Abstract
Background and Aims
Transgenics are used to demonstrate a causal relationship between ethylene insensitivity of a seedling legume plant, the level of ethylene receptor gene expression, lateral root growth and Mesorhizobium loti-induced nodule initiation.
Methods
Lotus japonicus plants expressing the dominant etr1-1 allele of the Arabidopsis thaliana gene encoding a well-characterized mutated ethylene receptor were created by stable Agrobacterium tumefaciens transformation. Single insertion, homozygous lines were characterized for symbiotic properties.
Key Results
Transgenic plants were ethylene insensitive as judged by the lack of the ‘Triple Response’, and their continued ability to grow and nodulate in the presence of inhibitory concentrations of ACC (1-aminocyclopropane-1-carboxylic acid; an ethylene precursor). Transgenic plants with high insensitivity to ACC had significantly fewer lateral roots and exhibited increased nodulation while showing no altered nitrate sensitivity or lack of systemic autoregulation. Whereas ACC-insensitive shoot growth and nodulation were observed in transformants, root growth was inhibited similarly to the wild type. Increased nodulation was caused by increased infection and a seven-fold increase in nodules developing between xylem poles. Bacteroid numbers per symbiosome increased about 1·7-fold in ethylene-insensitive plants.
Conclusions
The study further demonstrates multiple roles for ethylene in nodule initiation by influencing root cell infections and radial positioning, independent of autoregulation and nitrate inhibition of nodulation.
Key words: Ethylene insensitivity, Lotus japonicus, symbiosis, phytohormone, nodulation, signal transduction
INTRODUCTION
Legume plants develop specialized nitrogen-fixing root structures through a symbiotic relationship with compatible bacteria, generally referred to as ‘rhizobia’ (Stacey et al., 2006). Reciprocal communication between plant and bacterium as well as environmental factors regulate the formation and function of such nodules (Caetano-Anollès and Gresshoff, 1991; Gresshoff, 1993, 2003; Oldroyd et al., 2001; Kinkema et al., 2006); many genetic and molecular components of this symbiosis have been defined (for example Krusell et al., 2002; Nishimura et al., 2002; Radutoiu et al., 2003; Searle et al., 2003; Ané et al., 2004), allowing predictions of chemical functions based on gene discovery.
The ontogeny of nodulation coexists with other developmental plant processes (Hirsch, 1992; Beveridge et al., 2003, 2007; Ferguson and Mathesius, 2003) and thus plant regulatory processes, such as those facilitated by phytohormones, play important roles. Physiological and developmental effects of phytohormones on nodulation have long been recognized and are extensively described (Guinel and LaRue, 1992; Penmetsa and Cook, 1997; Caba et al., 1999; Wopereis et al., 2000; Ma et al., 2002; Nukui et al., 2004; Biswas et al., 2009). However, the mechanisms of phytohormone regulation of nodule initiation remain relatively obscure. Fundamental to this issue is the question of whether a phytohormone affects a process through a direct regulatory effect, or influences a general plant growth capability, which in turn affects or effects the specific development.
The gaseous plant hormone ethylene and its precursor ACC (1-aminocyclopropane-1-carboxylic acid) are some of the earliest phytohormonal signals associated with the regulation of nodule number (Guinel and LaRue, 1992; Suganuma et al., 1995; Schmidt et al., 1999; Nukui et al., 2000; Sugawara et al., 2006). For example, treatment with ethylene or ACC inhibited nodulation in a wide range of legumes. In contrast, exposure to ethylene action ‘inhibitors’, for example AVG (aminoethoxyvinylglycine), and silver ions increased nodulation in many tested legumes. Such inhibitors also partially restored nodulation in selected low-nodulating pea mutants (Guinel and LaRue, 1992).
Analysis of ethylene action on nodulation has been aided by mutants and transgenics (Heidstra et al., 1997; Penmetsa et al., 2003, 2008; Nukui et al., 2004). Endogenous ethylene reduced the formation of nodule primordia (Zaat et al., 1989) and the accumulation of mRNA of ACC oxidase (the enzyme catalysing the last step of ethylene biosynthesis) in cells opposite phloem poles of Vicia sativa (Heidstra et al., 1997). As nodule primordia normally form opposite protoxylem poles, these authors speculated that ACC-derived ethylene produced opposite phloem suppressed nodule-associated cell division, possibly via modulation of critical localized cytokinin biosynthesis (cf. Vogel et al., 1998; Wopereis et al., 2000). In a pioneering study, Cook's group (Penmetsa and Cook, 1997; Penmetsa et al., 2008) isolated an ethylene-insensitive legume mutant and recognized the ‘sickle’ phenotype, stemming from increased infection and nodulation in the zone free of autoregulation of Medicago truncatula seedlings. The affected gene has now been shown to be orthologous to the arabidopsis EIN2 gene (Penmetsa et al., 2008). Ethylene attenuated the response of Medicago root hairs to lipo-oligosaccharide Nod-factor, leading to altered infection success (Oldroyd et al., 2001). These observations strengthened the correlative nexus between ethylene and nodule inhibition.
