ensuring vein fate induction, given that ectopic activation of either pathway is sufficient. The shaded region includes values of Δt and the ratio of time scales, ɛ, that are consistent with symmetric bistable switching from transient activation of either of the two signaling pathways, X or Y. The cross-activation thresholds, σ1=σ2=0.5, the exogenous signal strength (ux or uy), and α are set to 1. (B) Plot of parameter space showing that for a given transient activation of time, ɛ, is bound and centered on 1, even for a large variation in the sensitivity ratio, α. (C) Pupae with transient expression of activated DPP signaling (TKVQD) by hs–GAL4 were heat-shocked for 30 min at 37°C at 18 h APF. Representative pupal wings (left) are shown with pMAD staining of indicated pupal stages and with a higher magnification of the boxed region (right) between L4 and L5. (D) Representative 22.5-h APF pupal wing between L4 and L5 with pMAD and dpERK staining of pupae bearing hsp70–Gal4 and UAS–tkvQD after 30-min heat-shock treatment at 18 h APF. (E, F) Quantification of ectopic pMAD-positive cell number in selected boxed areas at different pupal stages for (E) hsp70>tkvQD (n=3 pupal wings) and (F) hsp70>dRafΔN (n=3), which were heat-shocked at 37°C for 30 min at 18 h APF. (G, H) Quantification of ectopic dpERK and pMAD intensities at different pupal stages of a selected boxed area between L4 and L5 of 18 h APF pupae for (G) hsp>tkvQD and (H) hsp70>dRafΔN that were heat-shocked at 37°C for 30 min at 18 h APF (n=4).
The EGFR–DPP positive feedback operates in bistable regime
One of the possible functions of the EGFR–DPP feedback loop is to generate bistability, but this regime will be realized only for a subset of network parameters (Ferrell and Xiong, 2001; Angeli et al, 2004). The ‘on' state of the positive feedback in the bistable regime would then maintain high levels of both EGFR and DPP signaling. In the absence of positive feedback, transient activation of any single pathway alone would be reversible and insufficient to ensure vein differentiation. However, in the presence of positive feedback, a sufficiently strong transient stimulation of either pathway might generate long-term activation of both pathways, which, as discussed previously, is a requirement for ensuring vein differentiation (Figure 2A–C). It should be noted, however, that the presence of the positive feedback motif is not sufficient for bistability. In vivo, the EGFR and DPP pathways are embedded in a more complex network wherein additional interactions can either suppress bistability or lead to more complex dynamics, such as oscillations (Tyson et al, 2003).
To determine whether the EGFF–DPP feedback loop in vivo operates in the bistable regime, we tested whether transient activation of either the EGFR or the DPP pathway induces long-term activation of the coupled system. Long-term activation can be assayed in the adult fly (after eclosion, approximately 120 h APF) by ectopic vein differentiation. We used the heat-shock-driven GAL4–UAS system to transiently activate either the DPP or the EGFR pathway by expressing tkvQD or DrafΔN, respectively, during early pupal growth. Although it is currently impossible to study the protein perdurance for TKVQD and DRAFΔN, we examined the kinetics of GAL4 expression due to hsp-driven expression of GAL4 (Supplementary Figure S6). For a 30-min heat shock at 37°C, we found that GAL4 expression is strongest at 20.5 h APF and then decreases to the level of background fluorescence by 22 h APF. We found that transient activation of either pathway at 18 h APF resulted in discrete ectopic vein cells in the adult wing (Figure 2D–F), showing that transient expression of TKVQD or DRAFΔN can produce long-lasting activation of both pathways in discrete cells, sufficient for inducing vein differentiation.
Transiently co-expressing a dominant-negative receptor for the second pathway, such as in combinations of TKVQD and EGFRDN or DRAFΔN and PUTΔI, respectively, not only resulted in significantly fewer ectopic veins, but also disrupted endogenous vein formation (Figure 2D–F). Failure to induce ectopic vein cell differentiation is consistent with a similar effect observed in experiments in which the pathways were perturbed in a persistent manner (Figure 1F). Thus, transient activation of either pathway can induce an irreversible transition from low to high signaling states in the DPP—EGFR-positive feedback circuit, which in turn leads to vein differentiation.
