Abstract
Rationale and objectives
We previously showed that systemic administration of the prototypical alpha-2 noradrenaline (NA) receptor antagonist yohimbine increases alcohol self-administration and reinstatement. Yohimbine also acts as an agonist of 5-hydroxytryptamine (5-HT) 5-HT1A receptors, which have been shown to be involved in alcohol seeking. Here, we determined the contributions of the alpha-2 and 5-HT1A properties of yohimbine to its effects on alcohol seeking.
Methods
The effects of lesions of the dorsal or ventral NA bundles with 6-OHDA on yohimbine-induced alcohol self-administration were first determined in male Wistar rats trained to self-administer alcohol (12% w/v, 0.19 ml per alcohol delivery), and then on reinstatement induced by yohimbine after extinction of the operant response. It was then determined whether the selective alpha-2 antagonist RS-79948 (0.1, 0.2, 0.4 mg/kg) would mimic the effects of yohimbine on self-administration and reinstatement. The effects of the alpha-2 receptor agonist clonidine, or the 5-HT1A antagonist WAY 100,635 were then determined on yohimbine-induced self-administration and reinstatement.
Results
Lesions of the NA systems did not affect yohimbine-induced alcohol self-administration or reinstatement, and RS-79948 did not mimic the effects of yohimbine. Clonidine did not significantly affect increased alcohol self-administration induced by yohimbine, but did attenuate its effects on reinstatement. Blockade of 5-HT1A receptors reduced both yohimbine-induced self-administration and reinstatement.
Conclusions
These results suggest that alpha-2 antagonist properties of yohimbine may play a role in the reinstatement of alcohol-seeking, but not self-administration. On the other hand, yohimbine's actions on 5-HT1A receptors contribute to its effects on both alcohol self-administration and reinstatement.
Studies using correlational methods in humans suggest that stressful life events are positively related to alcohol consumption and relapse (Brown et al. 1995; Cooper et al. 1992; Sinha 2001). The reasons for this relationship are not well understood. A recent focus of our research has been on the effects of the pharmacological stressor yohimbine on alcohol seeking. Yohimbine, a prototypical alpha-2 antagonist, activates the ascending noradrenaline (NA) systems by blocking inhibitory alpha-2 autoreceptors on the cell bodies of the NA neurons. It produces stress-like effects in humans and laboratory animals through this noradrenergic activation (File 1986; Krystal et al. 1994; Southwick et al. 1999). Yohimbine is notable in that it is one of the few compounds that both increases alcohol self-administration and induces reinstatement of alcohol seeking (Le et al. 2005; Marinelli et al. 2007). This drug has also been found to induce reinstatement of responding in monkeys and rats trained to self-administer cocaine (Fletcher et al. 2008; Kupferschmidt et al. 2008; Lee et al. 2004), methamphetamine (Shepard et al. 2004) and palatable food (Ghitza et al. 2006; Ghitza et al. 2007; Nair et al. 2006).
The neural mechanisms underlying the effects of yohimbine on drug seeking have received little attention. In monkeys, yohimbine-induced reinstatement of cocaine-seeking was shown to be blocked by clonidine, an alpha-2 agonist, and mimicked by RS-79948, a highly selective antagonist of alpha-2 receptors (Lee et al. 2004). These studies suggest that the effects of yohimbine on cocaine seeking are mediated by NA. In the case of alcohol, we showed that the increased alcohol self-administration and reinstatement of alcohol seeking (Le et al. 2005) induced by yohimbine is mediated by corticotropin-releasing factor (CRF) (Marinelli et al. 2007). While yohimbine is most often used as an alpha-2 receptor antagonist, it also has significant activity at other receptors, most notably as an agonist of 5-hydroxytryptamine (5-HT) 5-HT1A receptors, which function as autoreceptors to reduce 5-HT cell firing and release (Hjorth and Sharp 1991; Millan et al. 2000). These receptors have been shown by us and others to be involved in alcohol seeking (Le et al. 2002; Tomkins et al. 1994a; Tomkins et al. 1994b). To our knowledge, there are no studies that have systematically examined the role of NA and 5-HT in the effects of yohimbine on drug or alcohol seeking.
The aim of the present series of experiments was therefore to determine the role of the NA and 5-HT projections in the effects of yohimbine on alcohol seeking. We first tested the effects of selective depletion of NA in different areas of the forebrain by the injection of 6-hydroxydopamine (6-OHDA) into the dorsal and ventral noradrenergic bundles (DNAB, VNAB) on yohimbine-induced alcohol seeking. We then examined whether a highly selective alpha-2 antagonist, RS-79948 would mimic the effects of yohimbine, and if the alpha-2 agonist, clonidine would block the effects of yohimbine on self-administration and reinstatement. Finally, since yohimbine also acts as an agonist at 5-HT1A receptors, we examined the effects of WAY 100,635, a selective antagonist of the 5-HT1A receptor on yohimbine-induced alcohol self-administration and reinstatement.
Materials and Methods
Subjects
Male Wistar rats, weighing 175-200 g at the start of the experiment were obtained from Charles River (Montreal, Quebec). The rats were individually housed under a 12:12 h light-dark cycle (light on from 7:00 a.m. to 7:00 p.m.). Food and water were freely available in the home cage and the temperature was maintained at 21± 1°C. The experimental procedures followed the “Principles of laboratory animal care” (NIH publication no. 85-23, 1996) and were approved by the local animal care committee.
Apparatus
The alcohol self-administration chambers were equipped with two levers, symmetrically centered on the side panel. Responding on one lever (an active lever) activated the infusion pump (Razel Scientific, St. Albans, VT), while presses on the other lever (an inactive lever) were recorded, but did not activate the pump. Activation of the infusion pump resulted in the delivery of 0.19 ml of a 12% alcohol w/v solution into a receptacle located between the two levers over a period of 5 sec. During the delivery of alcohol, a stimulus light above the active lever was turned on, white noise was produced through a speaker, and lever presses were not reinforced.
Lesions of the dorsal or ventral noradrenergic bundles with 6-OHDA
Animals received 6-OHDA lesions after they responded stably for 12 % alcohol at FR3. The lesion procedure was based on previous work (Sahakian et al. 1983). Rats were injected with pargyline (50 mg/kg, i.p.) 30 min before the 6-OHDA infusions to enhance the neurotoxic effect of the 6-OHDA, and were then anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and placed in a stereotaxic frame. Bilateral injectors (28 gauge) were directed toward the DNAB and VNAB in separate groups of rats using the following coordinates: DNAB, AP: -6.0 mm, ML: ±0.8 mm, DV: -6.3 mm from skull, incisor bar -2.4 mm; VNAB, AP: -6.6 mm, ML: ±2.0 mm, DV: -8.2 and -9.2 mm from skull, incisor bar +5.0 mm. Infusions of 2 μL of vehicle or 6-OHDA (DNAB 2 μg/μL; VNAB 3 μg/μL in 0.1% ascorbic acid/0.9% saline) were made over 8 min (0.25 μL/min) using a Hamilton syringe connected to a microinfusion pump (Razel). The injectors were kept in place for an additional 5 min in order to limit diffusion upwards along the injector tract.
