Abstract
Porous Si exhibits a number of properties that make it an attractive material for controlled drug delivery applications: The electrochemical synthesis allows construction of tailored pore sizes and volumes that are controllable from the scale of microns to nanometers; a number of convenient chemistries exist for the modification of porous Si surfaces that can be used to control the amount, identity, and in vivo release rate of drug payloads and the resorption rate of the porous host matrix; the material can be used as a template for organic and biopolymers, to prepare composites with a designed nanostructure; and finally, the optical properties of photonic structures prepared from this material provide a self-reporting feature that can be monitored in vivo. This paper reviews the preparation, chemistry, and properties of electrochemically prepared porous Si or SiO2 hosts relevant to drug delivery applications.
Keywords: Porous silicon, Small molecule drug delivery, Nanotechnology, Photonic crystal, Cancer, Protein therapy
1. Introduction
Porous Si has been investigated for applications in microelectronics, optoelectronics, [1–4] chemical [5,6] and biological [7–10] sensors, and biomedical devices [11]. The in vivo use of porous Si was first promoted by Leigh Canham, who demonstrated its resorbability and biocompatibility in the mid 1990s [12–15]. Subsequently, porous Si or porous SiO2 (prepared from porous Si by oxidation) host matrices have been employed to demonstrate in vitro release of the steroid dexamethasone [16], ibuprofen [17], cis-platin [18], doxorubicin [19], and many other drugs [20]. The first report of drug delivery from porous Si across a cellular barrier was performed with insulin, delivered across monolayers of Caco-2 cells [21]. An excellent review of the potential for use of porous Si in various drug delivery applications has recently appeared [20].
An emerging theme in porous Si as applied to medicine has been the construction of microparticles (“mother ships”) with sizes on the order of 1–100 µm that can carry a molecular or nanosized payload, typically a drug.With a free volume that can be in excess of 80%, porous Si can carry cargo such as proteins, enzymes [22–29], drugs [16–20,30,31], or genes. It can also carry nanoparticles, which can be equipped with additional homing devices, sensors, or cargoes. In addition, the optical properties of nanocrystalline silicon can be recruited to perform various therapeutic or diagnostic tasks—for example, quantum confined silicon nanostructures can act as photosensitizers to produce singlet oxygen as a photodynamic therapy [32–35]. A long-term goal is to harness the optical, electronic, and chemical properties of porous Si that can allow the particles to home to diseased tissues such as tumors and then perform various tasks in vivo. These tasks include detecting, identifying, imaging, and delivering therapies to the tissue of interest. In this work we review the chemistry of porous Si that allows the incorporation of drug pay-loads, homing devices, optical features for imaging, and sensors for detection of various physical changes.
2. Preparation of porous Si
2.1. Electrochemical etching
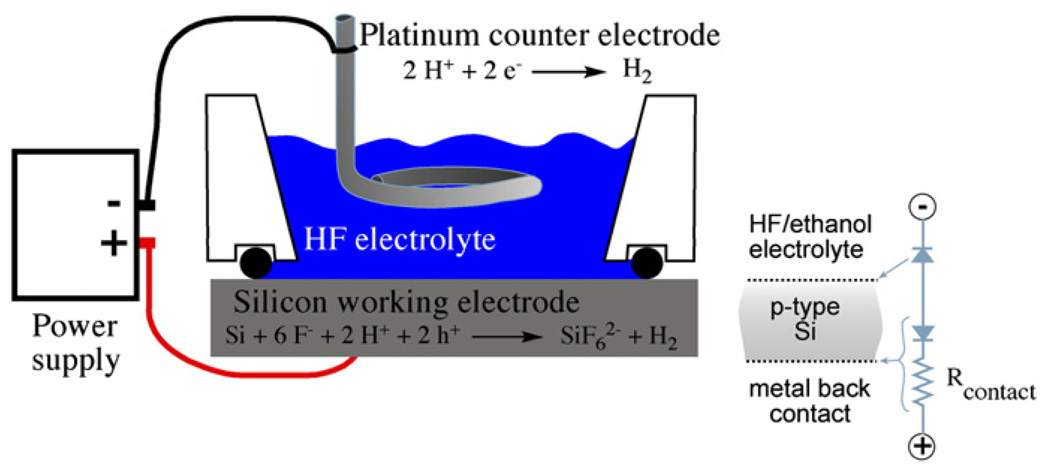
Porous Si is a product of an electrochemical anodization of single crystalline Si wafers in a hydrofluoric acid electrolyte solution. Pore morphology and pore size can be varied by controlling the current density, the type and concentration of dopant, the crystalline orientation of the wafer, and the electrolyte concentration in order to form macro-, meso-, and micropores [36]. Pore sizes ranging from 1 nmto a few microns can be prepared.
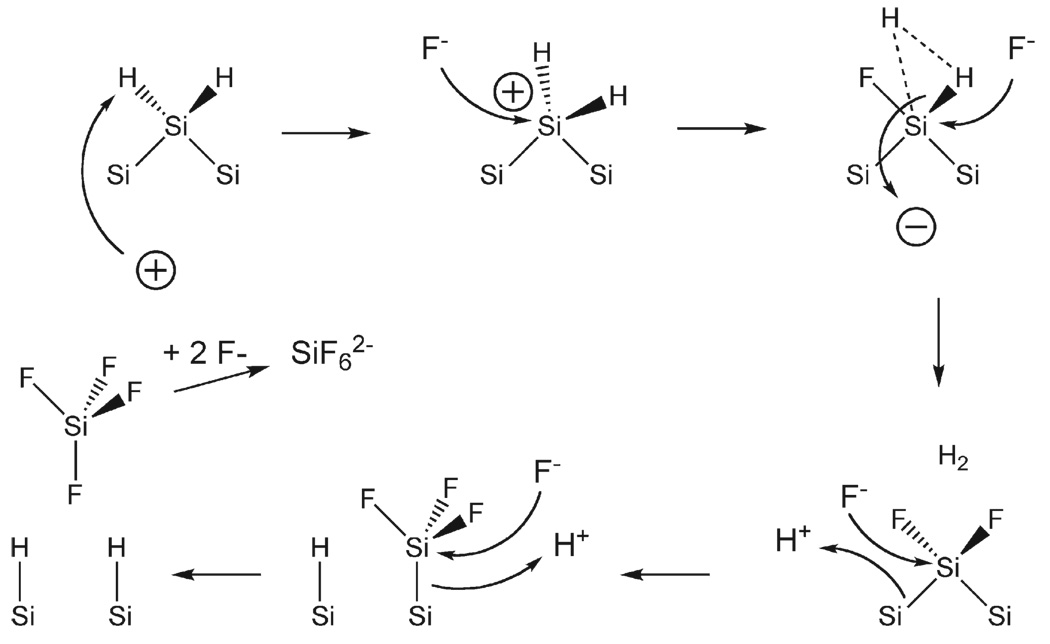
The mechanism of pore formation is not generally agreed upon, but it is thought to involve a combination of electronic and chemical factors [37]. The type of dopant in the original silicon wafer is important because it determines the availability of valence band holes that are the key oxidizing equivalents in the reaction shown in Fig. 1. In general the relationships of dopant to morphology can be segregated into four groups based on the type and concentration of the dopant: n-type, p-type, highly doped n-type, and highly doped p-type. By “highly doped,” we mean dopant levels at which the conductivity behavior of the material is more metallic than semiconducting. For n-type silicon wafers with a relatively moderate doping level, exclusion of valence band holes from the space charge region determines the pore diameter. Quantum confinement effects are thought to limit pore size in moderately p-doped material. For both dopant types the reaction is crystal face selective, with the pores propagating primarily in the <100> direction of the single crystal. A simplified mechanism for the chemical reaction is shown in Fig. 2 [38,39]. The electrochemically driven reaction requires an electrolyte containing hydrofluoric acid. Application of anodic current oxidizes a surface silicon atom, which is then attacked by fluoride. The net process is a 4 electron oxidation, but only two equivalents are supplied by the current source. The other two equivalents come from reduction of protons in the solution by surface SiF2 species. Pore formation occurs as Si atoms are removed in the form of SiF4, which reacts with two equivalents of F− in solution to form
Fig. 1.
Schematic of the etch cell used to prepare porous Si. The electrochemical half-reactions are shown, and the equivalent circuit for etching of a p-type Si wafer is shown at right.
Fig. 2.
Mechanism of Si oxidation during the formation of porous Si (adapted from reference [38]).
The porosity of a growing porous Si layer is proportional to the current density being applied, and it typically ranges between 40 and 80%. Pores form only at the Si/porous Si interface, and once formed, the morphology of the pores does not change significantly for the remainder of the etching process. However, the porosity of a growing layer can be altered by changing the applied current. The film will continue to grow with this new porosity until the current changes. This feature allows the construction of layered nanostructures simply by modulating the applied current during an etch. For example, one-dimensional photonic crystals consisting of a stack of layers with alternating refractive index can be prepared by periodically modulating the current during an etch [40–42].
The ability to easily tune the pore sizes and volumes during the electrochemical etch is a unique property of porous Si [37] that is very useful for drug delivery applications. Other porous materials generally require a more complicated design protocol to control pore size, and even then, the available pore sizes tend to span a limited range. With electrochemically prepared porous Si, control over porosity and pore size is obtained by adjusting the current settings during the etch. Typically, larger current density produces larger pores. Large pores are desirable when incorporating sizable molecules or drugs within the pores. Pore size and porosity is important not only for drug loading; it also determines degradation rates of the porous Si host matrix [43]. Smaller pores provide more surface area and expose more sites for attack of aqueous media. The smaller porous filaments within the film yield greater dissolution rates, providing a convenient means to control degradation rates of the porous Si host.
For in vivo applications, it is often desired to prepare porous Si in the form of particles. The porous layer can be removed from the Si substrate with a procedure commonly referred to as “electropolishing” or “lift-off.” The etching electrolyte is replaced with one containing a lower concentration of HF and a current pulse is applied for several seconds. The lower concentration of HF results in a diffusion limited situation that removes silicon from the crystalline Si/porous Si interface faster than pores can propagate. The result is an under-cutting of the porous layer, releasing it from the Si substrate [37]. The freestanding porous Si film can then be removed with tweezers or a vigorous rinse. The film can then be converted into microparticles by ultrasonic fracture. Conventional lithography [44,45] or microdroplet patterning [46,47] methods can also be used if particles with more uniform shapes are desired.
2.2. Stain etching
Stain etching is an alternative to the electrochemical method for fabrication of porous Si powders. The term stain etching refers to the brownish or reddish color of the film of porous Si that is generated on a crystalline silicon material subjected to the process [48]. In the stain etching procedure, a chemical oxidant (typically nitric acid) replaces the power supply used in the electrochemically driven reaction. HF is a key ingredient, and various other additives are used to control the reaction [49]. Stain etching generally is less reproducible than the electrochemical process, although recent advances have improved the reliability of the process substantially [50]. Furthermore, stain etching cannot be used to prepare stratified structures such as double layers or multilayered photonic crystals. However, porous Si powders prepared by stain etch are now commercially available (http://vestaceramics.net), and a few additional vendors are poised to enter the market. For the biomedically inclined researcher this eliminates the need to set up a complicated and hazardous electrochemical etching system, and it should stimulate the growth of the field.
3. Chemistry of porous Si
3.1. Biocompatibility and reactions of biological relevance
Silicon is an essential trace element that is linked to the health of bone and connective tissues [51]. The chemical species of relevance to the toxicity of porous Si are silane (SiH4) and dissolved oxides of silicon; three important chemical reactions of these species are given in Eq. (1)–(3). The surface of porous Si contains Si–H, SiH2, and SiH3 species that can readily convert to silane [52, 53]. Silane is chemically reactive (Eq. (1)) and toxic, especially upon inhalation [54,55]. Like silane, the native SiHx species on the porous Si surface readily oxidize in aqueous media. Silicon itself is thermodynamically unstable towards oxidation, and even water has sufficient oxidizing potential to make this reaction spontaneous Eq. (2). The passivating action of SiO2 and Si–H (for samples immersed in HF solutions) make the spontaneous aqueous dissolution of Si kinetically slow. Because of its highly porous nano-structure, oxidized porous Si can release relatively large amounts of silicon-containing species into solution in a short time. The soluble forms of SiO2 exist as various silicic acid compounds with the ortho-silicate ion as the basic building block (Eq. (3)), and these oxides can be toxic in high doses [56–58]. Because the body can handle and eliminate silicic acid, the important issue with porous Si-based drug delivery systems is the rate at which they degrade and resorb [12,14,15,59,60]. The work of Bayliss, Canham, and others established the relatively low toxicity of porous Si in various cellular and live animal systems [21, 61–66]. The low toxicity, degradation properties, and solubility of the degradation byproducts of porous Si have generated much interest in its use in controlled drug delivery systems.
