Abstract
Epidermal growth factor receptor (EGFR) kinase inhibitors induce dramatic clinical responses in a subset of non-small cell lung cancer (NSCLC) patients with advanced disease, and such responses are correlated with the presence of somatic activating mutations within the EGFR kinase domain. Consequently, one of these inhibitors, erlotinib, has been FDA-approved as a second- or third-line treatment for chemotherapy-refractory advanced NSCLC. However, responses are typically relatively short-lived due to acquired drug resistance, prompting studies to determine whether first-line treatment with EGFR inhibitors could provide greater clinical benefit. NSCLC-derived cell lines have provided a powerful system for modeling EGFR mutation-correlated sensitivity to EGFR inhibitors and for modeling mechanisms of acquired drug resistance that are observed clinically. In a cell culture model of an erlotinib-sensitive EGFR mutant NSCLC cell line, we tested the hypothesis that prior exposure to platinum agents, a standard component of NSCLC chemotherapy treatment, affects the subsequent response to erlotinib. Indeed, NSCLC cells initially selected for growth in cisplatin exhibit 5-fold reduced sensitivity to erlotinib, even after propagating the cisplatin-treated cells in the absence of cisplatin for several months. This lingering effect of cisplatin exposure appears to reflect changes in PTEN tumor suppressor activity and persistent EGFR-independent signaling through the PI-3 kinase/AKT survival pathway. These pre-clinical findings suggest that first-line chemotherapy treatment of EGFR mutant NSCLCs may reduce the benefit of subsequent treatment with EGFR kinase inhibitors, and should prompt further clinical investigation of these inhibitors as a first-line therapy in NSCLC.
INTRODUCTION
Non-small cell lung cancer (NSCLC) is the leading cause of cancer death worldwide. The prognosis for most patients with advanced NSCLC remains poor despite significant advances in medical oncology. Such patients typically experience modest clinical benefit from standard platinum-based chemotherapy treatments, associated with a limited increase in overall survival (1). The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) erlotinib (Tarceva) yields a modest increase in survival when administered to unselected NSCLC patients following chemotherapy and was hence approved for this indication by the FDA (Food and Drug Administration) in 2004 (2). However, recent studies have demonstrated that a subset (10–20%) of NSCLC patients treated with EGFR TKIs experience striking clinical responses, which, in some cases, lead to durable remissions (3–5). Significantly, those responses are well correlated with the presence of a class of somatic activating mutations within the EGFR kinase domain (6–8), paving the way for recent genotype-based trials aimed at improving the overall response rate by pre-selecting patients that are more likely to respond to these agents in the first-line setting (9–11). Although none of the genotype-directed studies reported thus far have included a comparison arm in their design, initial results are promising, with response rates and durations being 2 to 3-fold better than those typically seen with standard chemotherapy (9–11).
While such clinical studies are encouraging, and the concept of utilizing a first-line treatment regimen that is targeted to a specific genetic lesion and is less toxic than conventional chemotherapy is appealing, there are significant considerations that need to be addressed before such an approach could be considered standard. Primarily, this strategy has not yet been compared to traditional chemotherapy in a randomized trial within a genotype-selected population, and consequently, its relative benefit has not yet been proven. Moreover, some have suggested that EGFR mutations are prognostic, not predictive factors for survival in the setting of EGFR-directed therapy, and are therefore not optimal for therapeutic decision-making (12, 13).
To further investigate the potential benefit of first-line EGFR TKI therapy in NSCLC, we examined the effect of a platinum-based chemotherapy agent on subsequent sensitivity to EGFR kinase inhibitors using a cell culture-based pre-clinical model. NSCLC-derived cell lines have proven a reliable model of clinical response to EGFR kinase inhibitors. Thus, most tumor cell lines harboring activating EGFR kinase domain mutations exhibit substantially increased sensitivity to gefitinib and erlotinib (14), and continuous exposure of these cells to kinase inhibitors eventually yields drug-resistant clones that have acquired resistance through mechanisms that have been observed clinically in EGFR TKI-treated NSCLC patients (15, 16). To determine whether standard chemotherapy treatment of EGFR mutation-positive NSCLC can impact the subsequent responsiveness to second-line treatment, we examined an EGFR mutant NSCLC-derived cell line. Exposing these cells to cisplatin substantially reduced their subsequent sensitivity to erlotinib, via a mechanism that involves persistent activation of the PI-3 kinase-AKT cell survival pathway. Moreover, the reduced sensitivity to erlotinib was observed even after cells had been maintained in cisplatin-free medium for several months. Our findings suggest that treatment with platinum-based agents may render EGFR-mutant NSCLC cells more resistant to EGFR kinase inhibitors than previously untreated cells. These findings provide further evidence to support a rationale for the use of EGFR kinase inhibitors as first-line therapy in NSCLC.
MATERIALS AND METHODS
Cell lines culture and pharmacologic inhibitors
PC9 NSCLC-derived cells expressing the EGFR exon 19 deletion mutation ( ΔE746-A750) were kindly provided by Dr Kazuto Nisho (National Cancer Center Hospital, Tokyo) and were maintained as previously described (17). Cisplatin-resistant PC9 cells were maintained in cisplatin containing RPMI 1640 with 10% fetal-bovine serum, penicillin and streptomycin (100U /ml and 100 g/ml, respectively). Erlotinib (from the MGH pharmacy) was re-suspended in DMSO at a stock concentration of 10 mM. Cisplatin was purchased from Calbiochem. LY 294002 was purchased from Cell Signaling Technology and re-suspended in DMSO at a stock concentration of 50mM. All inhibitors were stored in small aliquots at −20 °C.
Generation of cisplatin-resistant PC9 cells
PC9 cells at 70% confluency were exposed to 5 µM cisplatin, equivalent to the empirically-determined IC80 of cisplatin for PC9 cells in a 96-hour survival assay. Fresh media containing drug was added to the cells every 3 days until cells reached confluency (approximately 3 months). These cells were subjected to subsequent cell survival assays and were found to be stably resistant to cisplatin when maintained in 5 µM cisplatin. This pool of cells was used for further biochemical characterization and cell survival assays.
Antibody studies
The rabbit polyclonal antibodies directed against phospho-EGFR (Tyr 1068) and EGFR were from Abcam and Santa Cruz Biotechnology, respectively. Rabbit polyclonal antibodies against phospho-p44/42 MAP kinase (Thr 202/Tyr 204), phospho-Akt (Ser 473), and antibodies directed against their non-phosphorylated counterparts were purchased from Cell Signaling Technology. The mouse monoclonal anti-c-K-Ras antibody was from Calbiochem. The rabbit monoclonal antibody directed against PTEN for immunofluorescence analysis was from Cell Signaling Technology. Secondary antibodies included HRP-conjugated anti-mouse and anti-rabbit antibodies, and were also from Cell Signaling Technology.