Analysis of brain monoamines
At the end of the experiment, the rats were decapitated, their brains quickly removed and tissue samples from the hypothalamus and hippocampus taken (-0.6 to -2.6 mm from bregma). The effectiveness of the lesion manipulation was tested by an HPLC assay for NA in the hippocampus and hypothalamus. The hippocampus is innervated solely by the DNAB projections originating in the locus coeruleus (LC) (Moore and Bloom 1979) and should only be affected by the DNAB lesion procedure. The hypothalamus is innervated by the VNAB projections originating from the lateral tegmental NA neurons and to a lesser degree from the LC neurons (Moore and Bloom 1979). Thus, the VNAB lesion should significantly decrease NA content in the hypothalamus, as previously found (Shaham et al. 2000).
The frozen tissue was placed in assay buffer, sonicated and then centrifuged at 4,000 rpm for 15 min. The supernatant was analyzed for amine content using two high-performance liquid chromatography systems with electrochemical detection HPLC-EC. The samples were loaded onto reverse-phase columns (15 × 0.46 cm; HAISIL C18, 5 μm) through manual injection ports (20 μl loop). Reduction currents for NA were measured with dual-channel ESA coulometric detectors. The mobile phase (sodium acetate 36 mM, SOS 3.1 mM, EDTA 100 μM, 5% acetonitrile, adjusted to pH 3.7 using glacial acetic acid) circulated through each closed system at a flow rate of 1.4 ml/min by Waters 515 HPLC pumps. The concentration of NA was estimated from peak height by comparison with injections of known amounts of pure standards using an EZChrom Chromatography Data System.
Drugs
Alcohol solution was prepared by diluting 95% ethanol (Commercial Alcohols Inc., Tiverton, ON, Canada) in tap water. Yohimbine HCl, WAY 100,635 maleate, clonidine HCl and 6-OHDA HBr were obtained from Sigma (St. Louis, MO). RS-79948 HCl was obtained from Tocris (Ellisville, MO). Yohimbine was dissolved in distilled water and 6-OHDA was dissolved in 0.1% ascorbic acid in saline. The vehicle for the other drugs was saline. The doses of yohimbine and RS-79948 were based on previous work (Le et al. 2005; Packard and Wingard 2004; White and Birkle 2001). Those for clonidine and WAY 100,635 were also based on published findings (Bagdy et al. 2001; Jackson et al. 1998; Shaham et al. 2000; Tomkins and O'Neill 2000).
Procedure
Alcohol self-administration training
Rats were trained to self-administer alcohol using methods described previously (Le et al. 1998). Briefly, rats were initially provided with access to alcohol solutions and water in modified Richter tubes for 30 min/day in drinking cages (Linseman 1987). Alcohol solutions were provided in escalating concentrations: 3% for the first 5 days, 6% for the next 8 days and 12% for the last 10-12 days. Subsequently, self-administration of alcohol was initiated on a fixed ratio-1 schedule of reinforcement (FR-1, each lever press is reinforced) for 10-14 days (1-h/day). The requirement for alcohol delivery was then increased to a FR-2 schedule for 5 sessions and then to a FR-3 schedule for 8-12 days until the rats demonstrated three days of stable responding for alcohol (variability of less than 20% from the mean). Twenty-three rats that consumed less than 0.4 g/kg/h of alcohol during alcohol training were excluded from the experiments.
Extinction of alcohol-reinforced behavior
The experimental procedures during the extinction sessions were the same as those during the self-administration sessions, with the exception that responding on the active lever did not lead to alcohol delivery, and the cue lights and speakers signaling delivery were disconnected. Tests for reinstatement commenced after 7-12 extinction sessions, after the rats reached the extinction criterion of fewer than 12 presses on the previously active lever during the 1 h session.
Test for reinstatement of alcohol seeking
Tests were conducted under the same conditions experienced during extinction.
Experiment 1
Effects of lesions of the DNAB or VNAB on yohimbine-induced alcohol seeking
Forty-eight rats were trained to self-administer alcohol as described previously and were then assigned to one of four groups (n=12/group), matched on the basis of alcohol self-administration. They underwent surgery for lesions of either the DNAB or VNAB with 6-OHDA, or sham lesions (vehicle injections) of the DNAB or VNAB. Animals were allowed to recover for 7 days prior to retraining for alcohol self-administration.
a. Self-administration
Once animals reacquired stable levels of alcohol self-administration, the effects of the DNAB or VNAB lesions on yohimbine-induced increases in alcohol self-administration were examined. A mixed design was used, with lesion site (DNAB or VNAB) and neurotoxin condition (vehicle, 6-OHDA) as between factors, and yohimbine dose (vehicle, 0.625, 1.25 mg/kg) as the within factor. Vehicle or one of the doses of yohimbine was administered 30 min prior to 1 h self-administration sessions, in counterbalanced order, with 2-3 daily self-administration sessions separating each dose. In this and the other experiments, animals were administered vehicle solutions in the 3 days prior to the test sessions, in order to habituate them to the injection procedure.
b. Reinstatement
After the tests of self-administration were completed, animals underwent extinction of responding for alcohol, and were then tested for the effects of the DNAB or VNAB lesions on the reinstatement of alcohol seeking induced by yohimbine. The same design and drug doses were used as in Experiment 1a. Vehicle or one of the doses of yohimbine was administered 30 min prior to the reinstatement test sessions, in counterbalanced order, with 2-3 daily extinction sessions separating each dose.
Experiment 2
Effects of yohimbine and RS-79948 on alcohol seeking
a. Self-administration
The effects of pretreatment with vehicle or different doses of yohimbine, or RS-79948 on alcohol self-administration were determined in two groups of rats (n = 14 rats/group) that were trained to self-administer alcohol. For each of the drugs, a within-subject design was used with the factor of dose (yohimbine: vehicle, 0.625, 1.25 and 2.5 mg/kg; RS-79948: vehicle, 0.1, 0.2 and 0.4 mg/kg). Rats were injected 30 min before the 1-h tests sessions with the appropriate vehicle or drug. Animals received the different doses of each drug in a counterbalanced order with 2-3 self-administration sessions between each dose.
b. Reinstatement
Two different groups of animals were used (n=12/group). They were trained to self-administer alcohol, and then responding for alcohol was extinguished in daily 1 h sessions. After reaching the criterion for extinction, the effects of pretreatment with vehicle or different doses of yohimbine or RS-79948 on reinstatement was determined with the within factor of yohimbine or RS-79948 dose (yohimbine: vehicle, 0.625, 1.25 and 2.5 mg/kg; RS-79948: vehicle, 0.1, 0.2 and 0.4 mg/kg). During testing, each rat was injected 30 min before the 1-h test sessions with vehicle or drug. Doses of yohimbine or RS-79948 were given in a counterbalanced order with 2-3 extinction sessions between each dose.