There are many conditions that affect the lifetime of biomaterials in vivo. In order to be a successful candidate, porous Si must be able to perform reproducibly and retain the physical and chemical properties needed for the particular application under the harsh biological conditions of the body (salinity, pH, and enzymatic activity). The chemistry of the nanomaterial is therefore of utmost importance.
| (1) |
| (2) |
| (3) |
Surface chemistry plays a large role in controlling the degradation properties of porous Si in vivo. Immediately after Si is electrochemically etched, the surface is covered with reactive hydride species. These chemical functionalities provide a versatile starting point for various reactions that determine the dissolution rates in aqueous media, allow the attachment of homing species, and control the release rates of drugs. The two most important modification reactions are chemical oxidation (Eq. (2)) and grafting of Si–C species.
3.2. Oxidation of porous Si
With its high surface area, porous Si is particularly susceptible to air or water oxidation. Once oxidized, nanophase SiO2 readily dissolves in aqueous media [10], and surfactants or nucleophiles accelerate the process [67,68]. Si–O bonds are easy to prepare on porous Si by oxidation, and a variety of chemical or electrochemical oxidants can be used. Thermal oxidation in air tends to produce a relatively stable oxide [69], in particular if the reaction is performed at >600°C [70]. Ozone oxidation, usually performed at room temperature, forms a more hydrated oxide that dissolves quickly in aqueous media [10]. Milder chemical oxidants, such as dimethyl sulfoxide (DMSO, Eq. (4)) [71] benzoquenone [72], or pyridine [73] can also be used for this reaction. Mild oxidants are sometimes preferred because they can improve the mechanical stability of highly porous Si films, which are typically quite fragile.
The mechanical instability of porous Si is directly related to the strain that is induced in the film as it is produced in the electro-chemical etching process [74], and the volume expansion that accompanies thermal oxidation can also introduce strain. Mild chemical oxidants presumably attack porous Si preferentially at Si–Si bonds that are the most strained, and hence most reactive [16]. As an alternative, nitrate is a stronger oxidant, and nitric acid solutions are used extensively in the preparation of porous Si particles from silicon powders by chemical stain etching [75].
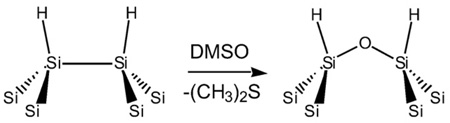 |
(4) |
Slow oxidation of the porous Si surface by dimethyl sulfoxide (DMSO), when coupled with dissolution of the newly formed oxide by HF, is a mild means to enlarge the pores in porous Si films [16]. Aqueous solutions of bases such as KOH can also be used to enlarge the pores after etching [76]. Electrochemical oxidation, in which a porous Si sample is anodized in the presence of a mineral acid such as H2SO4, yields a fairly stable oxide [77]. Oxidation imparts hydrophilicity to the porous structure, enabling the incorporation and adsorption of hydrophilic drugs or biomolecules within the pores. Aqueous oxidation in the presence of various ions including Ca2+ generates a calicified form of porous Si [12,15] that has been shown to be bioactive and is of particular interest for in vivo applications. Calcification can be enhanced by application of a DC electric current [14].
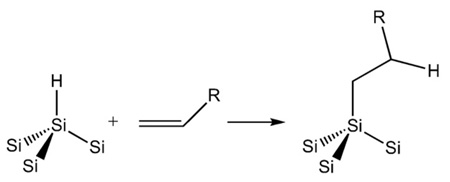
3.3. Hydrosilylation to produce Si–C bonds
Carbon directly bonded to silicon yields a very stable surface species. First recognized by Chidsey and coworkers [78], Si–C bonded species possess greater kinetic stability relative to Si–O due to the low electronegativity of carbon. Silicon can readily form 5- and 6-coordinate intermediates, and an electronegative element such as oxygen enhances the tendency of silicon to be attacked by nucleophiles. Si–C bonds are usually formed on hydride-terminated porous Si surfaces by hydrosilylation (Eq. (5)), a reaction first demonstrated on porous Si by Buriak [79–81] and extensively studied by Boukherroub, Chazalviel, Lockwood, and others.[60,82–88] Hydrosilylation involves reaction of an alkene (usually terminal) or alkyne with a Si–H bond. On porous Si, the reaction can be thermal [85], photochemical [89,90], or Lewis acid catalyzed [80,81].
 |
(5) |
Thermal hydrosilylation provides a means to place a wide variety of organic functional groups on a crystalline Si or porous Si surface [80]. The main requirement of the reaction is that the silicon surface contains Si–H species so they can react with a terminal alkene on the organic fragment. Thus it is important to use freshly etched porous Si and to exclude oxygen and water from the reaction mixture. Conventional Schlenk or vacuum line techniques should be employed [91].
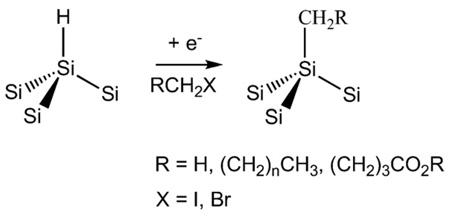
3.4. Chemical or electrochemical grafting of Si–C bonds
As an alternative to hydrosilylation, covalently attached layers can be formed on porous Si surfaces using Grignard and alkyl- or aryllithium reagents [78,92–98]. Electrochemical oxidation of methyl-Grignards [99] on porous Si and electrochemical reduction of phenyldiazonium salts [100] on single crystal Si have been shown to yield dense monolayers of methyl and phenyl groups, respectively. Electrochemical reduction of organohalides (Eq. (6)) has also been demonstrated as an effective grafting technique [101]. The electro-chemical approach allows the attachment of a methyl group to the Si surface, which is not possible with the hydrosilylation route. Because of their ease of application and dramatic improvements in stability, hydrosilylation and electrochemical grafting of alkyl halides are useful reactions for the preparation of biointerfaces.
 |
(6) |
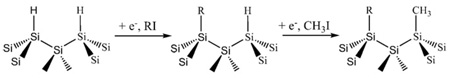
It is important to note that porous Si modification reactions do not provide 100% surface coverage; they merely decorate the surface with the functional group. Thus infrared spectra show a large amount of surface Si–H groups remaining after hydrosilylation or electrochemical grafting reaction. The electrochemical method allows one to minimize residual Si–H species by “endcapping” the surface with small methyl groups following modification with a functional species (Eq. (7)) [102]. The endcapping reaction can also be performed on a hydrosilylated porous Si surface. The doubly modified (methyl endcapped) surfaces exhibit the greatest stability in aqueous media [102]. It is still an open question as to why the modification reactions impart such stability to the material; for example Buriak's hydrosilylation reaction makes porous Si stable to hydroxide solutions of pH>10 [81]. Unmodified (exclusively H-terminated) porous Si dissolves rapidly under such conditions. The stability probably derives from a combination of two factors: the Si–C bond is kinetically inert due to the low electronegativity of carbon and the attached organic species (typically a hydrocarbon chain 8 or more carbons long) is sufficiently hydrophobic that aqueous nucleophiles are excluded from the vicinity of their Si atom target.
 |
(7) |
Reaction of porous Si with gas phase acetylene generates highly carbonized porous Si that is possibly the most stable form of Si–C modified porous Si [103–105]. This material is referred to as thermally carbonized porous Si, or TCPSi [30]. It has been extensively investigated by Salonen and coworkers, with many publications of relevance to drug loading and delivery [20,30,31,106–109].
3.5. Conjugation of biomolecules to modified porous Si
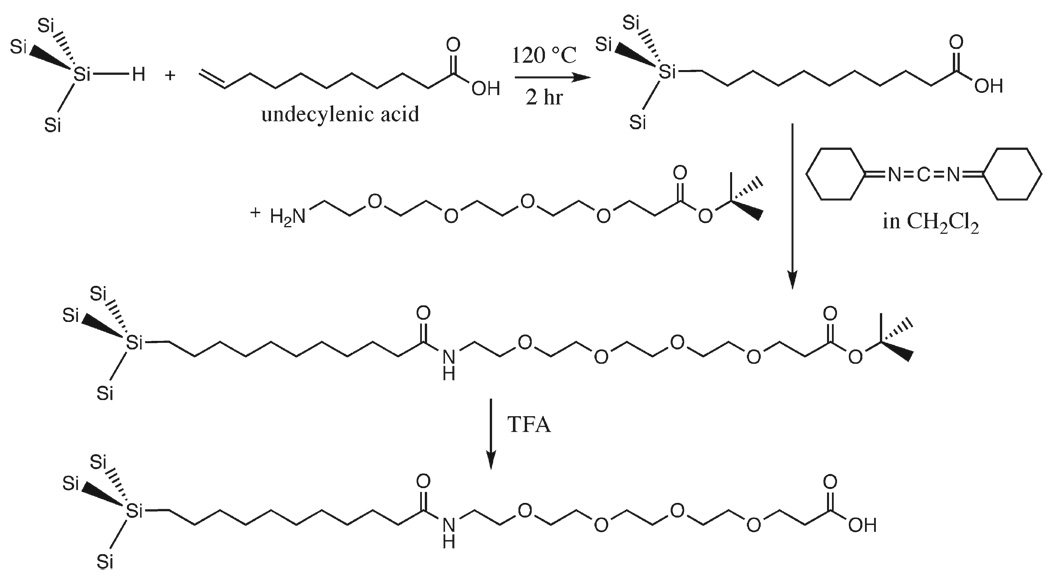
Carbon grafting stabilizes porous Si against dissolution in aqueous media, but the surface must still avoid the non-specific binding of proteins and other species that can lead to opsonization or encapsulation. Reactions that place a polyethylene glycol (PEG) linker on a porous Si surface have been employed to this end (Fig. 3) [110, 111]. A short-chain PEG linker yields a hydrophilic surface that is capable of passing biomolecules into or out of the pores without binding them strongly [112]. The distal end of the PEG linker can be modified to allow coupling of other species, such as drugs, cleavable linkers, or targeting moieties, to the material [110,112].
Fig. 3.
Adding a linker to porous Si via hydrosilylation. The short-chain PEG linker yields a hydrophilic surface that minimizes non-specific binding effects (adapted from reference [110]).
The oxides of porous Si are easy to functionalize using conventional silanol chemistries [76, 110, 113]. When small pores are present (as with p-type samples), monoalkoxydimethylsilanes (RO–Si(Me)2–R′) can be more effective than trialkoxysilanes ((RO)3Si–R′) as surface linkers [113]. This is because trialkoxysilanes oligomerize and clog smaller pore openings, especially when the reagent is used at higher concentrations.
Whereas Si–C chemistries are robust and versatile, chemistries involving Si–O bonds represent an attractive alternative two reasons. First, the timescale in which highly porous SiO2 is stable in aqueous media is consistent with many short-term drug delivery applications— typically 20 min to a few hours. Second, a porous SiO2 sample that contains no additional stabilizing chemistries is less likely to produce toxic or antigenic side effects. If it is desired that the porous Si material be stable in vivo for long periods (for example, an extended release formulation or an in vivo biosensor), Si–C chemistries such as hydrosilylation with endcapping [102] or thermal carbonization with acetylene [30] is preferred. If a longer-lived oxide matrix is desired, silicon oxides formed at higher temperatures (>700 °C) are significantly more stable in aqueous media than those formed at lower temperatures or by ozone oxidation [114].
4. Loading and controlled release of drugs with porous Si
Providing a controlled and localized release of therapeutics within the body are key objectives for increasing efficacy and reducing the risks of potential side effects [115–119]. The low toxicity of porous Si and porous SiO2, the high porosity, and the relatively convenient surface chemistry has spurred interest in the use of this system as a host, or “mother ship” for therapeutics, diagnostics, or other types of payloads. Various approaches to load a molecular payload into a porous Si host have been explored, and they can be grouped into the following general categories: covalent attachment, physical trapping, and adsorption.
4.1. Incorporating a payload within the porous nanostructure by covalent attachment
Covalent attachment provides a convenient means to link a biomolecular capture probe to the inner pore walls of porous Si for biosensor applications [85,110], and this approach can also be used to attach drug molecules. As was pointed out in the previous section, linking a biomolecule via Si–C bonds tends to be a more stable route than using Si–O bonds due to the susceptibility of the Si–O species to nucleophilic attack.