Symmetry constrains a model on positive feedback between the EGFR and DPP pathways
The most striking result of the transient-activation assay is the relative symmetry in the number of detected ectopic veins as a function of heat-shock induction time between the DPP (Figure 2E) and EGFR pathways (Figure 2F). In each case, the growth in the number of ectopic veins and saturation levels of ectopic differentiated vein cells is roughly similar for a given duration of heat shock, which potentially provides insight into the signaling dynamics of the coupled signaling system. As a first step towards exploring this effect, we analyzed a simple mathematical model. As the effects of pathway cross-activation were found to be of cell-autonomous nature (Figure 1, Supplementary Figures S1–S3), a lumped model is appropriate.
In the model, the two components, X and Y, correspond to the DPP and EGFR signaling pathways, respectively (Figure 2A). In the absence of stimuli, each signaling pathway relaxes with a characteristic time constant (τX, τY), and increases linearly in amplitude in response to an exogenous input (UX, UY), corresponding to either constitutively active TKVQD or EGFRλ, which can be switched on either transiently, as in the heat-shock experiment, or in a sustained manner, as in experiments with the en–GAL4 driver. Cross-activation of either pathway is approximated as a non-linear step function that is triggered when the other component is greater than a certain threshold value (ΣXY, ΣYX). A step function was chosen for the sake of simplicity to limit the number of parameters for the model. Choosing a smoothly varying Hill function (n>1) does not significantly change the results discussed below (Supplementary Figure S7). The amplitude of cross-activation of component X by Y is given by AX and the amplitude of cross-activation of component Y by X is described by AY. The model is described by the following equations:
The maximal levels of X and Y in the absence of exogenous inputs (UX=UY=0) are given by AXτX and AYτY, respectively. Using these to rescale the levels of X and Y, rescaling time by τX (τ≡t/τX), and both UX and UY by AX (ux=UX/AX and uy=UY/AX), reduces the number of free parameters in the model:
![]()
where σ1=∑XY/AYτY and σ2=∑YX/AXτX are the dimensionless cross-activation thresholds, and ɛ=τY/τX is the ratio of relaxation time scales in the two pathways; α=Axτx/Ayτy is the ratio of the maximal levels of X and Y stimulation and provides a measure of the difference in the sensitivity to external inputs for the two pathways.
In the experiments described in the previous section, both pathways were activated by heat shocks of identical duration and it was found that activation of either of the pathways led to ectopic veins, interpreted as the ‘on' state in our simple model. Analyzing the model, we found that such symmetric responses require that the two pathways operate on similar timescales (shaded gray area in Figure 3A). For a given duration of stimulus that is just long enough to ensure vein differentiation by activating one pathway (Δtmin<Δtstimulus≪Δtvein differentiation), the ratio of time scales is bound, even for large values of α, which can be viewed as a measure of the differences of the sensitivities of the EGFR and DPP systems to a heat-shock stimulation (Figure 3B).
Thus, we interpret the co-activation state of the EGFR and DPP pathways in the provein cells as the ‘on' state of the positive feedback system and predict that the two pathways are operating on similar time scales. To test this prediction, we examined the expressions of pMAD and dpERK at various pupal stages after the activation of the two pathways by expressing TKVQD or dRafΔN for a short period of time (30-min heat shock at 18 h APF, Figure 3C). Co-staining of pMAD and dpERK shows strong co-localization (Figure 3D). The number of ectopic pMAD-positive cells as well as intensity levels of pMAD and dpERK show similar temporal dynamics (Figure 3E–H) when ectopically stimulating either the DPP or the EGFR pathway, consistent with the model's prediction of equal pathway decay rates and stimulation sensitivities. The ‘off' state of low PMAD/low dpERK is similarly reached by transiently downregulating either the EGFR or the DPP signaling pathways. The switching dynamics to the ‘off' state are also symmetric with respect to perturbations of either pathway (Supplementary Figure S8).