Cell harvesting and protein analysis
Cells grown under the described conditions were lysed in a solution (1% NP40; 20 mM TRIS-HCl pH 7.5; 2 mM EDTA; 137 mM NaCl, 10% glycerol) containing the protease inhibitors Aprotinin, Leupeptin, and PMSF, and the phosphatase inhibitors NaF and Na3VO4,. The lysate was cleared by centrifugation at 15,000 rpm for 20 minutes and the protein samples were quantified by BCA protein assay (Pierce). 15 µgs of total protein were re-suspended in Laemmli sample buffer and the proteins were separated by electrophoresis on 10% SDS-polyacrylamide protein gels. Proteins were transferred to nitrocellulose membranes (0.45 µm Protean- Schleicher & Scheull) and the non-specific protein binding sites were blocked by incubating filters in 5% non-fat dry milk re-suspended in Tris-Buffered-Saline (TBS) containing 0.1% Tween (TBS-T). Filters were then incubated overnight with the appropriate primary antibody in TBS-T containing 5% bovine serum albumin (BSA). The next day the filters were washed three times in TBS-T and incubated with the respective HRP-conjugated secondary antibodies for 1 hour at room temperature. The filters were then washed three times in TBS-T and the specific protein bands were visualized by Supersignal West Pico chemiluminescence (Pierce).
The in vivo Ras activation status was examined using the GST-Raf RBD pull-down assay. Cells grown under the described conditions were lysed in 500 µL of MLB buffer, consisting of 20 mM Tris.HCl pH 7.5, 150 mM NaCl, 10mM MgCl2, 10% glycerol, 1% NP-40 and 0.25% sodium deoxycholate. The following inhibitors were added: 25mM NaF, 1mM Na Vanadate, 10 µg/ml Aprotinin and 10 µg/ml Leupeptin. Lysates were transferred to pre-chilled 1.5 ml Eppendorf tubes, rocked at 4 °C for 15 minutes and cleared by centrifugation at 14,000 rpm for 5 minutes at 4 °C. 50 µl of each sample was normalized with BCA protein assay and used for loading control subsequently. 5 µL of a GST-Raf RBD–sepharose bead slurry (a stock concentration of 2 µg/µl) was added to the remainder of each lysate, and samples were incubated at 4°C on a rocker for 30 minutes. The beads were spun down at 4000 rpm for 30 seconds at 4°C, the supernatant was aspirated, and beads were washed several times with MLB. Protein was extracted from beads in 10 µl of 2X SDS sample buffer, samples were heated at ~95°C for 5 minutes, and were resolved by 12% SDS-PAGE, transferred to PVDF, subsequently processed as described above and immunoblotted with K-Ras antibody.
Cell survival assays
Approximately 30,000 cells were plated in a well of a 12-well cluster dish. 24 h after plating, media was removed from the wells and replaced with media containing drugs. The experiments, unless otherwise stated, were performed over 72 hours and terminated by fixation of cells for 15 min at room temperature with 4% formaldehyde in PBS. Cells were then washed twice with PBS and stained with the fluorescent nucleic acid stain, Syto60 (700 nM) in PBS; excitation and emission wavelengths of 652 and 678nm, respectively; Molecular Probes) for 15 min at room temperature. The dye was then removed and the cells were washed once with PBS. Quantitation of fluorescent signal intensity was carried out at 700nm, using an Odyssey Infrared Imager (Li-Cor Biosciences). Each experiment was performed in quadruplicate and the results shown represent the average of the four values in comparison with untreated controls.
Clonogenic assays
To assess the effects of erlotinib on the respective cell lines, approximately 105 cells corresponding to each cell line were plated in a 100 mm culture dish. The cell lines include PC9, cisplatin-resistant PC9, PTEN-infected cisplatin-resistant PC9, GFP-infected cisplatin-resistant PC9 and cisplatin-resistant PC9 (cultured in cisplatin-free media). Erlotinib (2 µM) was added the day after the cells were plated, and fresh media with erlotinib was replaced every 3 days. These assays were performed over 10 days, after which time the experiment was terminated by fixing the cells with 4% formaldehyde, and cells were stained with Syto 60 for quantitation. Assays that examined the effects of LY294002 were performed in a similar manner, except that LY294002 was added in addition to erlotinib during each drug and media change. All experiments were performed in triplicate.
Expression of PTEN in PC9 cells by Lentiviral Transduction
Recombinant GFP-expressing (control) or PTEN-expressing lentiviral particles were generated in 293T cells by co-transfection of 1 µg pLenti-6V5-PTEN plus 0.9 µg pCMVΔ8.91 and 0.1 µg pMD.G-VSV-G into two wells of a 6 well plate. The day before infection, cisplatin-resistant PC9 cells were plated at a density of 1 × 105 cells per well of a 6-well plate. The cells were then infected by adding 0.5 ml of GFP or PTEN-expressing lentiviruses per well in the presence of 8 µg/ml polybrene. Infection was enhanced by centrifugation of the cells at 1200 × g for 1 h at 32°C. Twenty-four hours later, the media was replaced with fresh complete growth medium. Lentiviral transduction was allowed to proceed for a further 48 h. GFP- or PTEN-expressing cells were selected by typsinizing the cells into 10 cm dishes and adding 10 µg/ml Blasticidin in complete growth medium for 72 h. PTEN expression was verified by immunoblotting.
RESULTS
EGFR mutant NSCLC cells exhibit reduced sensitivity to erlotinib following prior treatment with cisplatin
To model the typical clinical experience of an EGFR mutation-positive NSCLC patient that involves first-line chemotherapy followed by second- or third-line EGFR TKI treatment, we utilized the PC9 human NSCLC-derived cell line. PC9 cells are derived from a previously untreated human NSCLC that harbors one of the recurrent in-frame deletions within EGFR exon 19 that gives rise to an activated kinase. These cells are exquisitely sensitive to EGFR TKIs, exhibiting an IC50 of ~30nM in a 72 hour cell viability assay (18). Thus, they appear to represent the subset of EGFR mutation-positive NSCLC patients that demonstrate a good clinical response to treatment with EGFR TKIs. We utilized this cell culture model to determine whether pre-treatment with a platinum-based drug, a standard component of traditional first-line therapy for NSCLC, would affect the response to subsequent treatment with erlotinib. Treatment of PC9 cells with 5 µM cisplatin for 3 days results in ~80% cell death, followed by the emergence of cells that are largely refractory to the cytotoxic actions of cisplatin and can be maintained in cisplatin indefinitely (Figure 1A). Thus, these cells appear to model a typical clinical response to cisplatin in EGFR mutation-positive NSCLC.