Experiment 3
Effects of clonidine on yohimbine-induced alcohol seeking
a. Self-administration
Three groups of rats (n= 10/group) trained to self-administer alcohol were used to evaluate the effects of pretreatment with different doses of clonidine on yohimbine-induced increases in alcohol self-administration. A mixed design was used, with the between factor of clonidine dose (vehicle, 20, 40 μg/kg) and within factor of yohimbine dose (vehicle, 1.25 mg/kg). Rats were injected with vehicle or yohimbine 15 min before administration of vehicle or clonidine, and 15 min later received a 1 h self-administration session. Animals received the vehicle and yohimbine tests in a counterbalanced order with 2-3 self-administration sessions between the tests.
b. Reinstatement
Two different groups of rats were used (n=14/group). They were trained to self-administer alcohol, and their responding for alcohol was then extinguished in daily 1 h sessions. After reaching the criterion for extinction, the effects of pretreatment with vehicle or different doses of clonidine on reinstatement induced by yohimbine (1.25 mg/kg) were determined. A mixed design was used, with the between factor of clonidine dose group (20 μg/kg, 40 μg/kg) and within factors of clonidine condition (vehicle, clonidine) and yohimbine condition (vehicle, 1.25 mg/kg). Animals were injected with vehicle or yohimbine, and 15 min later with clonidine or its vehicle, and 15 min later received a 1 h reinstatement test. Animals received the vehicle and yohimbine tests in a counterbalanced order with 2-3 extinction sessions between the tests.
Experiment 4
Effects of WAY 100,635 on yohimbine-induced alcohol seeking
a. Self-administration
The effects of pretreatment with vehicle or different doses of WAY 100,635 on yohimbine-induced increases in alcohol self-administration were determined in two groups of rats (n=14/group) that were trained to self-administer alcohol. A mixed design was used, with the between factor of yohimbine dose (vehicle, 1.25 mg/kg) and within factor of WAY 100,635 dose (vehicle, 0.1, 0.3, 1.0 mg/kg). Rats were injected the vehicle or yohimbine 15 min before administration of vehicle or WAY 100,635, and 15 min later received a 1 h self-administration session. Animals received the vehicle and different doses of WAY 100,635 in a counterbalanced order with 2-3 self-administration days separating them.
b. Reinstatement
Four different groups of animals were used (n=10/group). They were trained to self-administer alcohol, and then responding for alcohol was extinguished in daily 1 h sessions. After reaching the criterion for extinction, the effects of pretreatment with vehicle or different doses of WAY 100,635 on reinstatement induced by yohimbine was determined. A mixed design was used, with the between factor of WAY 100,635 dose (vehicle, 0.1, 0.3, 1.0 mg/kg) and the within factor of yohimbine dose (vehicle, 1.25 mg/kg). Rats were injected the vehicle or yohimbine 15 min before administration of vehicle or WAY 100,635, and 15 min later received a 1 h reinstatement test. Animals received the vehicle and yohimbine in a counterbalanced order with 2-3 extinction days separating them.
Results
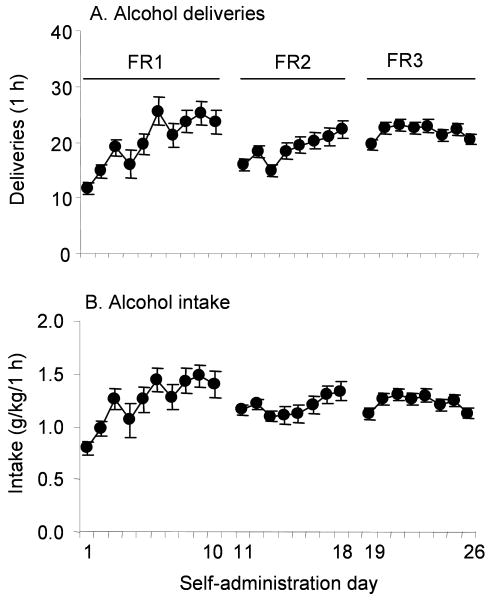
Figure 1 shows the mean number of alcohol deliveries (A) and intake (B) during alcohol self-administration training.
Figure 1.
Alcohol self-administration of animals in the experiments. A, alcohol deliveries and B, intake during training for self-administration training at fixed ratio (FR) 1, 2 and 3. Data are presented as means (±sem). n=207.
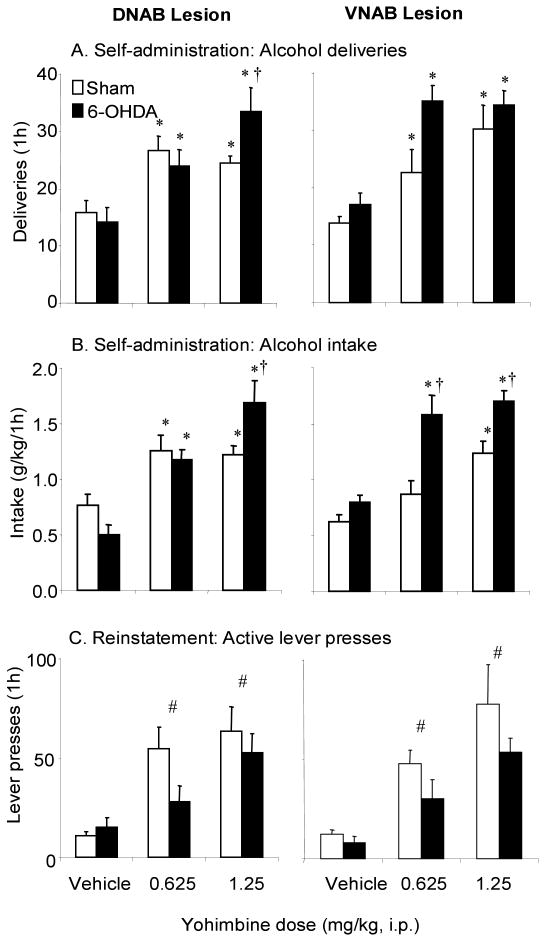
Experiment 1: Effects of lesions of the DNAB or VNAB on yohimbine-induced alcohol seeking
Analysis of lesion effects on regional NA
Table 1 shows the effects of 6-OHDA infusions into the DNAB or VNAB on hippocampal and hypothalamic NA. Six animals with less than 50% depletion of hippocampal or hypothalamic NA in, respectively, the DNAB and VNAB 6-OHDA groups, and one animal in the DNAB 6-OHDA group that had over 50% depletions of NA in both regions were excluded from behavioral analysis. ANOVA of hippocampal NA levels with the between factors of site (DNAB, VNAB) and neurotoxin condition (Vehicle, 6-OHDA) revealed significant main effects of site (F(1,32)=27.06, p<0.05), neurotoxin condition (F(1,32)=133.98, p<0.05) and a significant site × neurotoxin condition interaction (F(1,32)=41.62, p<0.05). Post-hoc tests showed animals infused with 6-OHDA into the DNAB had significantly lower hippocampal NA than did those infused with vehicle or with 6-OHDA into the VNAB (p's<0.05). There was also a small, but significant effect of VNAB infusions of 6-OHDA on levels of hippocampal NA (p<0.05).