The versatility of the hydrosilylation reaction for preparing functional porous Si surfaces was recognized early in the history of porous Si surface chemistry [80]. One of the more common approaches is to graft an organic molecule that contains a carboxyl species on the distal end of a terminal alkene as was presented above in Fig. 3 [85]. The alkene end participates in the hydrosilylation reaction, bonding to the Si surface and leaving the carboxy-terminus free for further chemical modification. A favorite linker molecule is undecylenic acid, which provides a hydrophobic 10 carbon aliphatic chain to insulate the linker from the porous Si surface [85,120]. The drug payload can be attached directly to the carboxy group of the alkene, or it can be further separated from the surface with a PEG linker as shown in Fig. 3 [110]. Due to the stability of the Si–C bond, hydrosilylation is one of the most robust means of attaching a payload to porous Si. The payload is only released when the covalent bonds are broken [112] or the supporting porous Si matrix is degraded. For drug delivery this introduces a complication in that the drug may not release from the linker, resulting in a modified version of the drug being introduced into the body. In addition, a drug may be susceptible to attack by silane generated during the degradation of the porous Si scaffolding [52] or by residual reactive species on the porous Si material itself [31]. Any studies involving porous Si (or with nanomaterials in general) need to incorporate activity assays to ensure that the released drug has not become inactivated.
4.2. Trapping a payload by oxidation
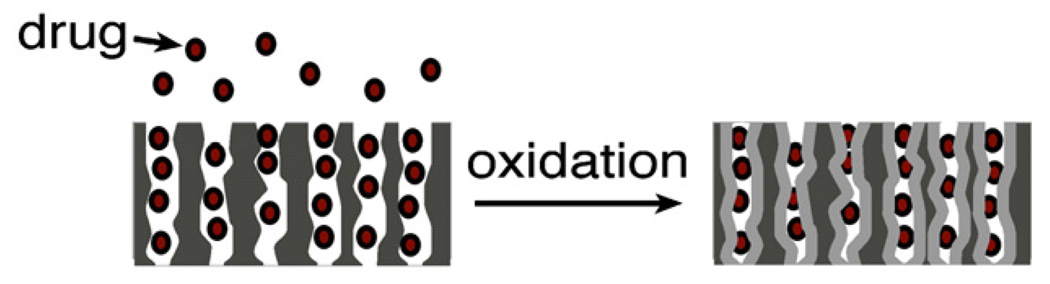
If the species to be trapped is relatively robust, it can be locked into place by oxidation of the porous Si host matrix. The locking procedure takes advantage of the fact that when porous Si is oxidized to SiO2 there is a volume expansion to accommodate the extra oxygen atoms, Fig. 4. This volume expansion serves to shrink the pores, trapping anything that happens to be in them at the time. Iron oxide (Fe3O4) nanoparticles have been loaded and locked into the porous nanostructure in this fashion, using aqueous ammonia to induce oxidation [121]. The high pH and nucleophilic nature of ammonia enhance oxidation of freshly etched porous Si in aqueous solutions [122]. Similar oxidation can be induced by vapor phase pyridine [123]. Nucleophilic groups present on drug payloads may also participate in this reaction [20], as can oxidizing species such as quinones [72].
Fig. 4.
Building a bottle around a ship. A molecular or nanoparticle payload can be trapped by partial oxidation of the porous Si host layer. Oxidation produces a volume expansion (Si to SiO2) that shrinks the pores, locking the payload in place. After [124]
The silicic acid generated during dissolution Eq. (3) can participate in sol–gel type reactions—essentially reprecipitation of the silicic acid, but in the form of various inorganic silicates. Common ions such as Ca2+ and Mg2+ in solution can participate in silicate precipitation reactions Eq. (8), and these types of precipitates are known to be bioactive [125–127].
| (8) |
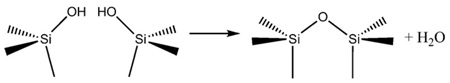
Once formed, mild thermal treatments can be used to dehydrate the oxide or silicate matrix. Heating tends to densify and rigidify the structure by forming strong Si–O–Si linkages (Eq. (9)).
 |
(9) |
4.3. Concentrating a payload by spontaneous adsorption
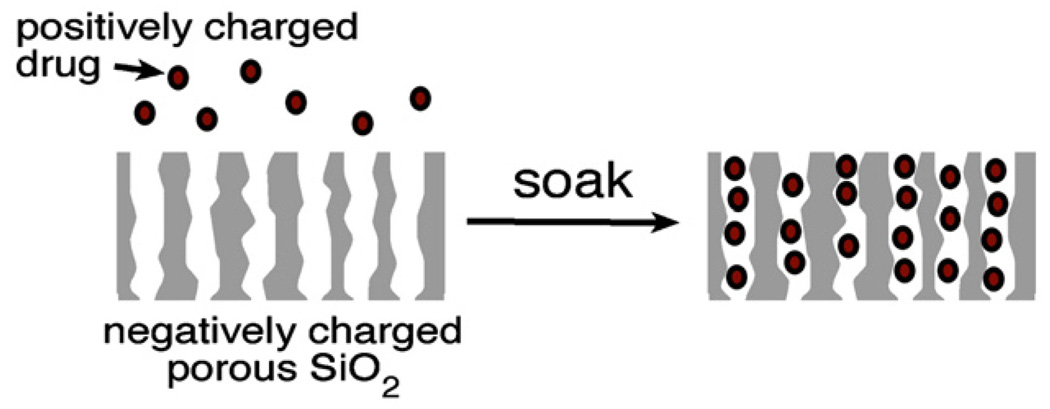
As-formed porous Si has a hydride-terminated surface that is very hydrophobic. Oxidized porous Si is hydrophilic, and chemically modified porous Si surfaces can be hydrophobic, hydrophilic, or both (amphiphilic), depending on the specific functional group(s) attached. The nature of the surface plays a critical role in determining the amount of drug that can be loaded and the rate at which it is released. Silicon oxide surfaces tend to present a negative surface charge to an aqueous solution due to the low pKa of SiO2 [128]. Often referred to as “electrostatic adsorption,” attractive coulombic forces from this negative surface provide a means to extract positively charged ions from solution and concentrate them at the interface. Whereas covalent attachment and oxidative trapping approaches described above tend to trap their payloads fairly irreversibly, electrostatic adsorption represents essentially an ion exchange mechanism that holds molecules more weakly. Electrostatics is a useful means to effect more rapid drug delivery, as opposed to covalent or physical trapping approaches that release drug over a period of days, weeks, or months [43,60].
The affinity of a porous Si particle for a particular molecule can be controlled with surface chemistry. The surface of oxidized porous Si has a point of zero charge at a pH of around 2 [128, 129], and so it presents a negatively charged surface to most aqueous solutions of interest, as depicted in Fig. 5. At the appropriate pH, porous SiO2 spontaneously adsorbs positively charged proteins such as serum albumin [130, 131], fibrinogen [132], protein A [70, 114, 133], immunoglobulin G (IgG) [134], or horseradish peroxidase [25], concentrating them in the process. For example, a 0.125 mg/mL solution of the monoclonal antibody bevacizumab (trade name Avastin, an anti-cancer drug) spontaneously concentrates in suitably prepared porous SiO2 by a factor of >100 (unpublished results).
Fig. 5.
Trapping of a positively charged drug payload in a porous SiO2 layer by ionic adsorption. Porous SiO2 (oxidized porous Si) has a negative surface charge; molecules with positive charges will spontaneously adsorb to the inner pore walls and surface. This method is commonly used to load proteins [70,114,130,132–134]
Porous Si can also be made hydrophobic, and hydrophobic molecules such as the steroid dexamethasone or serum albumin can be loaded into these nanostructures [16, 135]. Hydrophilic molecules can also be loaded into such materials with the aid of the appropriate surfactant [21]. The native hydride surface of porous Si is hydrophobic. Though it is not particularly stable in aqueous media, it has been used for short-term loading and release studies [21, 31]. Because water is excluded from these hydrophobic surfaces, aqueous degradation and leaching reactions tend to be slow. The grafting of alkanes to the surface by hydrosilylation is commonly used to prepare materials that are stable in biological media; this stability derives in large part from the ability of the hydrophobic moieties to locally exclude water or dissolved nucleophiles, as was discussed above in the chemistry section [43,60].
5. Composites of porous Si and polymers
Hybrid materials, in which the payload consists of an organic polymer or a biopolymer, forms an additional class of host/payload systems. Composites are attractive candidates for drug delivery devices because they can display a combination of advantageous chemical and physical characteristics not exhibited by the individual constituents. Advances in polymer [136] and materials [137] chemistries have greatly expanded the design options for nanomaterial composites in the past few years, and synthesis of materials using nanostructured templates has emerged as a versatile technique to generate ordered nanostructures [138]. Templates consisting of microor mesoporous membranes [139,140], zeolites [141], and crystalline colloidal arrays [142–144] have been used, and many elaborate electronic, mechanical, or optical structures have resulted.
5.1. Porous Si as a template
Porous Si is an attractive candidate for use as a template because of the tunability of the porosity and average pore size. Additionally, elaborate 1, 2, and 2.5-dimensional photonic crystals are readily prepared in porous Si [145]. Porous Si composites show great promise for improving the mechanical stability and control over release rates of a delivery system. Polymers that have been incorporated into porous Si include polylactide [146], polydimethylsiloxane [147], polyethylene [146], polystyrene [146], polycaprolactone [148], zein (a biopolymer derived from maize) [149], and poly(N-isopropylacrylamide) [150]. Either the composite itself or a nanostructure derived from the composite by removal of the porous Si template can be used (Fig. 6) [146]. Porous Si combined with a biocompatible polymer has been shown to yield improved control over drug release kinetics and improved stability in aqueous media [146], and the use of biopolymers that are selectively cleaved by specific proteases [112,149] provides the possibility of tissue-specific action.
Fig. 6.
Fabrication of a nanostructured composite from a porous Si template. A variety of solution- or melt-processible organic and biopolymers can be solution-cast or injection-molded into a porous Si or porous SiO2 host. The composite can be used as-formed, or the template can be removed by chemical dissolution. If the template is removed, the polymer castings often replicate the nanostructure of the master. Use of these castings as vapor sensors, deformable and tunable optical filters, and as self-reporting, bioresorbable materials has been demonstrated [146].
Removal of the porous Si or porous SiO2 template from a polymer or biopolymer imprint can sometimes be achieved by chemical dissolution using aqueous KOH or HF, respectively, providing a freestanding porous polymer film with the optical characteristics of the master. Whether or not the process replicates the nanostructure of the master is highly dependant on the processing conditions and the type of polymer used. Also, the ability of the polymer to release from the master is highly dependant on the interfacal chemistry and tortuosity of the pore network.
The concept of placing a polymeric material within a porous Si matrix was first demonstrated in the early 1990s [151]. Two synthetic approaches have emerged: either the polymer is synthesized within the porous matrix [151–154], or a pre-formed polymer is infused into the matrix by melt- or solution-casting [46,47,146,147,155]. For drug delivery applications, it is important to use a biocompatible polymer, and hydrogels are of particular interest [29,150]. Hydrogels are commonly used in ophthalmologic devices, biosensors, biomembranes, and controlled drug delivery [156].Water-swollen, crosslinked polymeric networks can undergo volume phase transitions in response to environmental changes such as pH [157,158], ionic strength [159], temperature [160], or electric fields [161]. Materials responsive to pH changes have been investigated extensively because they are applicable to many drug delivery and biosensing schemes.