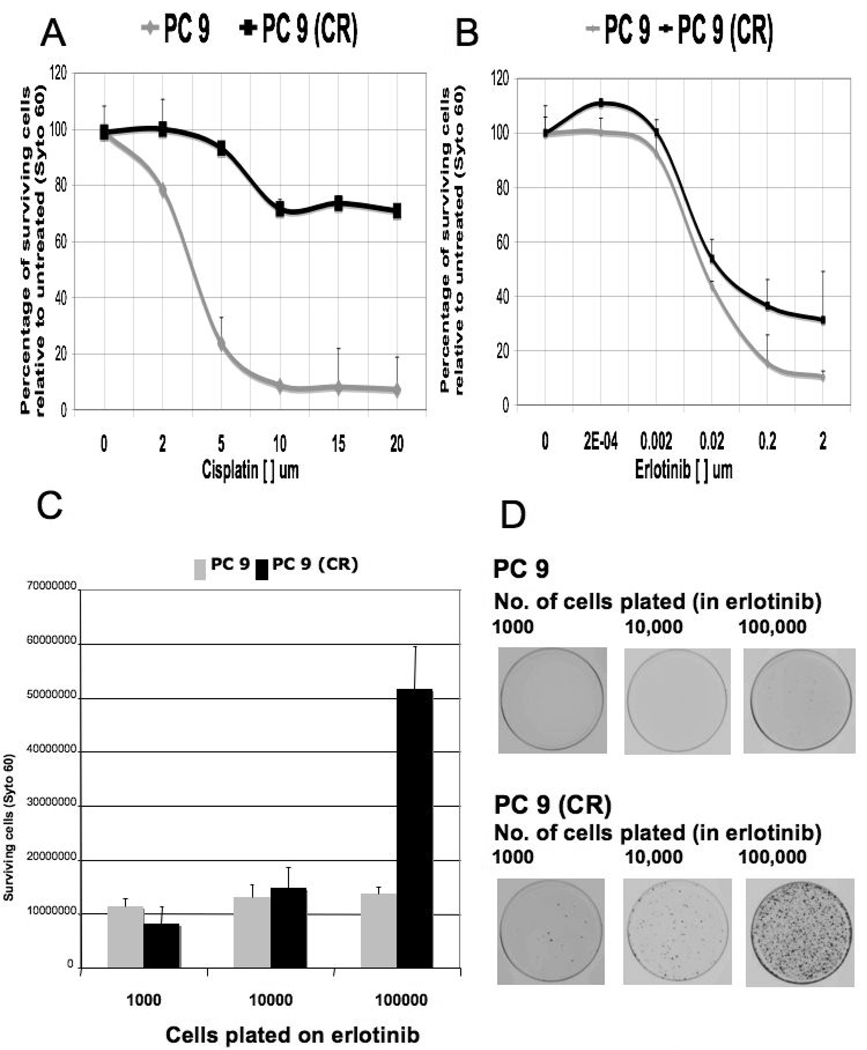
Figure 1. Sensitivity of PC9 and cisplatin-resistant PC9 cell lines to cisplatin and erlotinib.
A. Survival curves of PC9 and cisplatin-resistant PC9 (CR) cells following 72-hour treatment with the indicated concentration of cisplatin. Each data point represents a percentage of surviving cells with respect to untreated cells, and is the average of 4 independently conducted experiments.
B. Survival curves of PC9 and PC9 (CR) cells following 72-hour treatment with the indicated concentration of erlotinib. Each data point represents a percentage of surviving cells with respect to untreated cells, and is the average of 4 independently conducted experiments. Note that at 2 micromolar erlotinib (pharmacologic concentration), the curves corresponding to PC9 and PC9 (CR) cells are significantly different.
C. Clonogenic assays were performed on PC9 and PC9 (CR) in the presence of 2 µM of erlotinib for 10 days. 1000, 10 000, and 100,000 cells of each respective cell line were plated in triplicate, and the number of surviving cells was assessed by Syto 60 staining. Error bars represent the standard deviation from the average value derived from 3 experiments.
D. A representative cell culture plate from each cell line tested in 1C is shown.
Next, we determined whether the cells that emerged following cisplatin exposure exhibit an altered sensitivity to erlotinib treatment relative to drug-naïve PC9 cells. As demonstrated (Figure 1B), the IC50 of erlotinib sensitivity for PC9 cells and cisplatin-resistant PC9 derivatives is approximately equal (30 nM), as measured in a 72-hour viability assay. However, the IC90 value for erlotinib sensitivity was significantly increased in the cisplatin-resistant cells, suggesting the existence of cell heterogeneity within the cisplatin-resistant population with regard to erlotinib sensitivity. To explore this difference further, we performed a similar comparison using a longer-term clonogenic survival assay. By plating varying numbers of PC9 or cisplatin-resistant PC9 cells (withdrawn from cisplatin) in the presence of 2 µM erlotinib (the clinical concentration), we observed a 5-fold increase in the number of erlotinib-resistant clones that emerge from the cisplatin-resistant PC9 cells relative to the parental PC9 cells during 10 days of continuous erlotinib exposure (Figure 1C, D). Together, these results suggest that pre-treatment of EGFR mutant NSCLC cells with cisplatin significantly reduces their sensitivity to subsequent exposure to erlotinib.
Erlotinib fails to suppress AKT activation in cisplatin-resistant PC9 cells
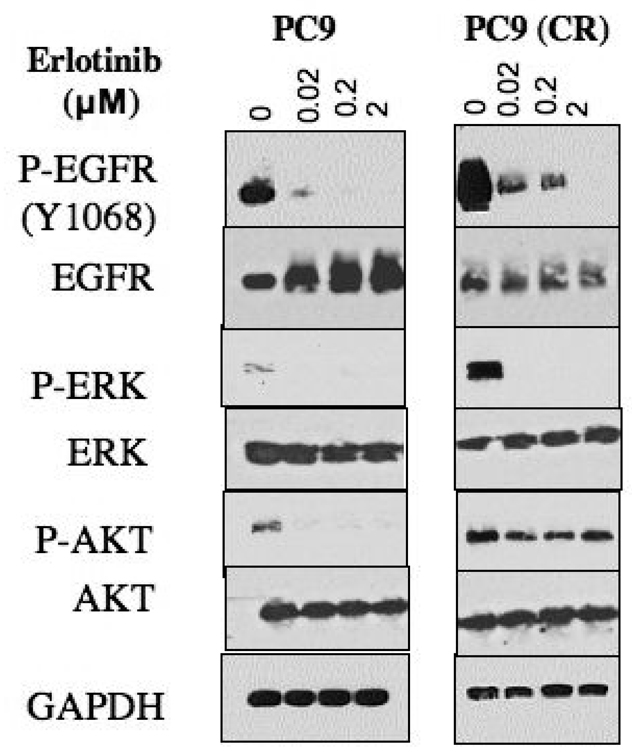
To begin to address the molecular basis for the reduced erlotinib sensitivity seen in cisplatin-resistant PC9 cells, we examined the signaling pathways in these cells that are associated with mutationally activated EGFR. We found that cisplatin-resistant cells exhibit significantly increased basal levels of EGFR phosphorylation, consistent with previous studies demonstrating the activation of EGFR kinase activity by chemotherapy drugs (19). Similarly, these cells exhibit elevated levels of phospho-AKT and phospho-ERK, two key downstream effectors of EGFR-mediated cell survival. Interestingly, upon treatment with erlotinib, cisplatin-resistant cells, like PC9 cells, rapidly experience suppressed phosphorylation of EGFR and ERK; however, they fail to demonstrate suppression of AKT phosphorylation, and phospho-AKT levels remain essentially unchanged even after exposure to 2 µM erlotinib for 4 hours (Figure 2). These results suggest that in cisplatin-resistant PC9 cells, activation of AKT, a key survival protein, becomes uncoupled from EGFR kinase activity.