Conclusions
To summarize, we have shown that vein cell differentiation requires simultaneous activation of both the EGFR and DPP pathways (in contrast to an earlier model (Sotillos and De Celis, 2005), which emphasizes the importance of DPP signaling in regulating differentiated veins). Our results strongly suggest that their synchronous activation is mediated by a bistable positive feedback loop that is formed by the stimulated production of each other's ligands. A phenomenological model of the system, constrained by the observed symmetric irreversible switching between the two states of differentiation, gives predictions on the bounds of system parameters. In particular, the observed symmetry in vein differentiation and signaling dynamics after equivalent short pulses of heat shock suggests that both pathways signal at similar rates, irrespective of the relative sensitivity of either pathway to heat shock.
Although our data suggest that the EGFR–DPP system can function as a vein-differentiation module throughout the pupal wing, further work is required to explore its region-specific regulation. For example, the loss of veins in response to transient inhibition of EGFR and DPP systems is limited to vein 4 in the heat-shock experiments (Figure 2D), indicating that the essential requirement of both the EGFR and DPP pathways in vein differentiation might be applicable to a subset of proveins. Our study highlights the subtleties associated with establishing the presence of bistable regimes in development, and the usefulness of simple phenomenological models to make prediction on signaling dynamics based on regulatory network topology.
One surprise from the heat-shock experiments was the decrease in the cluster size of ectopic pMAD/dpERK cells at long time periods (Figure 3C–E), suggesting a negative feedback signal, which limits the spread of diffusing ligands. One such negative feedback mechanism is Notch signaling, which is found in the proveins on either side of pMAD-expressing cells (Supplementary Figure S4A–C). In support of this model, we found that flip-out clones of TKVQD induced ectopic N expression in the cells (2–3 cells in the radial direction) surrounding TKVQD-expressing cells (Supplementary Figure S4D–I). Furthermore, ectopic NECN clones lead to ectopic vein differentiation and increased pMAD signaling (Supplementary Figure S5A–L). However, Ni clones lead to loss of pMAD (Supplementary Figure S5M–O). On the basis of the initial spread of ectopic pMAD cells at intermediate time points, the negative feedback created by increasing N levels must be operating at a slower time scale (Supplementary Figure S5P).
Materials and methods
Fly strains and genetics
Stocks used in the study are UAS–tkvQD (tkvQ253D) (Nellen et al, 1996), UAS–DrafΔN (Brand and Perrimon, 1994), UAS–EGFRDN (Freeman, 1996), UAS–putDN (putΔI) (Haerry et al, 1998), UAS–EGFRλ (Queenan et al, 1997), UAS–rho (Xiao et al, 1996), UAS–NECN (provided by S Artavanis-Tsakonas), UAS–Ni (provided by S Artavanis-Tsakonas), UAS–dpp (42B4, Bloomington), UAS–sspi (provided by T Schüpbach), dppP10638 (provided by T Kornberg), rhoAA69 (Nambu et al, 1990), and hsp70–flp; ACT5C>y+>GAL4 UAS–GFP (Ito et al, 1997). To express UAS transgenes in random clones, we crossed hsp70–flp; ACT5C>y+>GAL4 UAS–GFP flies to UAS transgene flies and raised the progenies at room temperature (22°C). Details are included in the Supplementary information text. In short, we induced clones in the resulting larvae at 96–120 h after egg laying (AEL; corresponding to late third-instar stage) by heat shocking for 20 min at 37°C in a water bath. This heat-shock regimen resulted in clone induction at the late third-instar stage, ensuring a large enough clone size without affecting A–P patterning or provein cell-fate determination as UAS transgenes are not immediately expressed in flip-out cells when vein determination takes place (data not shown).