5.2. Locking the polymer in a porous Si template
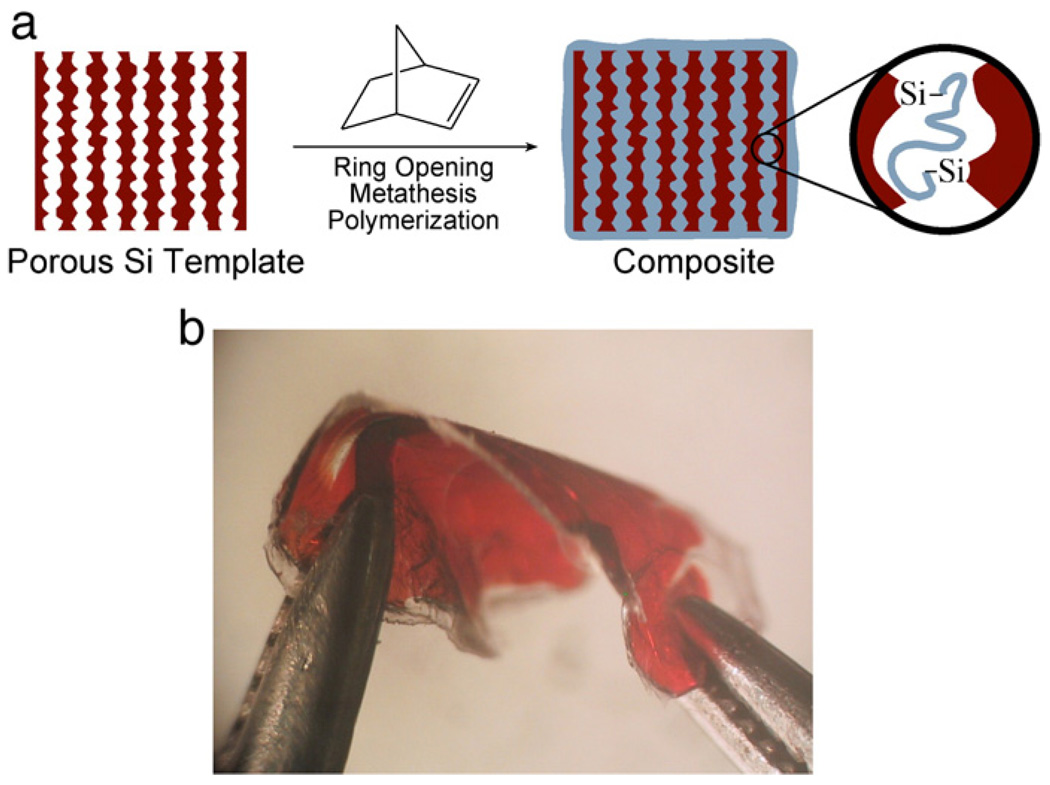
Since porous Si consists of a delicate matrix of nanocrystalline domains, its mechanical stability is an issue, especially for applications in which the material is thermally cycled or subjected to mechanical stresses. Chemical crosslinking of a porous Si template can be achieved with the correct choice of polymerization conditions. First demonstrated in 2003 [152], such methods take advantage of the reactivity of the hydride-terminated porous Si surface. For example, porous Si treated with a ruthenium ring-opening metathesis polymerization catalyst followed by norbornene produces a flexible, stable composite in which poly(norbornene) is covalently attached to the porous Si matrix (Fig. 7). The method follows the procedure of Lewis and Grubbs to graft polymers onto crystalline Si surfaces using a ring-opening metathesis polymerization catalyst [162,163].
Fig. 7.
In-situ polymerization and crosslinking of a porous Si template. The catalyzed reaction generates a composite porous Si/polymer matrix in which the polymer is covalently attached to porous Si via Si–C bonds. The chemical and mechanical stability of the chemically crosslinked porous Si matrix is significantly improved relative to the porous Si film alone. After [152].
The ring-opening metathesis polymerization (ROMP) reaction acts on rings containing double bonded carbon, and the C=C double bonds are conserved through the process [162]. A significant amount of this unsaturated polymer becomes covalently attached by hydrosilylation with the Si–H species on the porous Si surface. If oxidized porous Si is used in the reaction, the lack of Si–H surface groups eliminates the possibility of covalent Si–C bond formation between the polymer and the template. Composites with lower mechanical and chemical stability result [152]. Thus both the weaving of a soft polymer network into the pores and the covalent attachment of this polymer to the matrix combine to provide the robust chemical and mechanical properties. Hydrosilylation can also be induced via a radical mechanism, and the radical initiators used to set a hydrogel polymer probably induce similar covalent attachment reactions between these polymers and their porous Si hosts [150].
6. In vivo monitoring using the optical properties of porous Si
Many material hosts have been developed for drug delivery, but few can ‘self-report’ on the amount of drug loaded or released. It is important to know these quantities when determining the efficacy of a treatment to identify when it is time to administer a new dose. The unique optical properties that can be engineered into porous Si provide a mechanism to perform such assays in vivo. Incorporation of molecules into a porous Si layer alters its index of refraction, and the spectrum obtained from a thin film or multilayer structure provides a measure of loading in the nanostructure. Primarily exploited for molecular binding assays [7,9,10,22,70,149,164–173], the optical spectrum can be monitored in vivo, allowing it to indicate the quantity of drug that has diffused out of the film [16] or the degree of degradation of the porous matrix [10]. The optical spectrum also provides a convenient means to characterize and quantify various drug loading or release concepts ex vivo. Detailed descriptions of the optical interference spectra and their interpretation appear elsewhere [6]; a brief summary will be presented here.
6.1. Principles of optical detection in porous Si films
The reflectivity spectrum of a thin film (Fig. 8) displays Fabry–Pérot interference frin1ges that correspond to constructive and destructive interference from light reflected at the different boundaries in the layered structure. The thickness of the layer and the refractive index (RI) contrast at each interface (air/porous Si and porous Si/crystalline Si) can be extracted from the fast Fourier transform (FFT) of this spectrum. Theoretically, a layered structure will yield a single peak in the FFT spectrum. The amplitude of the peak is related to the index contrast at each of the interfaces, and the position of a peak yields the product of optical thickness, or nL (where n is refractive index and L is thickness) [113]. Since the refractive index can be related to the mass loading of a layer by application of the Bruggeman effective medium model [174], this method can be used to monitor molecular in- or exfiltration. We refer to this method as RIFTS, for Reflective Interferometric Fourier Transform Spectroscopy [70,114,175].
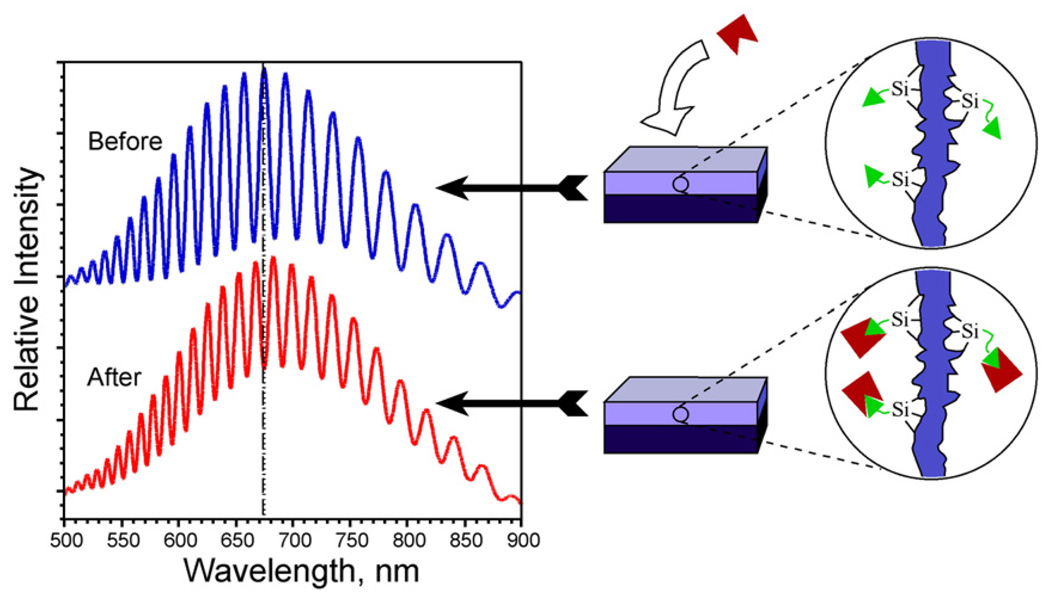
Fig. 8.
Schematic demonstrating the change in a reflectance spectrum from a single layer of porous Si upon introduction of a molecular species into the porous matrix. The change in refractive index of the composite film results in a red shift of the Fabry–Pérot interference fringes. The reverse process can also be monitored, yielding a blue shift in the spectrum.
More complicated structures, such as rugate filters, Bragg stacks, and microcavities can be prepared, but the basic principle of detection remains the same: a shift in the characteristic optical reflectivity spectrum corresponds to a change in refractive index of the porous layer, which is related to its composition. Shifts in the spectrum occur when molecules are transported into or out of the layer. A shift can also occur when the porous Si film oxidizes. Porous Si has a refractive index of approximately 2.1, and the index of oxidized porous Si ranges from this value down to a value of ~ 1.6, depending on the degree of oxidation. In an aqueous system, molecules removed from the pores are replaced by water. Since water has an index of 1.33 and most biomolecules have an index of 1.5–1.7, release of molecules from the pores results in a blue shift in the spectrum. For the same reason, dissolution of the porous Si matrix also results in a blue shift of the spectrum. The sensitivities of the various optical structures (Fabry–Pérot, rugate, Bragg stack, microcavity), in terms of spectral shift vs analyte concentration in the pores, are similar [176].
6.2. Monitoring a porous Si fixture in vivo
The optical interference spectrum used to assess loading can be measured with inexpensive and portable instrumentation such as a CCD spectrometer or a diode laser interferometer [177,178]. This is useful, for example, in monitoring porous Si particles in the vitreous of rabbit eyes for ocular drug delivery (Fig. 9) [146]. Removal of drug or dissolution of the particle results in a change in the refractive index of the porous film that is observed as a wavelength shift in the reflection spectrum. In clinical situations, we have shown that this color change can be qualitatively followed with the use of a fundus camera or quantitated by spectroscopy in the living eye. The presence of DNA [9,179], human IgG [10], bovine serum albumin [130], dexamethasone [16], caffeine [146], and many other molecules has been detected (in ex vivo systems) using this methodology. The high surface area and optical interferometric means of detection lead to sensitivities comparable to surface plasmon resonance (SPR) for many of these systems [173,176].
Fig. 9.
Photograph of porous Si microparticles in a live rabbit eye. The particles were prepared as multilayered photonic crystals (rugate filters), and they appear as brightly colored flecks that can be seen floating the vitreous. The color of the microparticles shifts to the blue as the particles degrade in vivo, providing a predictive metric to the clinician.
The optical spectrum of a porous Si photonic crystal can be read through human tissue (up to 1 mm in thickness), demonstrating the feasibility of the in vivo self-reporting system [146]. The optical method is particularly useful for monitoring of intraocular drug release either in the form of porous Si particles or composite films (Fig.10). The porous Si microparticles displayed in Fig. 9 demonstrate their potential as self-reporting drug delivery devices for treatment of diseases such as age-related macular degeneration, where there is an important need for long acting intraocular drug delivery systems [180].
Fig. 10.
Light microscope image of porous Si microparticles. These particles were prepared as multilayered photonic crystals (rugate filters) and display various spectral colors depending on the periodicity of their layered nanostructure. Nominal particle size is 50 µm.
7. Medical applications of porous Si
The suitability and efficacy of various forms of porous Si are being assessed for medical applications, and some are currently in clinical trials. The incorporation of anti-cancer therapeutics [19,181], anti-inflammatory agents [16,31], analgesics [31], and medicinally relevant proteins and peptides has been demonstrated [21]. The oral administration of porous Si to provide a dietary supplement of silicon [182] has also been assessed [183]. Porous Si drug delivery devices have taken the form of particles [25,184,185], films [16], chip implants [186,187], composite materials [146,150], and microneedles [188, 189]. Much work has focused on microparticle systems [21,25,30,31,106–108,190–193] due to their relative ease of fabrication, administration, and their compatibility with existing drug delivery concepts.
7.1. Particle formulations
Particles have the potential for percutaneous or intravenous administration depending on their size. Foraker et al. have demonstrated delivery of insulin across Caco-2 cell monolayers [21], while Salonen and coworkers have investigated the drug release kinetics from porous Si microparticles for applications in oral delivery [31]. The latter workers have investigated the interactions between various drugs with the different porous Si surface chemistries in detail [20]. The incorporation of superparamagnetic iron oxide nanoparticles, of interest for Magnetic Resonance Imaging (MRI) has been demonstrated [25,121], and remote RF heating (by Néel relaxation) of such structures has been reported [185]. The amount of drug that can be loaded into a porous Si microparticle is large due to its relatively large free volume. For example, a single cubic particle 10 µm on an edge and with a porosity of 80% yields a maximumfree volume of 0.8 pL [25]. Because they can concentrate molecules within their nanostructure and protect them from the body's natural immunological responses, the particles can potentially carry a much higher dose than could be allowed with a free drug injection. The particles can also be manufactured with well-defined shapes and dimensions, allowing the reproducible loading and release of precise quantities [44–46].