Figure 2. Persistent AKT activation in cisplatin-resistant PC9 cells.
Cell lysates from PC9 and PC9 (CR) treated with increasing concentrations of erlotinib for 4 hours were analyzed by SDS-PAGE followed by immunoblotting using antibodies directed against the indicated signal transduction proteins.
Cisplatin exposure has long-term consequences for subsequent erlotinib treatment
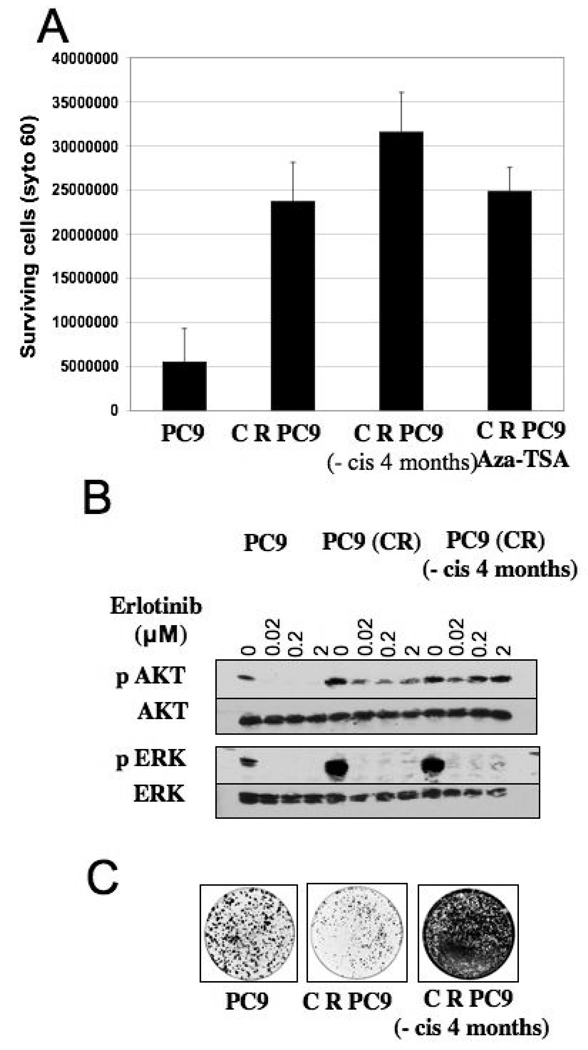
NSCLC patients who experience disease control with cisplatin typically undergo a period of time (a “drug holiday”) during which they are untreated, prior to initiating second- or third-line treatment with another agent, such as an EGFR TKI, at subsequent disease progression. Therefore, we wanted to determine whether withdrawal of cisplatin from cisplatin-resistant PC9 cells for several months would still impact subsequent sensitivity to erlotinib. Interestingly, after 4 months of culture in drug-free medium, the cisplatin-resistant cells retain their reduced sensitivity to erlotinib, as measured in the clonogenic survival assay. This observation suggests that rapidly reversible epigenetic mechanisms, such as DNA methylation, are unlikely to account for the observed effects of cisplatin. Indeed, treatment of these cells with the chromatin modifying agents Azacytidine (Aza) and Trichostatin-A (TSA) failed to affect erlotinib sensitivity (Figure 3A). These findings suggest that cisplatin treatment of erlotinib-sensitive NSCLC cells leads to reduced erlotinib sensitivity that is maintained even several months following cisplatin withdrawal.
Figure 3. Lingering effect of cisplatin exposure on erlotinib sensitivity.
A. Clonogenic assays were performed in PC9, PC9 (CR), PC9 (CR maintained in cisplatin-free media) and PC9 (CR) (treated with 1 µM Azacytidine and 30 nM Trichostatin-A) cells. 100,000 cells corresponding to each cell line were plated and 2 µM erlotinib was added the following day. Cells were maintained in erlotinib for the 10 day course of the experiment. The graph represents the number of surviving cells as measured by Syto 60 staining in the respective cell lines. Error bars correspond the standard deviation from the average value derived from three independent experiments.
B. Cell lysates from PC9, PC9 (CR) and PC9 (CR maintained in cisplatin-free media) treated with increasing concentrations of erlotinib for 4 hours were analyzed by SDS-PAGE followed by immunoblotting using antibodies directed against the indicated signal transduction proteins.
C. 1000 cells each of PC9, PC9 (CR), and PC9 (CR maintained in cisplatin-free media) were plated on a 100 mm dish and grown in 10% FBS. Cells were subjected to Syto 60 staining after one week to assess their respective proliferative rates. Note the increased staining seen in cisplatin-resistant cells following withdrawal of cisplatin.
Next, we examined the status of phospho-AKT in the cisplatin-resistant cells that had been removed from cisplatin for 4 months. In these cells, as was seen in the cisplatin-resistant cells immediately following cisplatin withdrawal, phospho-AKT levels are elevated, and significantly, they are not suppressed by erlotinib treatment (Figure 3B). This observation suggests that persistent downstream activation of the AKT survival pathway may contribute to reduced erlotinib sensitivity as a long term consequence of cisplatin exposure, even several months following discontinuation of treatment. These findings are also consistent with previous studies that have demonstrated persistent activation of the AKT pathway in cells resistant to EGFR tyrosine kinase inhibitors (15, 16, 18, 20). Interestingly, the cisplatin-resistant cells cultured in cisplatin-free media also exhibit a significantly increased proliferative rate (Figure 3C), raising the possibility that cisplatin exposure enriches the population for tumor cells that grow more aggressively.