Table 1.
Effects of 6-OHDA infusions into the DNAB or VNAB on levels of noradrenaline (NA) in the hippocampus and hypothalamus. Results are expressed as ng/g NA (fresh tissue weight); degree of depletion is also shown as percentage of the vehicle condition. * Significant differences from the vehicle condition (p<0.05; Neumann Keuls test).
| Hippocampus | Hypothalamus | |
|---|---|---|
| DNAB Vehicle | 0.57 ± 0.02 | 2.36 ± 0.11 |
| DNAB 6-OHDA | 0.07 ± 0.02* | 1.81 ± 0.20* |
| % Vehicle | 11.83 ± 3.52 | 76.69 ± 8.30 |
| VNAB Vehicle | 0.53 ± 0.02 | 2.10 ±0.16 |
| VNAB 6-OHDA | 0.39 ± 0.04* | 0.64 ± 0.07* |
| % Vehicle | 74.11 ± 7.46 | 30.28 ± 3.29 |
ANOVA of hypothalamic NA levels showed significant main effects of site (F(1,32)=25.09, p<0.05), neurotoxin condition (F(1,32)=49.63, p<0.05) and a significant site × neurotoxin condition interaction (F1,32)=10.22, p<0.05). Compared to animals infused with vehicle, those infused with 6-OHDA into the VNAB had significantly lower levels of hypothalamic NA (p<0.05). There was a small, but significant effect of DNAB 6-OHDA on NA levels in the hypothalamus (p<0.05).
a. Self-administration
Figure 2 shows the effects of lesions of the DNAB (left panels) or VNAB (right panels) on increases in alcohol self-administration induced by yohimbine for alcohol deliveries (A) and intake (B). ANOVA of alcohol deliveries with the between factors of site and neurotoxin condition with the within factor of yohimbine dose revealed significant main effects of yohimbine dose (F(2,64)=42.36, p<0.05) and a yohimbine dose × site × neurotoxin condition interaction (F(2,64)=3.93, p<0.05). For intake, ANOVA showed significant main effects of neurotoxin condition (F(1,32)=7.17, p<0.05), yohimbine dose (F(2,64)=58.35, p<0.05) and interactions of site × neurotoxin condition (F(1,32)=5.16, p<0.05), yohimbine dose × neurotoxin condition (F(2,64)=5.98, p<0.05) and yohimbine dose × site × neurotoxin condition (F(2,64)=3.55, p<0.05). To follow up these significant three-way interactions, two-way ANOVAs with the between factor of neurotoxin condition (vehicle, 6-OHDA) and within factor of yohimbine dose (vehicle, 0.625, 1.25 mg/kg) were subsequently done separately on the data from the DNAB and VNAB groups. For the DNAB groups, analysis of alcohol deliveries and intake revealed significant neurotoxin condition × yohimbine dose interactions (deliveries: F(2,32)=4.62; intake: F(2,32)=7.32, p's<0.05); post-hoc analysis showed that these effects were attributable to the higher number of alcohol deliveries and greater intake of alcohol in the DNAB group compared to the sham lesioned group at the 1.25 mg/kg dose of yohimbine (p<0.05).
Figure 2.
Effects of lesions of the DNAB (left panels) or VNAB (right panels) with 6-OHDA on yohimbine-induced increases in alcohol self-administration (A, B) and reinstatement (C). Data are presented as mean (±sem) alcohol deliveries (A), alcohol intake (B) and for reinstatement, active lever presses (C). Open bars, sham lesions; closed bars, 6-OHDA lesions. The doses of yohimbine used are on the x-axis of Figure C. Self-administration (A, B): * Significant differences from the vehicle condition (p<0.05). † Significant differences from the sham lesion group (p<0.05). Reinstatement (C): # significant difference from vehicle condition. n= 7-11 rats/group.
Similar analyses done on the VNAB groups showed significant effects of yohimbine dose for alcohol deliveries (F(2,32)=20.59, p<0.05) and intake (F(2,32)=23.56, p<0.05), as yohimbine significantly increased alcohol self-administration. For intake, there was also a significant effect of neurotoxin condition (F(1,32)=20.37, p<0.05), as, overall, animals with VNAB lesions had higher intake than those with sham lesions.
ANOVA of active responses revealed significant main effects of yohimbine dose (F(2,64)=43.67, p<0.05), and a yohimbine dose × site × neurotoxin condition interaction (F(2,64)=5.35, p<0.05), while for inactive responses, there was a significant yohimbine dose × site interaction (F(2,64)=3.52, p<0.05) (data not shown). For the DNAB groups, analysis of active responses in this way revealed significant neurotoxin condition × yohimbine dose interactions (F(2,32)=6.36, p<0.05). Analysis of active responses in the VNAB groups showed significant effects of yohimbine dose (F(2,32)=20.39), p<0.05) (not shown).
There were no significant main effects or interaction in the ANOVAs done on inactive lever pressing (p's>0.05).
b. Reinstatement
The effects of lesions of the DNAB (left panel) or VNAB (right panel) on yohimbine-induced reinstatement of alcohol seeking are shown in Figure 2C. Mixed ANOVA of active responses with the between factors of site and neurotoxin condition and the within factor of yohimbine dose revealed a significant effect of yohimbine dose (F(2,64)=34.03, p<0.05) as yohimbine induced a significant degree of reinstatement; this was not significantly affected by the lesions. Analysis of inactive responses showed a significant main effect of yohimbine dose (F(2,64)=5.47, p<0.05) and a significant yohimbine dose × site × neurotoxin condition interaction (F(2,64)=3.52, p<0.05), that occurred due to lower levels of responding the DNAB lesion group compared to the sham lesioned group at the 1.25 mg/kg dose (not shown).