7.2. Cancer treatment
Some of the most advanced clinical studies have been performed by pSiMedica, inc. using porous Si as a brachytherapy device for the treatment of cancer [11,186,187]. In this work, percutaneous implants of porous Si particles (on the order of 20 µm in size) containing radioactive 32P provide local radiation to the tumor. The radioactive isotope is synthesized in the porous Si material by elemental transmutation of Si, induced by exposure to high energy neutrons emanating from a nuclear reactor. After delivery of the radiation dose, the device resorbs into the body as the Si implant hydrolyzes to silicic acid (Eqs. (2) and (3)), thereby requiring no further surgery to recover the device. Although silicic acid can be cytotoxic [56–58,64,65], apparently the resorption rate is slow enough under physiologic conditions that the concentration of free silicic acid does not reach toxic levels.
Anti-cancer therapeutics have been successfully incorporated into porous Si. Delivery of cis-platin, doped into calcium phosphate/porous Si films has been demonstrated in simulated body fluid [194], and doxorubicin loaded porous Si films have shown cytotoxic effects towards human colon adenocarcinoma cell lines [19]. In addition, photoexcitation of quantum confined silicon nanostructures in aqueous aerated media has been shown to produce singlet oxygen [32–35]. The ability of the porous Si nanostructure to degrade into relatively harmless silicic acid byproducts makes porous Si an attractive alternative to molecular (typically porphyrin-based [195]) sensitizers for photodynamic therapy [196,197].
8. Summary and prospects
Porous Si microparticles offer a number of properties of interest for controlled drug delivery: First, nanostructured materials based on silicon are promising platforms for pharmaceutical applications because they provide low toxicity. Their ability to degrade in the body presents fewer challenges for chronic use than, for example, carbon nanotubes which are not metabolized and so must be excreted after administration.
Second, the electrochemical means of fabrication allows one to “dial in” the properties of surface area, free volume, and pore size. Pores can be generated anywhere from a few nanometers to several hundreds of nanometers in diameter.
Third, the surface of freshly prepared porous Si is easily modified via convenient chemistry with a large range of organic or biological molecules (drugs, peptides, antibodies, proteins, etc.), allowing flexibility in the engineering of release profiles.
Fourth, the optical properties of porous Si provide a useful dimension for in vivo sensing or therapeutics. Porous Si can display fluorescence deriving from Si quantum dot structures that are produced during the etch [1], and it can be prepared with unique optical reflectivity spectra. These features allow porous Si to exhibit a signal that is affected in a predictable way when exposed to environmental changes, presenting possibilities for the development of advanced functional systems that incorporate sensors for diagnostic or therapeutic functions.
Finally, the ease with which porous Si can be integrated into well-established Si microelectronics fabrication techniques could also lead to more sophisticated, active devices for medical applications [65,89,198]. The possibility of placing active electronic circuit components into the silicon-based particles is another feature of silicon that is yet to be exploited for in vivo use.
Acknowledgements
The authors thank Dr. Sadik Esener, Dr. Stephen Howell, Dr. Danielle Jandial, Dr. Jean-Marie Devoioselle, Dr. Frederique Cunin, Jennifer Park, and Elizabeth Wu for helpful discussions. Financial support from the National Science Foundation, grant # DMR-0503006 (MJS), NIH grant EYO-7366 (WRF) and Research to Prevent Blindness, Inc. (WRF) is gratefully acknowledged. MJS is a member of the Moores UCSD Cancer Center and the UCSD NanoTUMOR Center under which this research was conducted and partially supported by NIH grant U54 CA 119335. EJA thanks the American Heart Association for a graduate fellowship.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Inorganic Nanoparticles in Drug Delivery”.
References
- 1.Collins RT, Fauchet PM, Tischler MA. Porous silicon: from luminescence to LEDs. Phys. Today. 1997;50:24–31. [Google Scholar]
- 2.Lazarouk S, Jaguiro P, Katsouba S, et al. Stable electroluminescence from reverse biased n-type porous silicon-aluminum Schottky junction device. Appl. Phys. Lett. 1996;68:2108–2110. [Google Scholar]
- 3.Steiner P, Kozlowski F, Lang W. Light-emitting porous silicon diode with an increased electroluminescence quantum efficiency. Appl. Phys. Lett. 1993;62:2700–2702. [Google Scholar]
- 4.Richter A, Steiner P, Kozlowski F, Lang W. Current-induced light emission from a porous Si device. IEEE Elec. Dev. Lett. 1991;12:691–692. [Google Scholar]
- 5.Foucaran A, Pascal-Delannoy F, Giani A, Sackda A, Combette P, Boyer A. Porous silicon layers used for gas sensor applications. Thin Solid Films. 1997;297:317–320. [Google Scholar]
- 6.Sailor MJ, Link JR. Smart dust: nanostructured devices in a grain of sand. Chem. Commun. 2005:1375–1383. doi: 10.1039/b417554a. [DOI] [PubMed] [Google Scholar]
- 7.Chan S, Fauchet PM, Li Y, Rothberg LJ, Miller BL. Porous silicon microcavities for biosensing applications. Phys. Status Solidi A—Appl. Res. 2000;182:541–546. [Google Scholar]
- 8.Thust M, Schoning MJ, Frohnhoff S, Arens-Fischer R. Porous silicon as a substrate material for potentiometric biosensors. Meas. Sci. Technol. 1996;7:26–29. [Google Scholar]
- 9.Lin VSY, Motesharei K, Dancil KS, Sailor MJ, Ghadiri MR. A porous silicon-based optical interferometric biosensor. Science. 1997;278:840–843. doi: 10.1126/science.278.5339.840. [DOI] [PubMed] [Google Scholar]
- 10.Dancil KPS, Greiner DP, Sailor MJ. A porous silicon optical biosensor: detection of reversible binding of IgG to a protein A-modified surface. J. Am. Chem. Soc. 1999;121:7925–7930. [Google Scholar]
- 11.Coffer JL, Whitehead MA, Nagesha DK, et al. Porous silicon-based scaffolds for tissue engineering and other biomedical applications. Phys. Status Solidi A—Appl. Mat. 2005;202:1451–1455. [Google Scholar]
- 12.Canham LT, Reeves CL, Loni A, et al. Calcium phosphate nucleation on porous silicon: factors influencing kinetics in acellular simulated body fluids. Thin Sol. Films. 1997;297:304–307. [Google Scholar]
- 13.Canham LT, Reeves CL, King DO, Branfield PJ, Crabb JG, Ward MCL. Bioactive polycrystalline silicon. Adv. Mater. 1996;8:850–852. [Google Scholar]
- 14.Canham LT, Newey JP, Reeves CL, et al. The effects of DC electric currents on the in-vitro calcification of bioactive Si wafers. Adv. Mater. 1996;8:847–849. [Google Scholar]
- 15.Canham LT. Bioactive silicon structure fabrication through nanoetching techniques. Adv. Mater. 1995;7:1033–1037. [Google Scholar]
- 16.Anglin EJ, Schwartz MP, Ng VP, Perelman LA, Sailor MJ. Engineering the chemistry and nanostructure of porous silicon Fabry-Pérot films for loading and release of a steroid. Langmuir. 2004;20:11264–11269. doi: 10.1021/la048105t. [DOI] [PubMed] [Google Scholar]
- 17.Charnay C, Begu S, Tourne-Peteilh C, Nicole L, Lerner DA, Devoisselle JM. Inclusion of ibuprofen in mesoporous templated silica: drug loading and release property. Eur. J. Pharm. Biopharm. 2004;57:533–540. doi: 10.1016/j.ejpb.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Coffer JL, Montchamp JL, Aimone JB, Weis RP. Routes to calcifled porous silicon: implications for drug delivery and biosensing. Phys. Status Solidi A—Appl. Res. 2003;197:336–339. [Google Scholar]
- 19.Vaccari L, Canton D, Zaffaroni N, Villa R, Tormen M, di Fabrizio E. Porous silicon as drug carrier for controlled delivery of doxorubicin anticancer agent. Microelectron. Eng. 2006;83:1598–1601. [Google Scholar]
- 20.Salonen J, Kaukonen AM, Hirvonen J, Lehto VP. Mesoporous silicon in drug delivery applications. J. Pharm. Sci. 2008;97:632–653. doi: 10.1002/jps.20999. [DOI] [PubMed] [Google Scholar]
- 21.Foraker AB, Walczak RJ, Cohen MH, Boiarski TA, Grove CF, Swaan PW. Microfabricated porous silicon particles enhance paracellular delivery of insulin across intestinal Caco-2 cell monolayers. Pharm. Res. 2003;20:110–116. doi: 10.1023/a:1022211127890. [DOI] [PubMed] [Google Scholar]
- 22.DeLouise LA, Miller BL. Enzyme immobilization in porous silicon: quantitative analysis of the kinetic parameters for glutathione-S-transferases. Anal. Chem. 2005;77:1950–1956. doi: 10.1021/ac0486185. [DOI] [PubMed] [Google Scholar]
- 23.Létant SE, Hart BR, Kane SR, Hadi MZ, Shields SJ, Reynolds JG. Enzyme immobilization on porous silicon surfaces. Adv. Mater. 2004;16:689–693. [Google Scholar]
- 24.Létant SE, Kane SR, Hart BR, et al. Hydrolysis of acetylcholinesterase inhibitors - organophosphorus acid anhydrolase enzyme immobilization on photoluminescent porous silicon platforms. Chem. Commun. 2005:851–853. doi: 10.1039/b412215a. [DOI] [PubMed] [Google Scholar]
- 25.Thomas JC, Pacholski C, Sailor MJ. Delivery of nanogram payloads using magnetic porous silicon microcarriers. Lab Chip. 2006;6:782–787. doi: 10.1039/b516121e. [DOI] [PubMed] [Google Scholar]
- 26.DeLouise LA, Kou PM, Miller BL. Cross-correlation of optical microcavity biosensor response with immobilized enzyme activity, Insights into Biosensor Sensitivity. Anal. Chem. 2005;77:3222–3230. doi: 10.1021/ac048144+. [DOI] [PubMed] [Google Scholar]
- 27.DeLouise LA, Miller BL. Quantatitive assessment of enzyme immobilization capacity in porous silicon. Anal. Chem. 2004;76:6915–6920. doi: 10.1021/ac0488208. [DOI] [PubMed] [Google Scholar]
- 28.Maia MDD, de Vasconcelos EA, Maia P, et al. Immobilization of urease on vapour phase stain etched porous silicon. Process Biochem. 2007;42:429–433. [Google Scholar]
- 29.Collins A, Mikeladze E, Bengtsson M, Kokaia M, Laurell T, Csoregi E. Interference elimination in glutamate monitoring with chip integrated enzyme microreactors. Electroanalysis. 2001;13:425–431. [Google Scholar]
- 30.Kaukonen AM, Laitinen L, Salonen J, et al. Enhanced in vitro permeation of furosemide loaded into thermally carbonized mesoporous silicon (TCPSi) microparticles. Eur. J. Pharm. Biopharm. 2007;66:348–356. doi: 10.1016/j.ejpb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Salonen J, Laitinen L, Kaukonen AM, et al. Mesoporous silicon microparticles for oral drug delivery: loading and release of five model drugs. J. Control. Release. 2005;108:362–374. doi: 10.1016/j.jconrel.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Lee C, Kim H, Cho YJ, Lee WI. The properties of porous silicon as a therapeutic agent via the new photodynamic therapy. J. Mater. Chem. 2007;17:2648–2653. [Google Scholar]