In contrast, moderately ethylene-insensitive mutants of soybean were not altered in their nodulation response (Schmidt et al., 1999). Also, the supernodulating soybean mutant nts382 (Carroll et al., 1985a, b), lacking autoregulation of nodulation through the mutational loss of a CLAVATA1-related leucine-rich repeat (LRR) receptor kinase (Searle et al., 2003), was not insensitive to ethylene (Caba et al., 1999) indicating that ethylene may not be directly involved in the control of nodulation via autoregulation. Like nts382, a hypernodulating mutant of Lotus japonicus (har1-1; cf. Krusell et al., 2002; Nishimura et al., 2002), mutated in the same LRR receptor kinase as nts382 of soybean, was ACC sensitive (Wopereis et al., 2000). To test the involvement of ethylene reception by transgenic approaches, Nukui et al. (2004) transferred a mutant ethylene receptor gene (CmERS1) from melon into L. japonicus and found that infection and nodule initiation increased.
One of its major ethylene receptor genes was cloned in Arabidopsis thaliana (Atetr1) and found to encode a two-component histidine protein kinase (reviewed in Chang et al., 1993; Chang and Shockey, 1999; Gamble et al., 2002). A single mutation at residue 65 (cysteine to tyrosine) caused a dominant mutation (etr1-1) leading to insensitivity in mutants (Chang et al., 1993). The paradigm for ethylene regulation in Arabidopsis was effectively transferred to other plant systems (Lanahan et al., 1994; Wilkinson et al., 1997; Klee, 2004), although processes such as nodulation could not be evaluated.
To further extend our knowledge of ethylene perception for Rhizobium-induced nodulation, we constructed ethylene-insensitive L. japonicus plants expressing this well-characterized Arabidopsis etr1-1 gene and showed that beside early infection and nodule initiation effects, ethylene also affects late symbiotic development.
MATERIALS AND METHODS
Plant transformation and culture
Lotus japonicus ecotype Gifu B-129-S10 (Handberg and Stougaard, 1992; Jiang and Gresshoff, 1997) was used for all transformation experiments and as a wild-type control. Transgenic plants were constructed (Stiller et al., 1997; Lohar et al., 2001) by co-cultivating dark-grown severed hypocotyl explants with Agrobacterium tumefaciens strains LBA4404 or GV3101. The etr1-1 cDNA construct was kindly provided by Dr C. Nessler (Texas A&M University); etr1-1 cDNA from Arabidopsis thaliana was cloned into pRTL2 vector behind dual 35S promoter and the 5′ untranslated leader of tobacco etch virus for high expression in higher plants. The promoter-leader cDNA-terminator portion of this plasmid was excised with HindIII, and cloned into the HindIII site of the binary vector pBIN19.
The vector was electroporated into both A. tumefaciens LBA4404 and GV3101. Plant transformants were selected on B5 medium (Gamborg, 1970) containing 5 mg L−1 geneticin base. Separated callus was used to define independent lines. Plants were grown under greenhouse conditions with supplemental light (16 h day/8 h night) and allowed to produce seed by selfing. Selfed seed lines were tested for phenotypic segregation for hypocotyl elongation by germinating on 25 µm ACC (see Table 1). T1 segregants segregated at a 3 : 1 (insensitive vs. sensitive) ratio. Further selection at the T2 stage identified lines that did not segregate for sensitivity to 25 µm ACC, and such lines were considered to be homozygous. Southern hybridization confirmed transgene integration in these lines. The homozygous independent transgenic lines with confirmed transgene integration were 4-6, 15-1, 23-13, 50-4, 50-7, 120-1, 125-13, 129-3 and 125-13.
Table 1.
Genetic segregation of ethylene-insensitive phenotype in transgenic Lotus japonicus expressing the dominant Atetr1-1 transgene
| Line | ACC insensitive (IS) | ACC sensitive (SE) | Ratio (IS : SE) | χ2 |
|---|---|---|---|---|
| 129 | 37 | 15 | 2·5 : 1 | 0·41 |
| 4 | 79 | 22 | 3·6 : 1 | 0·56 |
| 15 | 14 | 5 | 3·1 : 1 | 0·02 |
| 23 | 102 | 34 | 3·0 : 1 | 0·00 |
| 125 | 108 | 37 | 2·9 : 1 | 0·02 |
| 50 | 121 | 39 | 3·1 : 1 | 0·03 |
| 120 | 61 | 28 | 2·17 : 1 | 2·15 |
Plants were grown on B&D agar plates (25 µm ACC) and scored for seedling growth response after 7 d growth in the dark.
Southern and northern hybridization
Leaf tissue (approx. 1 g) was ground to a powder in liquid nitrogen and DNA was isolated as described (Dellaporta et al., 1983). For Southern blots, 8 µg genomic DNA was digested by EcoRI and separated on 0·8 % agarose gels then transferred to Zeta Probe GT Nylon membranes (Bio-Rad).