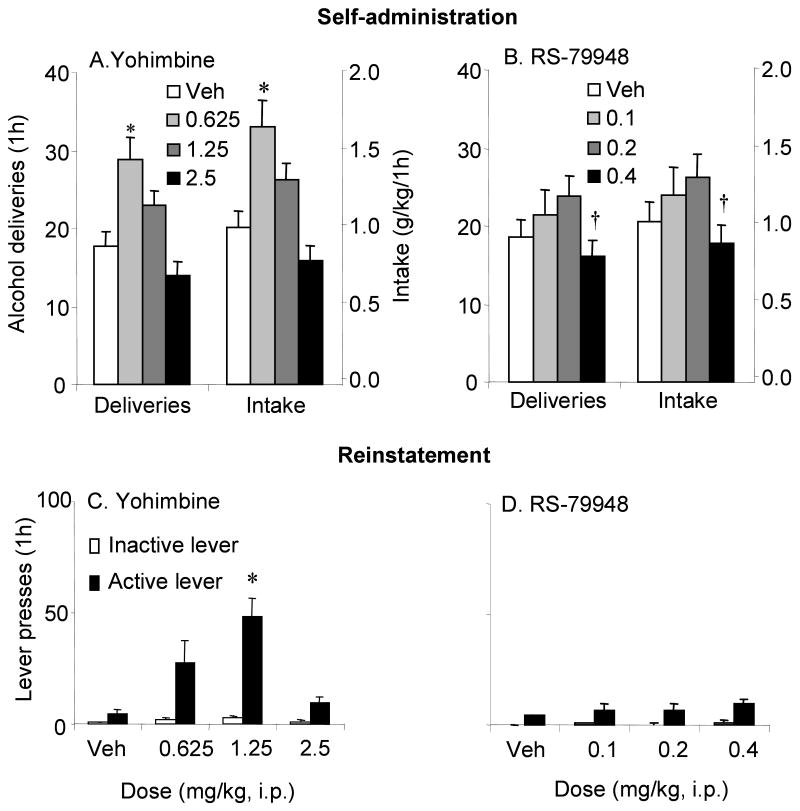
Experiment 2: Effects of yohimbine and RS-79948 on alcohol seeking
a. Self-administration
The top panel of Figure 3 shows the effects of yohimbine (A) and RS-79948 (B) on alcohol self-administration. The one-way ANOVAs with the within factor of yohimbine done on alcohol deliveries, intake and active lever responses revealed significant effects of yohimbine dose (deliveries: F(3,39)=10.99; intake: F(3,39)=11.59; active responses: F(3,39)=12.22, p's<0.05). Post hoc analyses revealed that for alcohol deliveries and intake the 0.625 dose was significantly greater than vehicle while both the 0.625 and 1.25 mg/kg doses of yohimbine significantly increased active responses compared to the saline condition. There was a significant effect of RS-79948 dose on number of alcohol deliveries (F(3,33)=3.31, p<0.05) and intake (F(3,33)=3.02, p<0.05), reflecting the fact that that the number of alcohol deliveries and intake in the 0.4 mg/kg dose condition were significantly lower than in the 0.2 mg/kg condition (p<0.05). As the active response data for the RS-79948 experiment was not normally distributed, it was analyzed with Friedman procedure. The analysis showed a significant effect of RS-79948 dose; Tukey post hoc analysis revealed significant differences between the 0.2 and 0.4 mg/kg conditions. Neither yohimbine nor RS-79948 significantly affected inactive lever responses (not shown).
Figure 3.
Effects of yohimbine (left panels) and RS-79948 (right panels) on alcohol self-administration (A, B) and reinstatement (C, D). Self-administration: Data are expressed as the number (±sem) of alcohol deliveries (A) and intake (B). In A and B, open bars represent vehicle, light grey the low dose, medium grey, the medium dose and black bars the high dose of respectively, yohimbine or RS-79948. The left and right y-axes of A and B represent alcohol deliveries and intake, respectively. * Significant differences from the vehicle condition (p<0.05). † Significant differences from the 0.2 mg/kg RS-79948 dose (p<0.05). Reinstatement: Data are expressed as the number of responses (±sem) on the inactive and previously active levers. Open bars, inactive lever; closed bars, active lever. * significant differences from the vehicle condition (p<0.05).n= 10-14 rats/drug.
b. Reinstatement
The lower panel of Figure 3 shows the effects of different doses of yohimbine (C) and RS-79948 (D) on reinstatement of alcohol seeking. The one way ANOVAs done with the factor of dose on active lever responses revealed significant effects in the yohimbine group (F(3,27)=9.54, p<0.05), since the 1.25 mg/kg dose of yohimbine produced significantly higher responding compared to the vehicle condition (p<0.05). RS-79948 did not significantly affect responding on the active lever. Neither drug significantly affected responding on the inactive lever during the reinstatement tests.
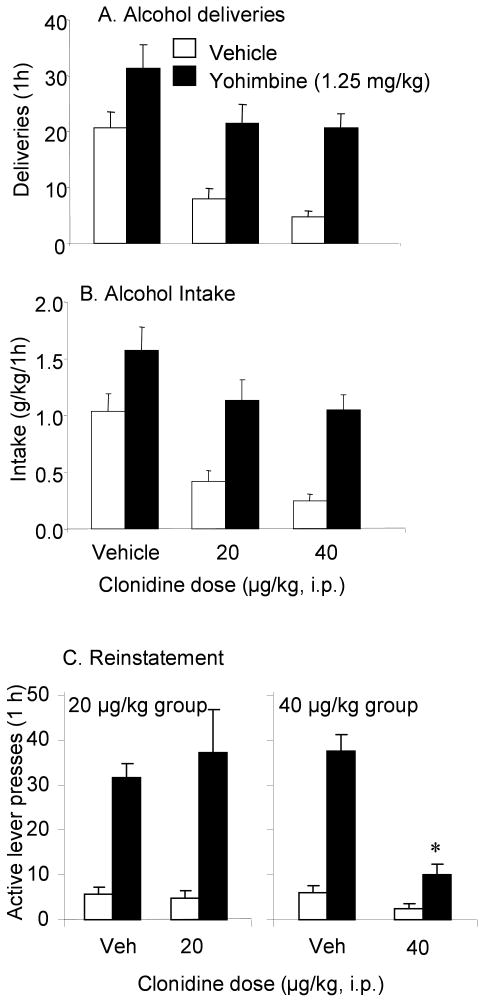
Experiment 3: Effects of clonidine on yohimbine-induced alcohol seeking
a. Self-administration
Figure 4 (A, B) shows the effects of clonidine on yohimbine-induced increases in self-administration. Mixed two way ANOVAs with the between factor of clonidine dose and the within factor of yohimbine dose done on alcohol deliveries (A) intake (B) and active lever responses (not shown), revealed significant main effects of clonidine dose and yohimbine dose (clonidine dose: deliveries: F(2,25)=8.74, intake: F(2,25)=7.61, active lever: F(2,25)=4.53; yohimbine dose: deliveries: F(1,25)=42.5, intake: F(1,25)=41.46, active lever: F(1,25)=27.35, p's<0.05) as overall, yohimbine increased self-administration and clonidine reduced it. The interaction of clonidine dose with yohimbine dose was not significant. ANOVA of inactive lever presses revealed no significant main effects or interaction (not shown).