- 33.Parkhutik V, Chirvony V, Matveyeva E. Optical properties of porphyrin molecules immobilized in nano-porous silicon. Biomol. Eng. 2007;24:71–73. doi: 10.1016/j.bioeng.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt R. Photosensitized generation of singlet oxygen. Photochem. Photobiol. 2006;82:1161–1177. doi: 10.1562/2006-03-03-IR-833. [DOI] [PubMed] [Google Scholar]
- 35.Chirvony V, Bolotin V, Matveeva E, Parkhutik V. Fluorescence and O–1(2) generation properties of porphyrin molecules immobilized in oxidized nano-porous silicon matrix. J. Photochem. Photobiol. A—Chem. 2006;181:106–113. [Google Scholar]
- 36.Canham L. Properties of porous silicon. In: Weiss BL, editor. EMIS Datareviews. vol. 18. London: Institution of Engineering and Technology; 1997. pp. 1–405. [Google Scholar]
- 37.Zhang XG. Morphology and formation mechanisms of porous silicon. J. Electrochem. Soc. 2004;151:C69–C80. [Google Scholar]
- 38.Lehmann V, Gosele U. Porous silicon formation: a quantum wire effect. Appl. Phys. Lett. 1991;58:856–858. [Google Scholar]
- 39.Lehmann V, Gosele U. Porous Si: quantum sponge structures grown via a self-adjusting etching process. Adv. Mater. 1992;4:114–116. [Google Scholar]
- 40.Vincent G. Optical properties of porous silicon superlattices. Appl. Phys. Lett. 1994;64:2367–2369. [Google Scholar]
- 41.Mazzoleni C, Pavesi L. Application to optical components of dielectric porous silicon multilayers. Appl. Phys. Lett. 1995;67:2983–2985. [Google Scholar]
- 42.Berger MG, Arens-Fischer R, Thoenissen M, et al. Dielectric filters made of porous silicon: advanced performance by oxidation and new layer structures. Thin Sol. Films. 1997;297:237–240. [Google Scholar]
- 43.Anderson SHC, Elliott H, Wallis DJ, Canham LT, Powell JJ. Dissolution of different forms of partially porous silicon wafers under simulated physiological conditions. Phys. Status Solidi A—Appl. Res. 2003;197:331–335. [Google Scholar]
- 44.Meade SO, Yoon MS, Ahn KH, Sailor MJ. Porous silicon photonic crystals as encoded microcarriers. Adv. Mater. 2004;16:1811–1814. [Google Scholar]
- 45.Meade SO, Sailor MJ. Microfabrication of freestanding porous silicon particles containing spectral barcodes. Phys. Status Solidi—Rapid Res. Lett. 2007;1:R71–R73. [Google Scholar]
- 46.Park JS, Meade SO, Segal E, Sailor MJ. Porous silicon-based polymer replicas formed by bead patterning. Phys. Status Solidi A—Appl. Mater. 2007;204:1383–1387. [Google Scholar]
- 47.Li YY, Kollengode VS, Sailor MJ. Porous silicon/polymer nanocomposite photonic crystals by microdroplet patterning. Adv. Mater. 2005;17:1249–1251. [Google Scholar]
- 48.Archer RJ. Stain films on silicon. J. Phys. Chem. Solids. 1960;14:104–110. [Google Scholar]
- 49.Kelly MT, Chun JKM, Bocarsly AB. High-efficiency chemical etchant for the formation of luminescent porous silicon. Appl. Phys. Lett. 1994;64:1693–1695. [Google Scholar]
- 50.de Vasconcelos EA, da Silva EF, dos Santos B, de Azevedo WM, Freire JAK. A new method for luminescent porous silicon formation: reaction-induced vapor-phase stain etch. Phys. Status Solidi A—Appl. Mat. 2005;202:1539–1542. [Google Scholar]
- 51.Jugdaohsingh R. Silicon and bone health. J. Nutr. Health Aging. 2007;11:99–110. [PMC free article] [PubMed] [Google Scholar]
- 52.Canham LT, Saunders SJ, Heeley PB, Keir AM, Cox TI. Rapid chemography of porous silicon undergoing hydrolysis. Adv. Mater. 1994;6:865–868. [Google Scholar]
- 53.Jay T, Canham LT, Heald K, Reeves CL, Downing R. Autoclaving of porous silicon within a hospital environment: potential benefits and problems. Phys. Status Solidi A—Appl. Res. 2000;182:555–560. [Google Scholar]
- 54.Omae K, Sakai T, Sakurai H, et al. Acute and subacute inhalation toxicity of silane 1000 ppm in mice. Arch. Toxicol. 1992;66:750–753. doi: 10.1007/BF01972626. [DOI] [PubMed] [Google Scholar]
- 55.Takebayashi T. Acute inhalation toxicity of high-concentrations of silane in male ICR mice. Arch. Toxicol. 1993;67:55–60. doi: 10.1007/BF02072036. [DOI] [PubMed] [Google Scholar]
- 56.Kawanabe K, Yamamuro T, Kotani S, Nakamura T. Acute nephrotoxicity as an adverse effect after intraperitoneal injection of massive amounts of bioactive ceramic powders in mice and rats. J. Biomed. Mater. Res. 1992;26:209–219. doi: 10.1002/jbm.820260207. [DOI] [PubMed] [Google Scholar]
- 57.Gorustovich AA, Monserrat AJ, Guglielmotti MB, Cabrini RL. Effects of intraosseous implantation of silica-based bioactive glass particles on rat kidney under experimental renal failure. J. Biomater. Appl. 2007;21:431–442. doi: 10.1177/0885328206068061. [DOI] [PubMed] [Google Scholar]
- 58.Lai W, Garino J, Flaitz C, Ducheyne P. Excretion of resorption products from bioactive glass implanted in rabbit muscle. J. Biomed. Mater. Res. Part A. 2005;75A:398–407. doi: 10.1002/jbm.a.30425. [DOI] [PubMed] [Google Scholar]
- 59.Canham LT, Reeves CL, Newey JP, et al. Derivatized mesoporous silicon with dramatically improved stability in simulated human blood plasma. Adv. Mater. 1999;11:1505–1507. [Google Scholar]
- 60.Canham LT, Stewart MP, Buriak JM, et al. Derivatized porous silicon mirrors: implantable optical components with slow resorbability. Phys. Status Solidi A— Appl. Res. 2000;182:521–525. [Google Scholar]
- 61.Bayliss SC, Harris PJ, Buckberry LD, Rousseau C. Phosphate and cell growth on nanostructured semiconductors. J. Mat. Sci. Lett. 1997;16:737–740. [Google Scholar]
- 62.Bayliss SC, Buckberry LD, Fletcher I, Tobin MJ. The culture of neurons on silicon. Sens. Actuators A 74. 1999:139–142. [Google Scholar]
- 63.Bayliss SC, Heald R, Fletcher DI, Buckberry LD. The culture of mammalian cells on nanostructured silicon. Adv. Mater. 1999;11:318–321. [Google Scholar]
- 64.Bayliss SC, Buckberry LD, Harris PJ, Tobin M. Nature of the silicon–animal cell interface. J. Porous Mater. 2000;7:191–195. [Google Scholar]
- 65.Mayne AH, Bayliss SC, Barr P, Tobin M, Buckberry LD. Biologically interfaced porous silicon devices. Phys. Status Solidi A—Appl. Res. 2000;182:505–513. [Google Scholar]
- 66.Chin V, Collins BE, Sailor MJ, Bhatia SN. Compatibility of primary hepatocytes with oxidized nanoporous silicon. Adv. Mater. 2001;13:1877–1880. [Google Scholar]
- 67.Bjorklund RB, Zangooie S, Arwin H. Adsorption of Surfactants in porous silicon films. Langmuir. 1997;13:1440–1445. [Google Scholar]
- 68.Canaria CA, Huang M, Cho Y, et al. The effect of surfactants on the reactivity and photophysics of luminescent nanocrystalline porous silicon. Adv. Funct. Mater. 2002;12:495–500. [Google Scholar]
- 69.Petrova-Koch V, Muschik T, Kux A, Meyer BK, Koch F, Lehmann V. Rapid thermal oxidized porous Si — the superior photoluminescent Si. Appl. Phys. Lett. 1992;61:943–945. [Google Scholar]
- 70.Pacholski C, Sartor M, Sailor MJ, Cunin F, Miskelly GM. Biosensing using porous silicon double-layer interferometers: reflective interferometric Fourier transform spectroscopy. J. Am. Chem. Soc. 2005;127:11636–11645. doi: 10.1021/ja0511671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song JH, Sailor MJ. Dimethyl sulfoxide as a mild oxidizing agent for porous silicon and its effect on photoluminescence. Inorg. Chem. 1998;37:3355–3360. [Google Scholar]
- 72.Harper TF, Sailor MJ. Using porous silicon as a hydrogenating agent: derivatization of the surface of luminescent nanocrystalline silicon with benzoquinone. J. Am. Chem. Soc. 1997;119:6943–6944. [Google Scholar]
- 73.Mattei G, Alieva EV, Petrov JE, Yakovlev VA. Quick oxidation of porous silicon in presence of pyridine vapor. Phys. Status Solidi A—Appl. Res. 2000;182:139–143. [Google Scholar]
- 74.Unagami T. Intrinsic stress in porous silicon layers formed by anodization in HF solution. J. Electrochem. Soc. 1997;144:1835–1838. [Google Scholar]
- 75.Kolasinski KW. Silicon nanostructures from electroless electrochemical etching. Curr. Opin. Solid State Mat. Sci. 2005;9:73–83. [Google Scholar]
- 76.Tinsley-Bown AM, Canham LT, Hollings M, et al. Tuning the pore size and surface chemistry of porous silicon for immunoassays. Phys. Status Solidi A—Appl. Res. 2000;182:547–553. [Google Scholar]
- 77.Létant SE, Content S, Tan TT, Zenhausern F, Sailor MJ. Integration of porous silicon chips in an electronic artificial nose. Sens. Actuators B. 2000;69:193–198. [Google Scholar]
- 78.Linford MR, Chidsey CED. Alkyl monolayers covalently attached to silicon surfaces. J. Am. Chem. Soc. 1993;115:12631–12632. [Google Scholar]
- 79.Buriak JM. Organometallic chemistry on silicon surfaces: formation of functional monolayers bound through Si–C bonds. Chem. Commun. 1999;12:1051–1060. [Google Scholar]
- 80.Buriak JM. Organometallic chemistry on silicon and germanium surfaces. Chem. Rev. 2002;102:1272–1308. doi: 10.1021/cr000064s. [DOI] [PubMed] [Google Scholar]
- 81.Buriak JM, Allen MJ. Lewis acid mediated functionalization of porous silicon with substituted alkenes and alkynes. J. Am. Chem. Soc. 1998;120:1339–1340. [Google Scholar]
- 82.Boukherroub R, Morin S, Wayner DDM, Lockwood DJ. Thermal route for chemical modification and photoluminescence stabilization of porous silicon. Phys. Status Solidi A—Appl. Res. 2000;182:117–121. [Google Scholar]
- 83.Boukherroub R, Morin S, Wayner DDM, et al. Ideal passivation of luminescent porous silicon by thermal, noncatalytic reaction with alkenes and aldehydes. Chem. Mater. 2001;13:2002–2011. [Google Scholar]
- 84.Boukherroub R, Petit A, Loupy A, Chazalviel JN, Ozanam F. Microwave-assisted chemical functionalization of hydrogen-terminated porous silicon surfaces. J. Phys. Chem. B. 2003;107:13459–13462. [Google Scholar]
- 85.Boukherroub R, Wojtyk JTC, Wayner DDM, Lockwood DJ. Thermal hydrosilylation of undecylenic acid with porous silicon. J. Electrochem. Soc. 2002;149:59–63. [Google Scholar]
- 86.Mattei G, Valentini V. In situ functionalization of porous silicon during the electrochemical formation process in ethanoic hydrofluoric acid solution. J. Am. Chem. Soc. 2003;125:9608–9609. doi: 10.1021/ja036228e. [DOI] [PubMed] [Google Scholar]
- 87.Sieval AB, Demirel AL, Nissink JWM, et al. Highly stable Si-C linked functionalized monolayers on the silicon (100) surface. Langmuir. 1998;14:1759–1768. [Google Scholar]
- 88.Sieval AB, Linke R, Zuilhof H, Sudholter EJR. High-quality alkyl monolayers on silicon surfaces. Adv. Mater. 2000;12:1457–1460. [Google Scholar]
- 89.Stewart MP, Buriak JM. Photopatterned hydrosilylation on porous silicon. Angew. Chem. Int. Ed. Engl. 1998;37:3257–3260. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3257::AID-ANIE3257>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 90.Stewart MP, Buriak JM. Exciton-mediated hydrosilylation on photoluminescent nanocrystalline silicon. J. Am. Chem. Soc. 2001;123:7821–7830. doi: 10.1021/ja011116d. [DOI] [PubMed] [Google Scholar]
- 91.Shriver DF, Drezdzon MA. The Manipulation of Air-Sensitive Compounds. 2nd ed. New York: John Wiley and Sons, Inc.; 1986. pp. 7–44. [Google Scholar]