Reduced erlotinib sensitivity following cisplatin treatment is correlated with reduced PTEN function and can be partially overcome by re-introduction of PTEN
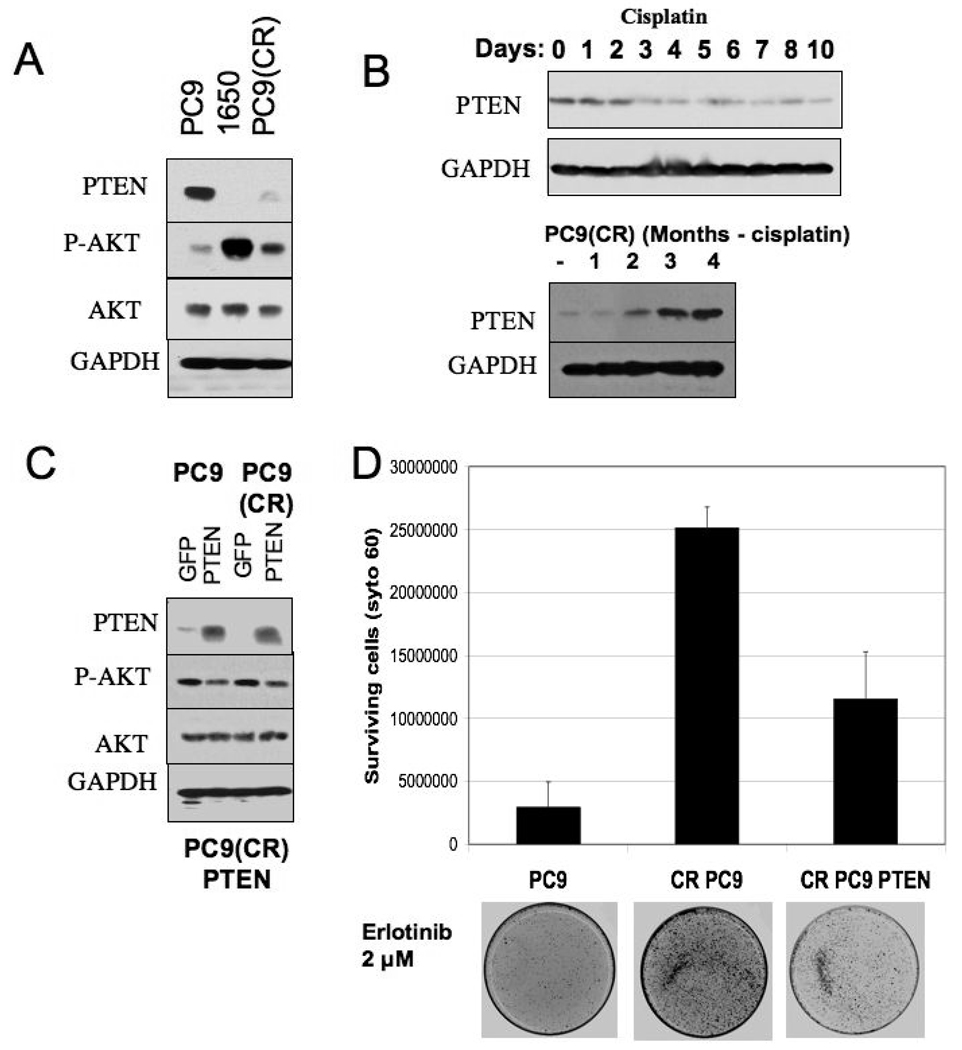
To pursue the mechanism underlying persistent AKT activation in the cisplatin-resistant PC9 cells, we examined the status of the PTEN tumor suppressor, a negative regulator of PI-3 kinase/AKT signaling that has been previously implicated in resistance to chemotherapy drugs, including cisplatin (21, 22), and is mutationally disrupted in a subset of NSCLCs (23). PC9 cells express readily detectable levels of PTEN protein, whereas another human NSCLC cell line (NCI-H1650) with an identical EGFR activating mutation, but which is ~100-fold less sensitive to erlotinib (Supplementary Figure 1), does not express PTEN, due to an inactivating mutation (Figure 4A). Notably, NCI-H1650 cells exhibit relatively high levels of phospho-AKT compared to PC9 cells, suggesting that PTEN loss may contribute to the difference in erlotinib sensitivity between NIH-H1650 and PC9 cells. Significantly, in the cisplatin-resistant PC9 cells, PTEN levels are barely detectable (Figure 4A), suggesting that PTEN attenuation may contribute to the persistent phospho-AKT levels seen in those cells upon treatment with erlotinib (Figure 3B).
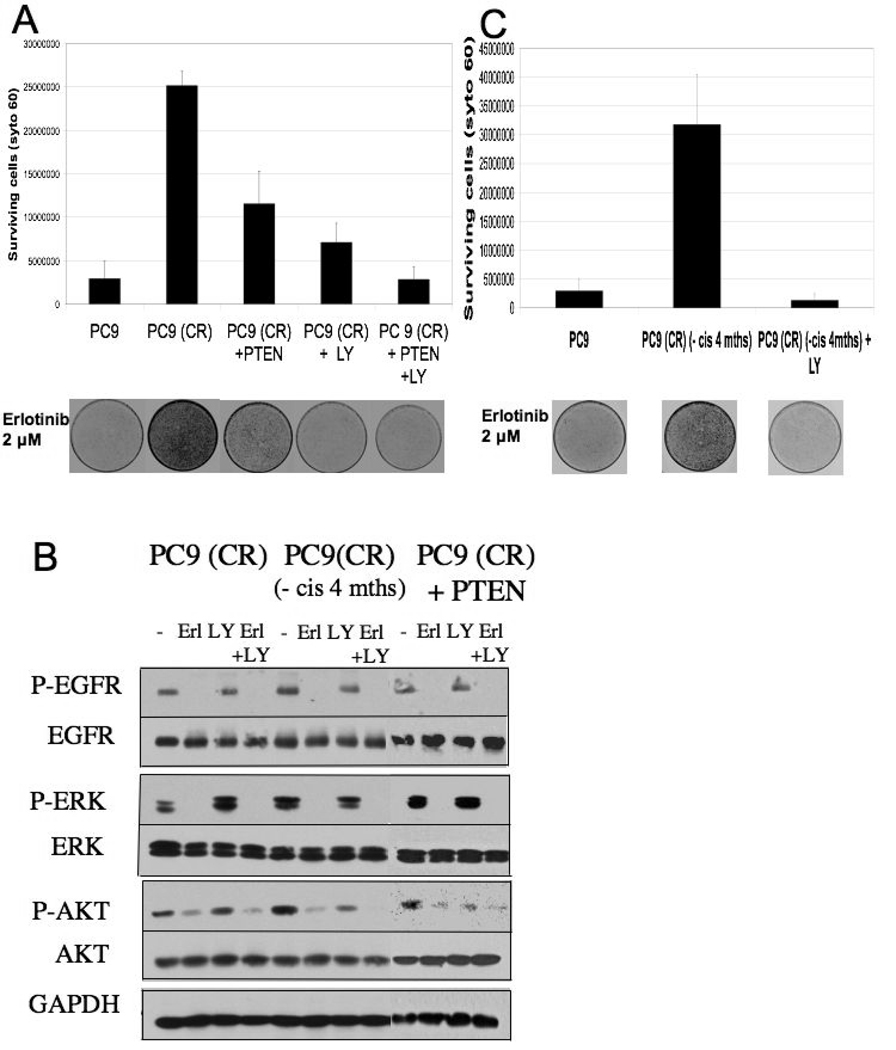
Figure 4. Reduced erlotinib sensitivity following cisplatin treatment is correlated with loss of PTEN expression and can be partially overcome by re-introduction of PTEN.