Figure 4.
Effects of clonidine on yohimbine-induced increases in alcohol self-administration (A, B) and reinstatement (C). For self-administration, data are expressed as the number (±sem) of alcohol deliveries (A) and intake (B). For reinstatement, data are presented as the mean number of responses (±sem) on the previously active lever in the 20 and 40 μg/kg clonidine groups (left and right panels, respectively)(C). Open bars, vehicle; closed bars, yohimbine (1.25 mg/kg). Doses of clonidine are presented on the x-axis. * Significant difference from the vehicle condition (p<0.05). n= 8-13 rats/group.
b. Reinstatement
Figure 4C shows the effects of clonidine on yohimbine-induced reinstatement. Mixed three way ANOVA with the between factor of clonidine group and within factors of clonidine condition and yohimbine condition on active lever responses revealed a significant three way interaction (F(1,20)=11.49, p<0.05). To explore this interaction, two way repeated measures ANOVA with the within factors of yohimbine condition and clonidine condition were done separately for the 20 μg/kg group and the 40 μg/kg clonidine groups. In the 20 μg/kg group, there was a significant effect of yohimbine dose (F(1,35)=34.9, p<0.05) since yohimbine significantly increased responding, that was unaffected by administration of this dose of clonidine. In the 40 μg/kg group, there were significant effects of yohimbine condition (F(1,51)=62.51, p<0.05), clonidine condition (F(1,51)=37.16, p<0.05) and a significant yohimbine condition × clonidine condition interaction (F(1,51)=32.31, p<0.05), reflecting the fact that yohimbine significantly increased responding, and 40 μg/kg clonidine significantly attenuated the yohimbine-induced increase (p<0.05). There were no significant effects or interactions in the analysis of inactive lever presses (not shown).
Experiment 4: Effects of WAY 100,635 on yohimbine-induced alcohol seeking
a. Self-administration
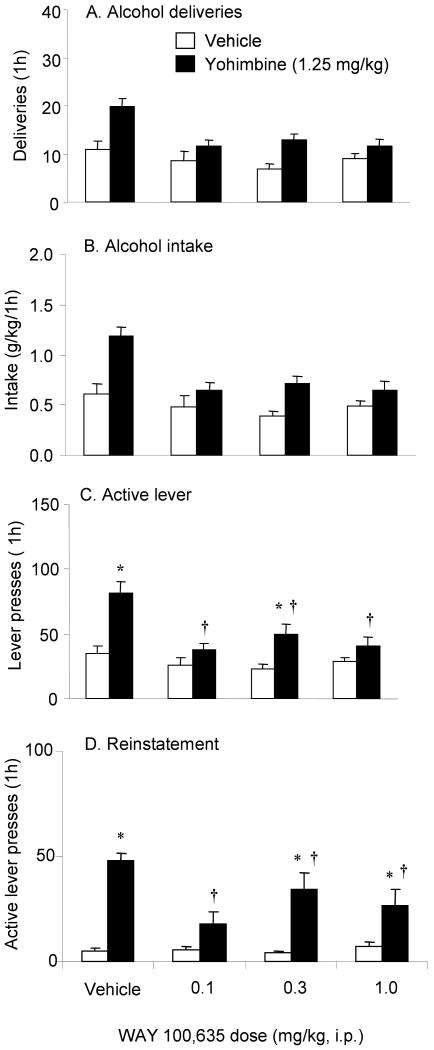
Figure 5 (A-C) shows the effects of WAY 100,635 on yohimbine-induced increases in alcohol seeking. Mixed two way ANOVAs with the between factor of yohimbine dose and the within factor of WAY 100,635 dose done on alcohol deliveries (A), intake (B) and active lever presses (C) revealed significant main effects of yohimbine dose and WAY 100,635 dose (yohimbine dose: deliveries: F(1,69)=16.07, intake: F(1,69)=19.97, active lever: F(1,69)=19.51; WAY 100,635 dose: deliveries: F(3,69)=6.02, intake: F(3,69)=7.78, active lever: F(3,69)=8.43, p's<0.05) as overall, alcohol self-administration was increased by yohimbine, and this was attenuated by WAY 100,635. The yohimbine dose × WAY 100,635 dose interaction was significant for active lever presses (F(3,69)=4.19, p<0.05). Post hoc analyses showed that all three doses of WAY 100,635 reduced yohimbine-induced increases in active lever presses. ANOVA of inactive lever presses revealed no significant main effects or interaction (not shown).
Figure 5.
Effects of WAY 100,635 on yohimbine-induced increases in alcohol self-administration (A-C) and reinstatement (D). For self-administration, data are expressed as the number (±sem) of alcohol deliveries (A), intake (B) and number of responses on the previously active lever (C). For reinstatement, data are presented as the mean number of responses (±sem) on the previously active lever (D). Open bars, vehicle; closed bars yohimbine (1.25 mg/kg). Doses of WAY 100,635 are on the x-axis. * Significant differences from the vehicle condition (p<0.05). † Significant differences from the yohimbine-WAY 100,635 vehicle condition (p<0.05). n= 10-14 rats/group.
b. Reinstatement
Figure 5D shows the effects of WAY 100,635 on yohimbine-induced reinstatement. Mixed two way ANOVA, with the between factor of WAY 100,635 dose and within factor of yohimbine dose on active lever responses revealed a significant WAY 100,635 dose × yohimbine dose interaction (F(3,32)=5.47, p<0.05), as WAY 100,635 attenuated the increases in active lever pressing induced by yohimbine. Post hoc analysis showed that animals in all three WAY 100,635 dose groups had lower levels of yohimbine-induced responding compared to animals administered yohimbine and the vehicle for WAY 100,635 (p's<0.05). There were no significant effects on inactive lever presses (not shown).
Discussion
The aims of the present report were to determine the involvement of the NA and 5-HT systems in the effects of yohimbine on alcohol seeking. We found that depletion of forebrain NA with 6-OHDA did not markedly affect yohimbine-induced increases in alcohol self-administration or reinstatement. Consistent with this, administration of a highly selective antagonist of alpha-2 adrenoceptors, RS-79948, did not mimic the effects of yohimbine on alcohol self-administration or reinstatement. The alpha-2 adrenoceptor agonist clonidine did not affect yohimbine-induced increases in self-administration, but did significantly attenuate its effects on reinstatement. These results clearly show that the effects of yohimbine on alcohol self-administration are not mediated via the NA systems. NA may, however, be involved to a certain extent in the effects of yohimbine on reinstatement, although this was not revealed with every type of manipulation we employed.
Since yohimbine also acts as an agonist of 5-HT1A receptors (Millan et al. 2000; Winter and Rabin 1992), we assessed whether the selective 5-HT1A antagonist, WAY 100,635 affected yohimbine-induced alcohol seeking. WAY 100,635 significantly reduced responding for alcohol during tests of self-administration and also significantly reduced yohimbine-induced reinstatement. These results therefore point to the importance of the 5-HT1A agonist properties of yohimbine in its stimulatory effects on alcohol seeking.