Immunocytochemistry and imaging
The following primary antibodies were used: monoclonal mouse anti-β-gal (1:1000, Promega), rabbit anti-pMAD (1:100, PS1, provided by P ten Dijke and C-H Heldin), monoclonal mouse anti-dpERK (1:200, Sigma), monoclonal mouse anti-Notch (C17.9C6, 1:100, DSHB), and mouse anti-GAL4 (DBD) (1:100, RK5C1, Santa Cruz Biotechnology). The following secondary antibodies were used: goat anti-mouse Alexa Fluor 488, 546 and 660 (1:200, Molecular Probes) and goat anti-rabbit Alexa Fluor 660 (1:200, Molecular Probes). Immunostaining of imaginal discs was carried out as described in Yan et al (2004), and detailed notes are provided in the Supplementary information text. Fluorescent images were obtained using a Leica confocal microscope and quantified by Photoshop image analysis. Adult wings were mounted in Halocarbon oil (Sigma) in order to preserve GFP activity; images were photographed using a Zeiss Axiophot microscope.
Supplementary Material
Supplementary Materials and Methods, Supplementary figures S1-S8
Acknowledgments
We thank Drs S Artavanis-Tsakonas, S Blair, D Bohmann, T Kornberg, J Nambu, T Schüpbach, P ten Dijke, C-H Heldin, and the Developmental Studies Hybridoma Bank (DSHB) at the University of Iowa, and the Bloomington Stock Center for fly stocks and reagents, and all the members of the Bohmann, Fleming, Jasper, and Li laboratories for helpful discussions. We also thank Drs H Land, L Silver-Morse, R Angerer, G Fitzpatric, D Bohmann, R Fleming, and G Reeves for suggestions and comments on the paper. JJZ is supported by the Fannie and John Hertz Foundation and the Princeton Wu fellowship. AS received the deKiewiet Fellowship of University of Rochester. This study was supported in part by NIH grants (R01GM077046, R01GM65774), an American Cancer Society Research Scholar Grant (RSG-06-196-01-TBE), and a Leukemia & Lymphoma Society Career Development Program Scholar Grant (1087-08) to WXL. This work has been supported by NIH grants P50 GM071508 and R01 GM078079 to SYS.
Footnotes
The authors declare that they have no conflict of interest.
References
- Angeli D, Ferrell JE, Sontag ED (2004) Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feed back systems. Proc Natl Acad Sci USA 101: 1822–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SS (2007) Wing vein patterning in drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol 23: 293–319 [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N (1994) Raf acts downstream of the EGF receptor to determine dorsoventral polarity during Drosophila oogenesis. Genes Dev 8: 629–639 [DOI] [PubMed] [Google Scholar]
- Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS (2000) Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127: 3947–3959 [DOI] [PubMed] [Google Scholar]
- de Celis JF (1997) Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development 124: 1007–1018 [DOI] [PubMed] [Google Scholar]
- de Celis JF (2003) Pattern formation in the Drosophila wing: the development of the veins. Bioessays 25: 443–451 [DOI] [PubMed] [Google Scholar]
- de Celis JF, Bray S, Garcia-Bellido A (1997) Notch signalling regulates veinlet expression and establishes boundaries between veins and interveins in the Drosophila wing. Development 124: 1919–1928 [DOI] [PubMed] [Google Scholar]
- Ferrell J, Xiong W (2001) Bistability in cell signaling: How to make continuous processes discontinuous, and reversible processes irreversible. Chaos 11: 221–236 [DOI] [PubMed] [Google Scholar]
- Ferrell JE (2002) Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol 14: 140–148 [DOI] [PubMed] [Google Scholar]
- Freeman M (1996) Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87: 651–660 [DOI] [PubMed] [Google Scholar]
- Freeman M, Gurdon JB (2002) Regulatory principles of developmental signaling. Annu Rev Cell Dev Biol 18: 515–539 [DOI] [PubMed] [Google Scholar]
- Guichard A, Biehs B, Sturtevant MA, Wickline L, Chacko J, Howard K, Bier E (1999) rhomboid and Star interact synergistically to promote EGFR/MAPK signaling during Drosophila wing vein development. Development 126: 2663–2676 [DOI] [PubMed] [Google Scholar]