A. Cell lysates from PC9, PC9 (CR) and 1650 cells were analyzed by SDS page followed by immunoblotting with PTEN and AKT antibodies. GAPDH serves as loading control.
B. Cell lysates from PC9 cells treated cisplatin for the indicated number of days were subjected to SDS- PAGE and immunoblotted for PTEN expression (upper panels). Cell lysates from PC9 (CR) maintained in cisplatin free media for increasing durations (1, 2, 3 and 4 months) were similarly analyzed (lower panels).
C. Cell lysates from GFP and PTEN-expressing lentivrus-infected PC9 and PC9 (CR) were analyzed for PTEN expression by immunoblotting.
D. 100,000 cells corresponding to PC9, PC9 (CR), and PC9 (CR PTEN-infected) lines were plated in a 100 mm dish for clonogenic assays. 2 µM erlotinib was added the following day and cells were maintained in media containing 2 µM erlotinib for the 10-day assay. The graph represents the number of surviving cells as measured by Syto 60 staining in the respective cell lines. Error bars represent the standard deviation from the average value derived from three independent experiments. A representative stained plate from each cell line is shown below.
To address the mechanism by which cisplatin affects PTEN, we examined the effects of cisplatin on PTEN expression and subcellular distribution. We observed that within a few days following cisplatin treatment of PC9 cells, PTEN protein levels are significantly reduced (Figure 4B, upper panel). PTEN regulation also appears to involve altered subcellular distribution in response to cisplatin. Thus, within 2 hours of cisplatin exposure, PC9 cells exhibit significant nuclear accumulation of PTEN (data not shown), a process that has been implicated in PTEN-mediated protection of the genome from cisplatin-mediated DNA damage (24). Notably, we observed that PTEN levels are gradually restored in the cisplatin-resistant PC9 cells during propagation in cisplatin-free media (Figure 4B, lower panel). Overall, it appears that PTEN regulation in response to cisplatin exposure is complex, and involves acute changes in protein distribution as well as longer-term changes in protein expression.
To directly address a potential role for reduced PTEN activity in the persistent phospho-AKT levels and reduced erlotinib sensitivity seen in the cisplatin-resistant PC9 cells, we restored high levels of PTEN in these cells via expression from a lentivirus. The virus-infected cells demonstrate a high level of PTEN expression relative to uninfected cells (Figure 4C), although their growth properties were not detectably affected (not shown). Significantly, in the clonogenic assay, the cells with restored PTEN levels exhibit significantly increased sensitivity to erlotinib; albeit, not to the same level as parental PC9 cells (Figure 4D). Notably, basal levels of phospho-AKT remain somewhat elevated in the PTEN virus-infected cisplatin-resistant cells compared to parental PC9 cells, suggesting that AKT is activated in these cells by an additional upstream pathway. This elevation of phospho-AKT may also be influenced by the increased nuclear PTEN localization in the PTEN-infected PC9 cells (not shown). The increased phospho-AKT may account for the fact that erlotinib sensitivity could not be completely restored in the cisplatin-resistant cells by expression of PTEN. Together, these findings suggest that the reduced PTEN function that arises during acquisition of resistance to cisplatin contributes to some, but not all of the subsequently reduced sensitivity to erlotinib.
PI-3 kinase inhibition, together with restoration of PTEN function, reverses resistance to erlotinib
Our findings thus far have demonstrated an important role for persistent AKT activation in the EGFR TKI sensitivity of cisplatin-resistant PC9 cells. Since PI-3 kinase is a major upstream activator of AKT, we examined the ability of a pharmacologic inhibitor of PI-3 kinase, LY294002, to modulate the erlotinib sensitivity of cisplatin-resistant PC9 cells. Indeed, we observed that the LY294002 restored a substantial level of erlotinib sensitivity in these cells (Figure 5A) and caused a corresponding reduction in phospho-AKT levels when combined with erlotinib (Figure 5B). Furthermore, PI-3 kinase inhibition in the PTEN-infected cisplatin-resistant cells completely restores sensitivity to erlotinib in these cells, rendering them as sensitive to erlotinib as parental PC9 cells (Figure 5A). The relevance of both a functional PTEN and PI-3 kinase inhibition in restoring erlotinib sensitivity is further supported by the observation that the cisplatin-resistant cells which had been take out of cisplatin for four months also exhibit restored erlotinib sensitivity following treatment with LY294002 alone (Figure 5C).
Figure 5. PI-3 kinase inhibition, together with restoration of PTEN function, reverses resistance to erlotinib.
A. To determine if PI-3 kinase inhibition has any effect on reversal of resistance to erlotinib, clonogenic assays were performed in triplicate on PC9, PC9 (CR) and PC9 (CR PTEN infected) cells. 100,000 cells were plated and 2 µM erlotinib +/− 2.5 µM LY 294002 was added the following day. The assay was carried out over 10 days and fresh media containing 2 µM erlotinib +/− 2.5 µM LY 294002 was replaced every 3 days. The graph represents the number of surviving cells as measured by Syto 60 staining in the respective cell lines. Error bars represent the standard deviation from the average value derived from three independent experiments. A representative stained plate from each cell line is shown below.
B. Cell lysates were derived from PC9 (CR), PC9 (CR PTEN infected) and PC9 (CR maintained in cisplatin-free media) treated with either erlotinib (2 µM), LY 294002 (2.5 µM) or the combination of the two drugs for 4 hours. These were subject to SDS-PAGE analysis and immunoblotting with the indicated antibodies.