Our results suggest a modest role for NA, and a greater one for 5-HT, in the effects of yohimbine on alcohol seeking. This is supported by a number of studies showing that other behavioral effects of yohimbine are not mediated by alpha-2 receptors, and may rely more on its effects on 5-HT1A receptors. For example, yohimbine blockade of prepulse inhibition of the startle response is primarily mediated by 5-HT1A receptors (Powell et al. 2005). It has also been shown that the selective 5-HT1A agonist, 8-OH-DPAT, generalizes to yohimbine in tests of drug discrimination (Winter and Rabin 1992; 1993).
5-HT1A receptors located on the cell bodies of 5-HT neurons function as inhibitory autoreceptors. Previous work suggests that inhibition of the ascending 5-HT projections, especially those originating the median raphe nucleus, exert a facilitatory effect on alcohol seeking. We previously reported that intra-median raphe infusions of the 5-HT1A agonist 8-OH-DPAT or the inhibitory GABA A agonist muscimol reinstate alcohol seeking (Le et al. 2008; Le et al. 2002), and both systemic (Tomkins et al. 1994a) and intra-raphe injections of 8-OH-DPAT (Tomkins et al. 1994b) increase alcohol consumption. Systemic injections of yohimbine reduce the release of 5-HT in the forebrain (Millan et al. 2000) to a degree similar to that seen following intra-raphe infusions of doses of 8-OH-DPAT that are effective in inducing alcohol seeking (Bonvento et al. 1992). Yohimbine may therefore produce its effects on alcohol seeking via its inhibitory actions on 5-HT cell firing and release, through stimulation of 5-HT1A receptors on the 5-HT cell bodies in the median raphe nucleus.
Methodological considerations
In Experiment 1, the average magnitude of the depletion of hypothalamic NA by the VNAB 6-OHDA lesions appeared to be relatively small (30% of control infused with vehicle), in comparison to the DNAB lesion-induced depletion of NA in the hippocampus (mean 11.83% of vehicle). It might be suggested that such a degree of depletion is not enough to rule out a role for the VNAB projections in alcohol seeking. Arguing against this is the finding that VNAB lesions that depleted the hypothalamus to 54% of controls caused a significant attenuation of footshock-induced reinstatement of heroin seeking (Shaham et al. 2000).
We noted slight increases produced by 6-OHDA lesions of both the DNAB and VNAB on alcohol self-administration, suggesting that the lesions may have enhanced sensitivity to yohimbine. In view of the fact that most studies analyzing the effects of noradrenergic lesions on alcohol self-administration found either reductions in intake or no effect (Corcoran et al. 1983; Melchior and Myers 1976; Richardson and Novakovski 1978), these modest, lesion-induced differences noted in the present study are difficult to interpret. One speculation is that NA depletion potentiated yohimbine-induced self-administration secondary to lesion-induced effects on other neurotransmitter systems, for example 5-HT.
Lesions did not affect reinstatement of alcohol seeking induced by yohimbine, and the selective alpha-2 antagonist RS 79948 did not mimic the effects of yohimbine on reinstatement. Therefore, the significant effects of clonidine on yohimbine-induced reinstatement of alcohol seeking are somewhat puzzling. The dose that we used has been shown to be free of sedative effects in tests of motor activity, and in operant-based procedures examining reinstatement of heroin or cocaine seeking (De Luca et al. 1999; Erb et al. 2000; Shaham et al. 2000). A speculative explanation is that these effects of clonidine on yohimbine-induced reinstatement may be specific to reinstatement of alcohol seeking. This possibility will be examined in more detail in future studies.
Related to this issue, we found that clonidine did reduce operant responding for alcohol under baseline conditions. One possible explanation for this is that the sedative effects of alcohol and clonidine are additive. Despite this, it is important to note that clonidine did not block the increases in alcohol self-administration induced by yohimbine, indicating that the alpha-2 antagonist properties of yohimbine are not responsible for its effects on self-administration.
A rival explanation of the effects of WAY 100,635 on yohimbine-induced increases in alcohol self-administration is it affected motor performance. This can be ruled out, however, as previous work shows that WAY 100,635 at the doses we employed either do not affect locomotor activity, or increase it slightly (Bagdy et al. 2001; Jackson et al. 1998).
Summary and conclusions
We have presented data on the relative roles of the NA and 5-HT systems in the effects of yohimbine on alcohol self-administration and reinstatement. Depletion of forebrain NA did not affect alcohol self-administration or reinstatement, while administration of RS-79948 a highly selective antagonist of alpha-2 adrenoceptors, failed to mimic the affects of yohimbine. Clonidine, the most widely-used alpha-2 agonist, did not significantly affect yohimbine-induced increases in alcohol self-administration but did attenuate reinstatement at the highest dose employed. These results suggest that the effects of yohimbine on alcohol self-administration are not mediated via the NA systems. The NA systems, may, however be involved in the effects of yohimbine on reinstatement of alcohol seeking, although this is not revealed with every type of pharmacological test technique.
Yohimbine also has significant activity at the 5-HT1A receptor, on which it acts as an agonist. Activation of these receptors, that would reduce 5-HT cell firing and release, has been shown to increase alcohol drinking and induce reinstatement of alcohol seeking (Le et al. 2002). In keeping with this previous work, we showed in the present study that blockade of 5-HT1A receptors with WAY 100,635 significantly attenuated responding for alcohol and significantly reduced the effects of yohimbine on reinstatement of alcohol seeking. These results suggest that the 5-HT1A agonist properties of yohimbine are involved in its effects on both aspects of alcohol seeking.
Taken together with our previous work, the present results with the pharmacological stressor yohimbine further emphasize the role of the 5-HT systems in stress-induced alcohol seeking. We have shown that the release of the stress-related peptide corticotrophin-releasing hormone (CRF) in the serotonergic median raphe nucleus is involved in the reinstatement of alcohol seeking induced by another stressor, intermittent footshock (Le et al. 2002). We are currently extending these results with experiments aimed at determining whether CRF in this nucleus is involved in the effects of yohimbine on alcohol seeking.
To summarize, 5-HT may play the predominant role in the effects of yohimbine on alcohol seeking. NA plays a considerably smaller role that may come into play in reinstatement. While inhibition of 5-HT activity by peripheral administration of low doses or intra-raphe infusion of 8-OH-DPAT have been shown to enhance alcohol self-administration (Tomkins et al. 1994a; Tomkins et al. 1994b) as well as reinstatement of alcohol seeking (Le et al. 2000), the magnitude of the effects induced inhibition of 5-HT is much smaller relative to that induced by yohimbine (Le et al. 2005). It is therefore possible that robust effects of yohimbine on alcohol self-administration and reinstatement may be related to its combined effects on the 5-HT and NA systems.