- 92.Linford MR, Fenter P, Eisenberger PM, Chidsey CED. Alkyl monolayers on silicon prepared from 1-alkenes and hydrogen-terminated silicon. J. Am. Chem. Soc. 1995;117:3145–3155. [Google Scholar]
- 93.Terry J, Linford MR, Wigren C, Cao R, Pianetta P, Chidsey CED. Determination of the bonding of alkyl monolayers to the Si(111) surface using chemical-shift, scanned-energy photoelectron diffraction. Appl. Phys. Lett. 1997;71:1056–1058. [Google Scholar]
- 94.Terry J, Linford MR, Wigren C, Cao RY, Pianetta P, Chidsey CED. Alkyl-terminated Si(111) surfaces: a high-resolution, core level photoelectron spectroscopy study. J. Appl. Phys. 1999;85:213–221. [Google Scholar]
- 95.Song JH, Sailor MJ. Functionalization of nanocrystalline porous silicon surfaces with aryllithium reagents: formation of silicon–carbon bonds by cleavage of silicon–silicon bonds. J. Am. Chem. Soc. 1998;120:2376–2381. [Google Scholar]
- 96.Song JH, Sailor MJ. Chemical modification of crystalline porous silicon surfaces. Comments Inorganic Chem. 1999;21:69–84. [Google Scholar]
- 97.Bansal A, Lewis NS. Stabilization of Si photoanodes in aqueous electrolytes through surface alkylation. J. Phys. Chem. B. 1998;102:4058–4060. [Google Scholar]
- 98.Bansal A, Li X, Lauermann I, Lewis NS, Li X, Yi SI, Weinberg WH. Alkylation of Si surfaces using a two-step halogenation/grignard route. J. Am. Chem. Soc. 1996;118:7225–7226. [Google Scholar]
- 99.Dubois T, Ozanam F, Chazalviel JN. Proc. Electro-chem. Soc. Canada, Montreal, Quebec: 1997. Stabilization of the porous silicon surface by grafting of organic groups: direct electrochemical methylation. [Google Scholar]
- 100.deVilleneuve CH, Pinson J, Bernard MC, Allongue P. Electrochemical formation of close-packed phenyl layers on Si(111) J. Phys. Chem. B. 1997;101:2415–2420. [Google Scholar]
- 101.Gurtner C, Wun AW, Sailor MJ. Surface modification of porous silicon by electrochemical reduction of organo halides. Angew. Chem. Int. Ed. 1999;38:1966–1968. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1966::AID-ANIE1966>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 102.Lees IN, Lin H, Canaria CA, Gurtner C, Sailor MJ, Miskelly GM. Chemical stability of porous silicon surfaces electrochemically modified with functional alkyl species. Langmuir. 2003;19:9812–9817. [Google Scholar]
- 103.Salonen J, Lehto VP, Bjorkqvist M, Laine E, Niinisto L. Studies of thermally-carbonize porous silicon surfaces. Phys. Status Solidi A—Appl. Res. 2000;182:123–126. [Google Scholar]
- 104.Salonen J, Laine E, Niinisto L. Thermal carbonization of porous silicon surface by acetylene. J. Appl. Phys. 2002;91:456–461. [Google Scholar]
- 105.Bjorkqvist M, Salonen J, Laine E, Niinisto L. Comparison of stabilizing treatments on porous silicon for sensor applications. Phys. Status Solidi A—Appl. Res. 2003;197:374–377. [Google Scholar]
- 106.Lehto VP, Vaha-Heikkila K, Paski J, Salonen J. Use of thermoanalytical methods in quantification of drug load in mesoporous silicon microparticles. J. Therm. Anal. Calorim. 2005;80:393–397. [Google Scholar]
- 107.Salonen J, Paski J, Vaha-Heikkila K, Heikkila T, Bjorkqvist M, Lehto VP. Determination of drug load in porous silicon microparticles by calorimetry. Phys. Status Solidi A—Appl. Mat. 2005;202:1629–1633. [Google Scholar]
- 108.Limnell T, Riikonen J, Salonen J, et al. The effect of different surface treatment and pore size on the dissolution of ibuprofen from mesoporous silicon particles. Eur. J. Pharm. Sci. 2006;28:S34–S34. [Google Scholar]
- 109.Heikkila T, Salonen J, Tuura J, et al. Evaluation of mesoporous TCPSi, MCM-41, SBA-15, and TUD-1 materials as API carriers for oral drug delivery. Drug Deliv. 2007;15:337–347. doi: 10.1080/10717540601098823. [DOI] [PubMed] [Google Scholar]
- 110.Schwartz MP, Cunin F, Cheung RW, Sailor MJ. Chemical modification of silicon surfaces for biological applications. Phys. Status Solidi A—Appl. Mat. 2005;202:1380–1384. [Google Scholar]
- 111.Kilian KA, Bocking T, Gaus K, Gal M, Gooding JJ. Si–C linked oligo(ethylene glycol) layers in silicon-based photonic crystals: optimization for implantable optical materials. Biomaterials. 2007;28:3055–3062. doi: 10.1016/j.biomaterials.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 112.Kilian KA, Böcking T, Gaus K, Gal M, Gooding JJ. Peptide-modified optical filters for detecting protease activity. ACS Nano. 2007;1:355–361. doi: 10.1021/nn700141n. [DOI] [PubMed] [Google Scholar]
- 113.Janshoff A, Dancil KPS, Steinem C, et al. Macroporous p-type silicon Fabry–Perot layers. Fabrication, characterization, and applications in biosensing. J. Am. Chem. Soc. 1998;120:12108–12116. [Google Scholar]
- 114.Pacholski C, Yu C, Miskelly GM, Godin D, Sailor MJ. Reflective interferometric Fourier transform spectroscopy: a self-compensating label-free immunosensor using double-layers of porous SiO2. J. Am. Chem. Soc. 2006;128:4250–4252. doi: 10.1021/ja056702b. [DOI] [PubMed] [Google Scholar]
- 115.Wise DL. Handbook of Pharmaceutical Controlled Release Technology. 1st ed. New York: Marcel Dekker Inc; 2000. [Google Scholar]
- 116.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 117.Braeckmans K, Smedt SCD, Leblans M, Pauwels R, Demeester J. Encoding micro-carriers: present and future technologies. Nat. Rev. Drug Discov. 2002;1:447–456. doi: 10.1038/nrd817. [DOI] [PubMed] [Google Scholar]
- 118.Ferrer-Montiel AV, Merino JM, Blondelle SE, PerezPaya E, Houghten RA, Montal M. Selected peptides targeted to the NMDA receptor channel protect neurons from excitotoxic death. Nat. Biotechnol. 1998;16:286–291. doi: 10.1038/nbt0398-286. [DOI] [PubMed] [Google Scholar]
- 119.St'astny M, Plocova D, Etrych T, Kovar M, Ulbrich K, Rihova B. HPMA-hydrogels containing cytostatic drugs. Kinetics of the drug release and in vivo efficacy. J. Control Release. 2002;81:101–111. doi: 10.1016/s0168-3659(02)00047-0. [DOI] [PubMed] [Google Scholar]
- 120.Boukherroub R, Wayner DDM, Sproule GI, Lockwood DJ, Canham LT. Stability enhancement of partially-oxidized porous silicon nanostructures modified with ethyl undecylenate. Nano Lett. 2001;1:713–717. [Google Scholar]
- 121.Dorvee JR, Derfus AM, Bhatia SN, Sailor MJ. Manipulation of liquid droplets using amphiphilic, magnetic 1-D photonic crystal chaperones. Nat. Matters. 2004;3:896–899. doi: 10.1038/nmat1253. [DOI] [PubMed] [Google Scholar]
- 122.Sweryda-Krawiek B, Chandler-Henderson RR, Coffer JL, Rho YG, Pinizzotto RF. A comparison of porous silicon and silicon nanocrystallite photo-luminescence quenching with amines. J. Phys. Chem. 1996;100:13776–13780. [Google Scholar]
- 123.Mattei G, Valentini V, Yakovlev VA. An FTIR study of porous silicon layers exposed to humid air with and without pyridine vapors at room temperature. Surf. Sci. 2002;502:58–62. [Google Scholar]
- 124.Dorvee JR, Sailor MJ, Miskelly GM. Digital microfluidics and delivery of molecular payloads with magnetic porous silicon chaperones. Dalton Trans. 2008 doi: 10.1039/b714594b. [DOI] [PubMed] [Google Scholar]
- 125.Kokubo T, Kim HM, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24:2161–2175. doi: 10.1016/s0142-9612(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 126.Saravanapavan P, Hench LL. Mesoporous calcium silicate glasses. I. Synthesis. J. Non-Cryst. Solids. 2003;318:1–13. [Google Scholar]
- 127.Salinas AJ, Vallet-Regi M, Izquierdo-Barba I. Biomimetic apatite deposition on calcium silicate gel glasses. J. Sol-Gel Sci. Technol. 2001;21:13–25. [Google Scholar]
- 128.Kosmulski M. Surface charge and zeta potential of silica in mixtures of organic solvents and water. In: Papirer E, editor. Adsorption on Silica Surfaces. New York: Marcel Dekker; 2000. pp. 363–364. [Google Scholar]
- 129.Parks GA. Isoelectric points of solid oxides solid hydroxides and aqueous hydroxo complex systems. Chem. Rev. 1965;65:177–198. [Google Scholar]
- 130.Collins BE, Dancil KP, Abbi G, Sailor MJ. Determining protein size using an electrochemically machined pore gradient in silicon. Adv. Funct. Mater. 2002;12:187–191. [Google Scholar]
- 131.Karlsson LM, Schubert M, Ashkenov N, Arwin H. Protein adsorption in porous silicon gradients monitored by spatially resolved spectroscopic ellipsometry. Thin Solid Films 455–456. 2004:726–730. [Google Scholar]
- 132.Arwin H, Gavutis M, Gustafsson J, Schultzberg M, Zangooie S, Tengvall P. Protein adsorption in thin porous silicon layers. Phys. Status Solidi, A Appl. Res. 2000;182:515–520. [Google Scholar]
- 133.Schwartz MP, Alvarez SD, Sailor MJ. A porous SiO2 interferometric biosensor for quantitative determination of protein interactions: binding of protein A to immunoglobulins derived from different species. Anal. Chem. 2007;79:327–334. doi: 10.1021/ac061476p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schwartz MP, Yu C, Alvarez SD, et al. Using an oxidized porous silicon interferometer for determination of relative protein binding affinity through non-covalent capture probe immobilization. Phys. Status Solidi, A Appl. Mater. 2007;204:1444–1448. [Google Scholar]
- 135.Tay L, Rowell NL, Poitras D, Fraser JW, Lockwood DJ, Boukherroub R. Bovine serum albumin adsorption on passivated porous silicon layers. Can. J. Chem.-Rev. Can. Chim. 2004;82:1545–1553. [Google Scholar]
- 136.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 137.Gurny R. Theme issue: nano- and microscaled drug carriers. Eur. J. Pharm. Biopharm. 2006;63:III–III. [Google Scholar]
- 138.Polarz S, Antonietti M. Porous materials via nanocasting procedures: innovative materials and learning about soft-matter organization. JCS Chem. Commun. 2002:2593–2604. doi: 10.1039/b205708p. [DOI] [PubMed] [Google Scholar]
- 139.Wirtz M, Parker M, Kobayashi Y, Martin CR. Template synthesized nanotubes for chemical separations and analysis. Chem. Eur. J. 2002;16:3572–3578. doi: 10.1002/1521-3765(20020816)8:16<3572::AID-CHEM3572>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 140.Hulteen JC, Martin CR. A general template-based method for the preparation of nanomaterials. J. Mater. Chem. 1997;7:1075–1087. [Google Scholar]
- 141.Moller K, Bein T. Inclusion chemistry in periodic mesoporous hosts. Chem. Mater. 1998;10:2950–2963. [Google Scholar]
- 142.Reese CE, Baltusavich ME, Keim JP, Asher SA. Development of an intelligent polymerized crystalline colloidal array colorimetric reagent. Anal. Chem. 2001;73:5038–5042. doi: 10.1021/ac010667j. [DOI] [PubMed] [Google Scholar]
- 143.Xu X, Majetich SA, Asher SA. Mesoscopic monodisperse ferromagnetic colloids enable magnetically controlled photonic crystals. J. Am. Chem. Soc. 2002;124:13864–13868. doi: 10.1021/ja026901k. [DOI] [PubMed] [Google Scholar]
- 144.Haynes CL, Van Duyne RP. Nanosphere lithography: a versatile nanofabrication tool for studies of size-dependent nanoparticle optics. J. Phys. Chem. B. 2001;105:5599–5611. [Google Scholar]