C. A similar assay as described in 5A was performed that included PC9 (CR) cells maintained in cisplatin-free media for 4 months. The graph represents the number of surviving cells as measured by Syto 60 staining in the respective cell lines. Error bars represent the standard deviation from the average value derived from three independent experiments. A representative stained plate from each cell line is shown.
Since the RAS pathway can activate AKT via PI-3 kinase, and K-RAS mutations are frequently detected in TKI-refractory NSCLC patients (25), we examined a potential role for K-RAS activation in the cisplatin-resistant cells. Interestingly, the levels of total as well as activated K-RAS are elevated in cisplatin-resistant PC9, relative to parental PC9 cells (Supplementary Figure 2A). To determine if an activated RAS pathway is responsible for the persistently activated AKT pathway after erlotinib treatment, we also examined activated (GTP-bound) RAS levels in parental PC9 and cisplatin-resistant PC9 cells after 4 hours of exposure to 1 µM erlotinib. (Supplementary Fig 2B) Although phospho-AKT remained elevated, activated K-RAS levels were efficiently down-regulated by erlotinib. This suggests that, although the elevated AKT activation in these cells may, in part, be contributed by the RAS pathway, the resistance to erlotinib is unlikely to be a consequence of increased K-RAS activation in the presence of erlotinib. Together, these findings suggest that activation of the PI-3 kinase pathway is largely responsible for the reduced erlotinib sensitivity seen in cisplatin-resistant PC9 cells, and that loss of PTEN expression and activation of an EGFR-independent pathway leading to PI-3 kinase activation both play an important role.
DISCUSSION
Cultured NSCLC cell lines provide a very useful system for modeling the clinical response to EGFR TKIs as well as mechanisms of acquired drug resistance. Since EGFR TKIs are typically administered as second- or third-line treatment in NSCLC, subsequent to platinum-based chemotherapy, we extended the standard use of cell culture modeling to examine the effect of cisplatin on subsequent treatment with an EGFR TKI. To our knowledge, these represent the first studies to model this frequently administered NSCLC treatment sequence in cell culture. Our overall findings indicate that exposure to cisplatin can lead to significant resistance to subsequent TKI challenge in EGFR-mutant NSCLC. Interestingly, while cisplatin pre-treatment does not detectably affect the IC50 of erlotinib in this model, the clonogenic growth potential of cisplatin-treated cells subsequently exposed to erlotinib is substantially increased. Extrapolating these cell culture findings to the clinical setting, this could indicate that while cisplatin treatment may not affect the rate of clinical responses to subsequent EGFR TKI therapy, it could lead to a reduced time to disease progression in cases where EGFR TKI responses are observed, due to a more rapid acquisition of TKI resistance. Indeed, EGFR mutation-positive NSCLC patients frequently respond to EGFR TKI therapy in the second- or third-line setting, but will ultimately develop resistance to therapy (26). Thus far, clinical studies to compare the time–to-progression following erlotinib treatment in the first-line versus later-line settings have not been reported, yet our findings suggest that a differential effect may exist and, if clinically validated, could have an important impact on first-line therapeutic decision-making.
The effects of cisplatin pre-treatment on EGFR-mutant NSCLC cells in the PC9 model are associated with persistently activated PI-3 kinase-AKT signaling, a well established tumor cell survival pathway that has been linked to drug resistance in a variety of contexts (26, 27). The persistent TKI-insensitive AKT activation observed in cisplatin-treated PC9 cells appears to involve at least two different mechanisms--reduced PTEN function and EGFR-independent AKT activation. Our results indicate that pharmacologic PI-3 kinase inhibition can efficiently suppress the alternatively activated AKT pathway, thereby rendering cells re-sensitized to EGFR TKIs when exposed to a combination of the PI-3 kinase inhibitor LY294002 and erlotinib. Several previous studies have similarly implicated PTEN and PI-3 kinase/AKT activity in cisplatin resistance in cancer cells (21, 22, 28, 29), consistent with the apparent role for PTEN and AKT in the NSCLC model described here.
The mechanism by which PTEN function is reduced in the cisplatin-treated PC9 cells appears to be complex and potentially involves changes in PTEN expression and subcellular localization, both of which have been previously implicated in PTEN regulation (30). Furthermore, the acute and longer-term effects of cisplatin treatment on these cells are distinct. Thus, within a few hours of cisplatin exposure, PTEN undergoes a gel mobility shift consistent with phosphorylation (not shown) and exhibits a nuclear accumulation by immunofluorescence. However, over a period of days following treatment, the PTEN subcellular distribution is not obviously different from that seen in untreated cells, whereas PTEN protein levels are clearly reduced (data not shown). The initial response to cisplatin may reflect a cellular mechanism that engages survival signaling acutely following a stressful stimulus, while the longer-term response may reflect the selection of a fraction of cells that are capable of sustaining such survival signaling.
The PI-3 kinase/AKT pathway is among the critical effectors of oncogenic EGFR, and persistent EGFR-independent signaling through this pathway appears to contribute to erlotinib resistance in some NSCLCs (16). This presumably explains why cisplatin-resistant PC9 cells, in which this pathway persists in the presence of erlotinib, exhibit erlotinib-resistant clonogenic cell survival. Moreover, the fact that AKT activation is seen in cisplatin-resistant cells even several months following cisplatin withdrawal is likely to account for the longer-term consequences of cisplatin exposure to subsequent EGFR TKI treatment. However, it remains unclear as to why the effects of cisplatin on this pathway persist following cisplatin withdrawal. Although we found that treatment of cisplatin-resistant PC9 cells with two different chromatin modifying agents did not affect subsequent erlotinib sensitivity, it is not possible to exclude a role for epigenetic regulation of components of this pathway, particularly when considering its reversible nature.
While erlotinib is currently approved for clinical use in the second- or third-line setting, our cell culture findings highlight a potential disadvantage associated with subjecting EGFR mutation-positive NSCLC patients to platinum-based therapy prior to treatment with EGFR TKIs. Notably, it has been reported that NSCLC patients with reduced PTEN expression experience a shorter time to tumor progression, and consequently, a worse prognosis (31, 32). Thus, if these pre-clinical findings accurately model the experience of many NSCLC patients, they would suggest that patients who have received recent platinum therapy may be more resistant to TKIs and may also be intrinsically more resistant to subsequent lines of therapy.
It is also interesting to consider how first-line EGFR TKI therapy might impact subsequent platinum therapy. The T790M EGFR kinase domain mutation, along with MET gene amplification, appear to account for 50–70% of acquired EGFR TKI resistance in NSCLC patients, and both mechanisms can promote increased signaling via the PI-3 kinase/AKT pathway (16, 33, 34). Taken together with the fact that PI-3 kinase/AKT signaling reportedly affects cisplatin sensitivity, these EGFR TKI resistance mechanisms that arise in the context of first-line TKI treatment, may similarly contribute to subsequent resistance to platinum-based agents.