Acknowledgments
This work was supported by a grant from the NIAAA (AA13108) to A.D. Lê. The authors would like to thank Dr. Yavin Shaham for his expert advice on this manuscript.
References
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Scatton B, Claustre Y, Rouquier L. Effect of local injection of 8-OH-DPAT into the dorsal or median raphe nuclei on extracellular levels of serotonin in serotonergic projection areas in the rat brain. Neurosci Lett. 1992;137:101–4. doi: 10.1016/0304-3940(92)90308-t. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P. Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J Abnorm Psychol. 1992;101:139–52. doi: 10.1037//0021-843x.101.1.139. [DOI] [PubMed] [Google Scholar]
- Corcoran ME, Lewis J, Fibiger HC. Forebrain noradrenaline and oral self-administration of ethanol by rats. Behav Brain Res. 1983;8:1–21. doi: 10.1016/0166-4328(83)90168-7. [DOI] [PubMed] [Google Scholar]
- De Luca LA, Jr, Nunes de Souza RL, Yada MM, Meyer EW. Sedation and need-free salt intake in rats treated with clonidine. Pharmacol Biochem Behav. 1999;62:585–9. doi: 10.1016/s0091-3057(98)00215-9. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- File SE. Aversive and appetitive properties of anxiogenic and anxiolytic agents. Behav Brain Res. 1986;21:189–194. doi: 10.1016/0166-4328(86)90236-6. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT(2C) Receptor Agonist Ro60-0175 Reduces Cocaine Self-Administration and Reinstatement Induced by the Stressor Yohimbine, and Contextual Cues. Neuropsychopharmacology. 2008;33:1402–12. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–96. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Nair SG, Golden SA, Gray SM, Uejima JL, Bossert JM, Shaham Y. Peptide YY3-36 decreases reinstatement of high-fat food seeking during dieting in a rat relapse model. J Neurosci. 2007;27:11522–32. doi: 10.1523/JNEUROSCI.5405-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth S, Sharp T. Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci. 1991;48:1779–86. doi: 10.1016/0024-3205(91)90216-x. [DOI] [PubMed] [Google Scholar]
- Jackson DM, Wallsten CE, Jerning E, Hu PS, Deveney AM. Two selective 5-HT1A receptor antagonists, WAY-100 635 and NDL-249, stimulate locomotion in rats acclimatised to their environment and alter their behaviour: a behavioural analysis. Psychopharmacology (Berl) 1998;139:300–10. doi: 10.1007/s002130050721. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney N, Kranzler HR, Charney DS. Specificity of ethanollike effects elicited by serotonergic and noradrenergic mechanisms. Arch Gen Psychiatry. 1994;51:898–911. doi: 10.1001/archpsyc.1994.03950110058008. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt DA, Tribe E, Erb S. Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Li Z, Fletcher PJ. Intra-median raphe nucleus (MRN) infusions of muscimol, a GABA-A receptor agonist, reinstate alcohol seeking in rats: role of impulsivity and reward. Psychopharmacology (Berl) 2008;195:605–15. doi: 10.1007/s00213-007-0943-4. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–73. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Shaham Y. Median raphe infusions of the 5-HT1a receptor agonist, 8-OH-DPAT, reinstate alcohol seeking in rats. Soc Neurosci Abstr. 2000;26:786. [Google Scholar]
- Le AD, Quan B, Juzytsch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–74. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–93. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Linseman MA. Alcohol consumption in free feeding rats: Procedure, genetic and pharmacokinetic factors. Psychopharmacology. 1987;92:254–261. doi: 10.1007/BF00177925. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–55. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Melchior CL, Myers RD. Genetic differences in ethanol drinking of the rat following injection of 6-OHDA, 5,6-DHT or 5,7-DHT into the cerebral ventricles. Pharmacol Biochem Behav. 1976;5:63–72. doi: 10.1016/0091-3057(76)90289-6. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Coge F, Galizzi JP, Boutin JA, Rivet JM, Dekeyne A, Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholaimine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Nair SG, Gray SM, Ghitza UE. Role of food type in yohimbine- and pellet-priming-induced reinstatement of food seeking. Physiol Behav. 2006;88:559–66. doi: 10.1016/j.physbeh.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Wingard JC. Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol Learn Mem. 2004;82:243–252. doi: 10.1016/j.nlm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Powell SB, Palomo J, Carasso BS, Bakshi VP, Geyer MA. Yohimbine disrupts prepulse inhibition in rats via action at 5-HT1A receptors, not alpha2-adrenoceptors. Psychopharmacology (Berl) 2005;180:491–500. doi: 10.1007/s00213-005-2193-7. [DOI] [PubMed] [Google Scholar]
- Richardson JS, Novakovski DM. Brain monoamines and free choice ethanol consumption in rats. Drug Alcohol Depend. 1978;3:253–64. doi: 10.1016/0376-8716(78)90079-0. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Robbins TW, Deeley RJ, Everitt BJ, Dunn LT, Wallace M, James WPT. Changes in body weight and food-related behaviour induced by destruction of the ventral or dorsal noradrenergic bundle in the rat. Neuroscience. 1983;10:1405–1420. doi: 10.1016/0306-4522(83)90122-7. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–9. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Paige S, Morgan CA, 3rd, Bremner JD, Krystal JH, Charney DS. Neurotransmitter alterations in PTSD: catecholamines and serotonin. Semin Clin Neuropsychiatry. 1999;4:242–8. doi: 10.153/SCNP00400242. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Higgins GA, Sellers EM. Low doses of the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH DPAT) increase ethanol intake. Psychopharmacology (Berl) 1994a;115:173–9. doi: 10.1007/BF02244769. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, O'Neill MF. Effect of 5-HT(1B) receptor ligands on self-administration of ethanol in an operant procedure in rats. Pharmacol Biochem Behav. 2000;66:129–36. doi: 10.1016/s0091-3057(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Sellers EM, Fletcher PJ. Median and dorsal raphe injections of the 5-HT1A agonist, 8-OH-DPAT, and the GABAA agonist, muscimol, increase voluntary ethanol intake in Wistar rats. Neuropharmacology. 1994b;33:349–58. doi: 10.1016/0028-3908(94)90065-5. [DOI] [PubMed] [Google Scholar]
- White DA, Birkle DL. The differential effects of prenatal stress in rats on the acoustic startle reflex under baseline conditions and in response to anxiogenic drugs. Psychopharmacology. 2001;154:169–176. doi: 10.1007/s002130000649. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. J Pharmacol Exp Ther. 1992;263:682–9. [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Antagonism of the stimulus effects of yohimbine and 8-hydroxydipropylaminotetralin. Pharmacol Biochem Behav. 1993;44:851–5. doi: 10.1016/0091-3057(93)90016-m. [DOI] [PubMed] [Google Scholar]