- 145.Thonissen M, Berger MG. Multilayer structures of porous silicon. In: Canham L, editor. Properties of Porous Silicon. London: Institution of Engineering and Technology; 1997. pp. 30–37. [Google Scholar]
- 146.Li YY, Cunin F, Link JR, et al. Polymer replicas of photonic porous silicon for sensing and drug delivery applications. Science. 2003;299:2045–2047. doi: 10.1126/science.1081298. [DOI] [PubMed] [Google Scholar]
- 147.Sirbuly DJ, Lowman GM, Scott B, Stucky GD, Buratto SK. Patterned microstructures of porous silicon by dry-removal soft lithography. Adv. Mater. 2003;15:149–152. [Google Scholar]
- 148.Mukherjee P, Whitehead MA, Senter RA, Fan DM, Coffer JL, Canham LT. Biorelevant mesoporous silicon/polymer composites: directed assembly, disassembly, and controlled release. Biomed. Microdevices. 2006;8:9–15. doi: 10.1007/s10544-006-6377-7. [DOI] [PubMed] [Google Scholar]
- 149.Orosco MM, Pacholski C, Miskelly GM, Sailor MJ. Protein-coated porous silicon photonic crystals for amplified optical detection of protease activity. Adv. Mater. 2006;18:1393–1396. [Google Scholar]
- 150.Segal E, Perelman LA, Cunin F, et al. Confinement of thermoresponsive hydrogels in nanostructured porous silicon dioxide templates. Adv. Funct. Mater. 2007;17:1153–1162. [Google Scholar]
- 151.Koshida N, Koyama H, Yamamoto Y, Collins GJ. Visible electroluminescence from porous silicon diodes with an electropolymerized contact. Appl. Phys. Lett. 1993;63:2655–2657. [Google Scholar]
- 152.Yoon MS, Ahn KH, Cheung RW, et al. Covalent crosslinking of 1-D photonic crystals of microporous Si by hydrosilylation and ring-opening metathesis polymerization. Chem. Commun. 2003:680–681. doi: 10.1039/b300937h. [DOI] [PubMed] [Google Scholar]
- 153.Lie LH, Patole SN, Hart ER, Houlton A, Horrocks BR. Photochemical reaction of diazomethane with hydrogen-terminated silicon surfaces. J. Phys. Chem. B. 2002;106:113–120. [Google Scholar]
- 154.Heinrich JL, Lee A, Sailor MJ. Porous silicon used as an initiator in polymerization reactions. Mat. Res. Soc. Symp. Proc. 1995;358:605–610. [Google Scholar]
- 155.Li YY, Cunin F, Sailor MJ, Link JR, Gao T. Nanostructured Casting of Organic and Bio-polymers in Porous Silicon Templates. San Diego: University of California; 2006. [Google Scholar]
- 156.Rerossi D, Kajiwara K, Osada Y, Yamauchi A. Polymer Gels: Fundamental and Biomedical Application. New York: Plenum; 1991. [Google Scholar]
- 157.Annaka M, Tanaka T. Multiple phases of polymer gels. Nature. 1992;355:430–432. [Google Scholar]
- 158.Dusek K, editor. Advances in Polymer Science. Berlin: Springer; 1993. Responsive Gels: Volume Transitions I. [Google Scholar]
- 159.Sakiyama T, Tsutsui T, Masuda E, Imamura K, Nakanishi K. Ionization characteristics of polyelectrolyte complex gels: analysis based on their swelling behaviors. Macromolecules. 2003;36:5039–5042. [Google Scholar]
- 160.Aoki T, Kawashima M, Katono H, et al. Temperature-responsive interpenetrating polymer networks constructed with poly(acrylic acid) and poly(N,N-dimethylacrylamide) Macromolecules. 1994;27:947–952. [Google Scholar]
- 161.Kim SJ, Lee CK, Lee YM, Kim IY, Kim SI. Electrical/pH-sensitive swelling behavior of polyelectrolyte hydrogels prepared with hyaluronic acid-poly(vinyl alcohol) interpenetrating polymer networks. React. Funct. Polym. 2003;55:291–298. [Google Scholar]
- 162.Trnka TN, Grubbs RH. The development of L2X2Ru=CHR olefin metathesis catalysts: an organometallic success story. Acc. Chem. Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- 163.Juang A, Scherman OA, Grubbs RH, Lewis NS. Formation of covalently attached polymer overlayers on Si(111) surfaces using ring-opening metathesis polymerization methods. Langmuir. 2001;17:1321–1323. [Google Scholar]
- 164.van Noort D, Welin-Klintstrom S, Arwin H, Zangooie S, Lundstrom I, Mandenius CF. Monitoring specific interaction of low molecular weight biomolecules on oxidized porous silicon using ellipsometry. Biosens. Bioelectron. 1998;13:439–449. doi: 10.1016/s0956-5663(97)00094-8. [DOI] [PubMed] [Google Scholar]
- 165.Snow PA, Squire EK, Russell PSJ, Canham LT. Vapor sensing using the optical properties of porous silicon Bragg mirrors. J. Appl. Phys. 1999;86:1781–1784. [Google Scholar]
- 166.Chan S, Li Y, Rothberg LJ, Miller BL, Fauchet PM. Nanoscale silicon microcavities for biosensing. Mater. Sci. Eng., C, Biomim. Supramol. Syst. 2001;15:277–282. [Google Scholar]
- 167.Anderson MA, Tinsley-Bown A, Allcock P, et al. Sensitivity of the optical properties of porous silicon layers to the refractive index of liquid in the pores. Phys. Status Solidi, A Appl. Res. 2003;197:528–533. [Google Scholar]
- 168.DeLouise LA, Miller BL, Haidaris C. Design of porous silicon biosensors for optical detection of pathogenic organisms causing skin infection. J. Invest. Dermatol. 2004;122:A136–A136. [Google Scholar]
- 169.Martín-Palma RJ, Torres-Costa V, Arroyo-Hernández M, Manso M, Pérez-Rigueiro J, Martínez-Duart JM. Porous silicon multilayer stacks for optical biosensing applications. Microelectronics J. 2004;35:45–48. [Google Scholar]
- 170.Tsargorodskaya A, Nabok AV, Ray AK. Ellipsometric study of the adsorption of bovine serum albumin into porous silicon. Nanotechnology. 2004;15:703–709. [Google Scholar]
- 171.Tinsley-Bown A, Smith RG, Hayward S, et al. Immunoassays in a porous silicon interferometric biosensor combined with sensitive signal processing. Phys. Status Solidi, A Appl. Mat. 2005;202:1347–1356. [Google Scholar]
- 172.Worsfold O, Voelcker NH, Nishiya T. Biosensing using lipid bilayers suspended on porous silicon. Langmuir. 2006;22:7078–7083. doi: 10.1021/la060121y. [DOI] [PubMed] [Google Scholar]
- 173.Ouyang H, DeLouise LA, Miller BL, Fauchet PM. Label-free quantitative detection of protein using macroporous silicon photonic bandgap biosensors. Anal. Chem. 2007;79:1502–1506. doi: 10.1021/ac0608366. [DOI] [PubMed] [Google Scholar]
- 174.Bohren CF, Huffman DR. Adsorption and Scattering of Light by Small Particles. New York: Wiley; 1983. p. 217. [Google Scholar]
- 175.Pacholski C, Sailor MJ. Sensing with porous silicon double layers: a general approach for background suppression. Phys. Status Solidi C. 2007;4:2088–2092. [Google Scholar]
- 176.Ouyang H, Christophersen M, Viard R, Miller BL, Fauchet PM. Macroporous silicon microcavities for macromolecule detection. Adv. Funct. Mater. 2005;15:1851–1859. [Google Scholar]
- 177.Gao J, Gao T, Sailor MJ. A porous silicon vapor sensor based on laser interferometry. Appl. Phys. Lett. 2000;77:901–903. [Google Scholar]
- 178.Gao J, Gao T, Li YY, Sailor MJ. Vapor sensors based on optical interferometry from oxidized microporous silicon films. Langmuir. 2002;18:2229–2233. [Google Scholar]
- 179.Chan S, Fauchet PM. Nanoscale microcavities for biomedical sensor applications. Proc. SPIE. 2000;3912:23. [Google Scholar]
- 180.Freeman WR, Falkenstein I. Avastin and new treatments for AMD; where are we? Retina. 2006;26:853–858. doi: 10.1097/01.iae.0000244722.35073.7c. [DOI] [PubMed] [Google Scholar]
- 181.Li X, John JS, Coffer JL, et al. Porosified silicon wafer structures impregnated with platinum anti-tumor compounds: fabrication, characterization, and diffusion studies. Biomed. Microdevices. 2000;2:265–272. [Google Scholar]
- 182.Macdonald HM, Hardcastle AE, Jugdaohsingh R, Reid DM, Powell JJ. Dietary silicon intake is associated with bone mineral density in premenopausal women and postmenopausal women taking HRT. J. Bone Miner. Res. 2005;20:S393–S393. [Google Scholar]
- 183.Canham LT. Nanoscale semiconducting silicon as a nutritional food additive. Nanotechnology. 2007;18 [Google Scholar]
- 184.Link JR, Sailor MJ. Smart dust: self-assembling, self-orienting photonic crystals of porous Si. Proc. Nat. Acad. Sci. 2003;100:10607–10610. doi: 10.1073/pnas.1233824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Park JH, Derfus AM, Segal E, Vecchio KS, Bhatia SN, Sailor MJ. Local heating of discrete droplets using magnetic porous silicon-based photonic crystals. J. Am. Chem. Soc. 2006;128:7938–7946. doi: 10.1021/ja0612854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Conner SE, Diettrich S, Goh A, et al. Brachysil: porous silicon as a novel 32P sealed source for cancer brachytherapy; Porous Semiconductors Science and Technology International Meeting 2006; Sitges — Barcelona; [Google Scholar]
- 187.Goh A, Chung A, Lo R, et al. A novel approach to brachytherapy in hepatocellular carcinoma using a phosphorus32 (32P) brachytherpay delivery device — a first-in-man study. Int. J. Radiation Oncology Biol. Phys. 2007;67:786–792. doi: 10.1016/j.ijrobp.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 188.Rajaraman S, Henderson HT. A unique fabrication approach for microneedles using coherent porous silicon technology. Sens. Actuator, B, Chem. 2005;105:443–448. [Google Scholar]
- 189.Ji J, Tay FEH, Miao JM, Iliescu C. Microfabricated microneedle with porous tip for drug delivery. J. Micromech. Microeng. 2006;16:958–964. [Google Scholar]
- 190.Heinrich JL, Curtis CL, Credo GM, Kavanagh KL, Sailor MJ. Luminescent colloidal Si suspensions from porous Si. Science. 1992;255:66–68. doi: 10.1126/science.255.5040.66. [DOI] [PubMed] [Google Scholar]
- 191.Bley RA, Kauzlarich SM, Davis JE, Lee HWH. Characterization of silicon nanoparticles prepared from porous silicon. Chem. Mater. 1996;8:1881–1888. [Google Scholar]
- 192.Limnell T, Riikonen J, Salonen J, et al. Surface chemistry and pore size affect carrier properties of mesoporous silicon microparticles. Int. J. Pharm. 2007;343:141–147. doi: 10.1016/j.ijpharm.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 193.Trewyn BG, Giri S, Slowing II, Lin VS. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem. Commun. 2007;31:3236–3245. doi: 10.1039/b701744h. [DOI] [PubMed] [Google Scholar]
- 194.Li X, Coffer JL, Chen Y, Pinizzotto RF, Newey J, Canham LT. Transition metal complex-doped hydroxyapatite layers on porous silicon. J. Am. Chem. Soc. 1998;120:11706–11709. [Google Scholar]
- 195.Aoudia M, Cheng GZ, Kennedy VO, Kenney ME, Rodgers MAJ. Synthesis of a series of octabutoxy- and octabutoxybenzophthalocyanines and photophysical properties of two members of the series. J. Am. Chem. Soc. 1997;119:6029–6039. [Google Scholar]
- 196.Fujii M, Minobe S, Usui M, et al. Generation of singlet oxygen at room temperature mediated by energy transfer from photoexcited porous Si. Phys. Rev. B. 2004;70 [Google Scholar]
- 197.Fujii M, Nishimura N, Fumon H, et al. Dynamics of photosensitized formation of singlet oxygen by porous silicon in aqueous solution. J. Appld Physics. 2006;100 [Google Scholar]
- 198.Nassiopoulos AG, Grigoropoulos S, Canham L, et al. Sub-micrometre luminescent porous silicon structures using lithographically patterned sub-strates. Thin Sol. Films. 1995;255:329–333. [Google Scholar]