In conclusion, we find that pre-treatment of EGFR TKI-sensitive PC9 NSCLC cells with cisplatin leads to significant resistance to subsequent TKI treatment via a persistently activated PI-3 kinase-AKT pathway, in part contributed by cisplatin-induced reduction in PTEN function. The addition of a pharmacologic PI-3 kinase inhibitor to an EGFR TKI can partially re-sensitize these cells to TKIs, suggesting a potential therapeutic strategy for reversing or delaying resistance to TKIs. Importantly, our findings also suggest that these effects of cisplatin can persist for at least several months following drug withdrawal. These potential issues associated with cisplatin treatment prior to EGFR TKI administration should prompt further consideration of first line EGFR TKI use in EGFR mutation-positive NSCLC patients.
ACKNOWLEDGEMENTS
We are grateful to members of the Settleman laboratory for helpful discussions during the course of this study. The work was supported by NIH RO1 CA115830 to and a V Foundation for Cancer Research award to J.S.
REFERENCES
- 1.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 3.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21(12):2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. Jama. 2003;290(16):2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 5.Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587–595. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 6.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 7.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 8.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue A, Suzuki T, Fukuhara T, et al. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol. 2006;24(21):3340–3346. doi: 10.1200/JCO.2005.05.4692. [DOI] [PubMed] [Google Scholar]
- 10.Asahina H, Yamazaki K, Kinoshita I, et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br J Cancer. 2006;95(8):998–1004. doi: 10.1038/sj.bjc.6603393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sequist LV, Martins R, Spigel D. First-line gefitinib in advanced non-small-cell lung cancer patients harboring somatic EGFR mutations. J Clin Oncol. 2008 doi: 10.1200/JCO.2007.14.8494. (in press). [DOI] [PubMed] [Google Scholar]
- 12.Shepherd FA, Rosell R. Weighing tumor biology in treatment decisions for patients with non-small cell lung cancer. J Thorac Oncol. 2007;2(Suppl 2):S68–S76. doi: 10.1097/01.JTO.0000269737.05962.a0. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd FA, Tsao MS. Unraveling the mystery of prognostic and predictive factors in epidermal growth factor receptor therapy. J Clin Oncol. 2006;24(7):1219–1220. doi: 10.1200/JCO.2005.04.4420. author reply 20-1. [DOI] [PubMed] [Google Scholar]
- 14.McDermott U, Sharma SV, Dowell L, et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A. 2007;104(50):19936–19941. doi: 10.1073/pnas.0707498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelman JA, Mukohara T, Zejnullahu K, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116(10):2695–2706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 17.Koizumi F, Shimoyama T, Taguchi F, Saijo N, Nishio K. Establishment of a human non-small cell lung cancer cell line resistant to gefitinib. Int J Cancer. 2005;116(1):36–44. doi: 10.1002/ijc.20985. [DOI] [PubMed] [Google Scholar]
- 18.Ono M, Hirata A, Kometani T, et al. Sensitivity to gefitinib (Iressa, ZD1839) in non-small cell lung cancer cell lines correlates with dependence on the epidermal growth factor (EGF) receptor/extracellular signal-regulated kinase 1/2 and EGF receptor/Akt pathway for prolifera. Mol Cancer Ther. 2004;3(4):465–472. [PubMed] [Google Scholar]
- 19.Van Schaeybroeck S, Kyula J, Kelly DM, et al. Chemotherapy-induced epidermal growth factor receptor activation determines response to combined gefitinib/chemotherapy treatment in non-small cell lung cancer cells. Mol Cancer Ther. 2006;5(5):1154–1165. doi: 10.1158/1535-7163.MCT-05-0446. [DOI] [PubMed] [Google Scholar]
- 20.Ando K, Ohmori T, Inoue F, et al. Enhancement of sensitivity to tumor necrosis factor alpha in non-small cell lung cancer cells with acquired resistance to gefitinib. Clin Cancer Res. 2005;11(24 Pt 1):8872–8879. doi: 10.1158/1078-0432.CCR-05-0811. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Choi EJ, Jin C, Kim DH. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol Oncol. 2005;97(1):26–34. doi: 10.1016/j.ygyno.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 22.Yan X, Fraser M, Qiu Q, Tsang BK. Over-expression of PTEN sensitizes human ovarian cancer cells to cisplatin-induced apoptosis in a p53-dependent manner. Gynecol Oncol. 2006;102(2):348–355. doi: 10.1016/j.ygyno.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 23.Soria JC, Lee HY, Lee JI, et al. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res. 2002;8(5):1178–1184. [PubMed] [Google Scholar]
- 24.Shen WH, Balajee AS, Wang J, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128(1):157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 25.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2(1):e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgillo F, Bareschino MA, Bianco R, Tortora G, Ciardiello F. Primary and acquired resistance to anti-EGFR targeted drugs in cancer therapy. Differentiation. 2007;75(9):788–799. doi: 10.1111/j.1432-0436.2007.00200.x. [DOI] [PubMed] [Google Scholar]
- 27.Ohmichi M, Hayakawa J, Tasaka K, Kurachi H, Murata Y. Mechanisms of platinum drug resistance. Trends Pharmacol Sci. 2005;26(3):113–116. doi: 10.1016/j.tips.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Gagnon V, Mathieu I, Sexton E, Leblanc K, Asselin E. AKT involvement in cisplatin chemoresistance of human uterine cancer cells. Gynecol Oncol. 2004;94(3):785–795. doi: 10.1016/j.ygyno.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Wu HJ, Wu HT, Weng DH, Xing H, Lu YP, Ma D. [Reversal of drug resistance in human ovarian cancer cells by wild-type PTEN gene and its mechanisms.] Zhonghua Fu Chan Ke Za Zhi. 2007;42(9):612–616. [PubMed] [Google Scholar]
- 30.Parsons R. Human cancer, PTEN and the PI-3 kinase pathway. Semin Cell Dev Biol. 2004;15(2):171–176. doi: 10.1016/j.semcdb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Buckingham LE, Coon JS, Morrison LE, et al. The prognostic value of chromosome 7 polysomy in non-small cell lung cancer patients treated with gefitinib. J Thorac Oncol. 2007;2(5):414–422. doi: 10.1097/01.JTO.0000268675.02744.b0. [DOI] [PubMed] [Google Scholar]
- 32.Lim WT, Zhang WH, Miller CR, et al. PTEN and phosphorylated AKT expression and prognosis in early-and late-stage non-small cell lung cancer. Oncol Rep. 2007;17(4):853–857. [PubMed] [Google Scholar]
- 33.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104(52):20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]