- Haerry TE, Khalsa O, O'Connor MB, Wharton KA (1998) Synergistic signaling by two BMP ligands through the SAX and TKV receptors controls wing growth and patterning in Drosophila. Development 125: 3977–3987 [DOI] [PubMed] [Google Scholar]
- Ingolia NT, Murray AW (2007) Positive-feed back loops as a flexible biological module. Curr Biol 17: 668–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D (1997) The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development 124: 761–771 [DOI] [PubMed] [Google Scholar]
- Laslo P, Spooner CJ, Warmflash A, Lancki DW, Lee HJ, Sciammas R, Gantner BN, Dinner AR, Singh H (2006) Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell 126: 755–766 [DOI] [PubMed] [Google Scholar]
- Li X, Carthew RW (2005) A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell 123: 1267–1277 [DOI] [PubMed] [Google Scholar]
- Lopes FJP, Vieira FMC, Holloway DM, Bisch PM, Spirov AV (2008) Spatial bistability generates hunchback expression sharpness in the Drosophila embryo. PLoS Comput Biol 4: e1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco E, Roch F, Noll E, Baonza A, Duffy J, Perrimon N (1999) A temporal switch in DER signaling controls the specification and differentiation of veins and interveins in the Drosophila wing. Development 126: 5739–5747 [DOI] [PubMed] [Google Scholar]
- Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, Cohen S, Desplan C (2005) The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell 122: 775–787 [DOI] [PubMed] [Google Scholar]
- Monod J, Jacob F (1961) General conclusions: teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb Symp Quant Biol 26: 389–401 [DOI] [PubMed] [Google Scholar]
- Nambu JR, Franks RG, Hu S, Crews ST (1990) The single-minded gene of Drosophila is required for the expression of genes important for the development of CNS midline cells. Cell 63: 63–75 [DOI] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K (1996) Direct and long-range action of DPP morphogen gradient. Cell 85: 357–368 [DOI] [PubMed] [Google Scholar]
- Paliwal S, Iglesias PA, Campbell K, Hilioti Z, Groisman A, Levchenko A (2007) MAPK-mediated bimodal gene expression and adaptive gradient sensing in yeast. Nature 446: 46–51 [DOI] [PubMed] [Google Scholar]
- Queenan AM, Ghabrial A, Schupbach T (1997) Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development 124: 3871–3880 [DOI] [PubMed] [Google Scholar]
- Reeves GT, Kalifa R, Klein DE, Lemmon MA, Shvartsman SY (2005) Computational analysis of EGFR inhibition by Argos. Dev Biol 284: 523–535 [DOI] [PubMed] [Google Scholar]
- Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O'Connor MB, Blair SS (2008) The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell 14: 940–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillos S, De Celis JF (2005) Interactions between the Notch, EGFR, and decapentaplegic signaling pathways regulate vein differentiation during Drosophila pupal wing development. Dev Dyn 232: 738–752 [DOI] [PubMed] [Google Scholar]
- Tyson JJ, Chen KC, Novak B (2003) Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol 15: 221–231 [DOI] [PubMed] [Google Scholar]
- Urban S, Lee JR, Freeman M (2002) A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. EMBO J 21: 4277–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Ferguson EL (2005) Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature 434: 229–234 [DOI] [PubMed] [Google Scholar]
- Xiao H, Hrdlicka LA, Nambu JR (1996) Alternate functions of the single-minded and rhomboid genes in development of the Drosophila ventral neuroectoderm. Mech Dev 58: 65–74 [DOI] [PubMed] [Google Scholar]
- Xiong W, Ferrell JEJ (2003) A positive-feedback-based bistable ‘memory module' that governs a cell fate decision. Nature 426: 460–465 [DOI] [PubMed] [Google Scholar]
- Yan SJ, Gu Y, Li WX, Fleming RJ (2004) Multiple signaling pathways and a selector protein sequentially regulate Drosophila wing development. Development 131: 285–298 [DOI] [PubMed] [Google Scholar]
- Yu K, Sturtevant MA, Biehs B, Francois V, Padgett RW, Blackman RK, Bier E (1996) The Drosophila decapentaplegic and short gastrulation genes function antagonistically during adult wing vein development. Development 122: 4033–4044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods, Supplementary figures S1-S8