Total RNA was isolated by grinding about 100 mg of leaf tissue in 1·5-mL Eppendorf tubes (Verwoerd et al., 1989). About 20 µg total RNA was separated on 1·0 % agarose gels containing 17 % formaldehyde (Sambrook et al., 1992), and transferred to Zeta Probe GT Nylon membranes.
A 2·2-kb NcoI-KpnI fragment containing the Atetr1-1 cDNA was used as a probe. Actin1 cDNA was used as a loading control in northern hybridizations. Random priming labelled probes as per the manufacturer's instructions (Promega Corp.) to a specific activity of approx. 1 × 108 counts min−1. Pre-hybridization and hybridization buffer contained 0·25 m sodium phosphate, pH 7·2, and 7 % sodium dodecyl sulfate (SDS). Hybridizations were carried out at 65 °C overnight followed by washing (once in solution I: 20 mm sodium phosphate, pH 7·2, 5 % SDS; and once in solution II; 20 mm sodium phosphate, pH 7·2, 1 % SDS; 45 min each at 65 °C).
Transgenic plant analysis
ACC sensitivity tests
Agar plates (1·4 %; sealed with Parafilm, and incubated upright in stacks of 10–12 plates) with half-strength B5 or B&D salts (Stiller et al., 1997) containing 25 µm ACC (added filter-sterilized) were used to germinate putative transgenic seeds in the dark for 7 d for ethylene insensitivity assay and segregation analysis. Hypocotyl length of at least 15 seedlings was determined for each independent line. Some lines had twisted/coiled hypocotyls in the presence or absence of ACC. These lines were always highly insensitive to ethylene and showed reduced hypocotyl thickness.
Infection and nodulation studies
Two-week-old seedlings grown in 1× B&D medium in vermiculite were inoculated with Mesorhizobium loti strain NZP2235 carrying a constitutive hemA:lacZ gene fusion to examine nodulation by lacZ staining (Boivin et al., 1990). Each nodulation experiment was repeated three times with 15–30 seedlings per line per experiment. Seedlings were uprooted 2 weeks after inoculation, and excised roots were stained. Whole stained roots were observed under a stereomicroscope, where infection threads, nodules and nodule foci were counted. Any externally visible bump was counted as a nodule. Foci therefore are internal to the root surface. The mean nodule and focus number was calculated for each line separately, and 95 % confidence intervals were estimated individually.
Nodulation sensitivity tests used 3-day-old pre-germinated seedlings on B&D agar plates inoculated with strain NZP2235 and proceeded for 32 d before determination of nodule number and plant growth characteristics. Plates were either sealed with Parafilm or vented daily to reduce ethylene accumulation in the plate. Plates were incubated upright in stacked formation with cardboard layers separating plates in a growth chamber (23 °C, 16 h light).
Histology and microscopy
For light microscopy, root samples were processed as described (Graham and Joshi, 1995). Specimens were fixed in HistoChoice (Amresco Inc., Solon, OH, USA), dehydrated in an isopropanol series, infiltrated with paraffin (Paraplast, MP56 C) and cast in specimen blocks. Ribboned sections were cut at 15 µm on a rotary microtome (Reichter-Jung, Vienna, Austria). Sections were observed under a light microscope and the number of nodule/nodule foci forming between or opposite protoxylem poles was recorded.
For electron microscopy, root segments were fixed in phosphate-buffered (pH 6·8) 3 % glutaraldehyde for 90 min, then post-fixed for 90 min in phosphate-buffered 2 % osmium tetroxide, all at room temperature. Samples were then dehydrated in an acetone step gradient and embedded in Spurr's resin. The tissue was sectioned (100 nm) and post-stained with uranyl acetate and lead citrate, before viewing with an Hitachi H-600 transmission electron microscope.
RESULTS
Arabidopsis etr1-1 confers ethylene insensitivity in transgenic L. japonicus
Selfed seeds from the primary transgenic plants transformed with the Arabidopsis etr1-1 gene were germinated on 25 µm ACC in the dark for 7 d and were observed for segregation of the ‘Triple Response’ phenotype. Segregation supported dominant monogenic inheritance for many independent transgenic lines tested (Table 1). Focus was on lines with Mendelian segregation ratios (insensitive vs. sensitive) suggesting single etr1-1 insertions (later confirmed by Southern blotting). Several ethylene-insensitive T1 plants from different T0 lines were selfed to produce homozygous T2 seeds. Eight stable lines originating from seven independent primary transformants with a range of ethylene insensitivity were selected for further phenotypic characterization. These lines were 120-1, 50-7, 125-13, 50-4, 4-6, 129-3, 23-13 and 15-1. (NB: line 129-3 hereafter is labelled ‘LjETR1-1’ depicting the homozygous dominant transgene condition and its ethylene-insensitive phenotype mediated by the Atetr1-1 gene.)
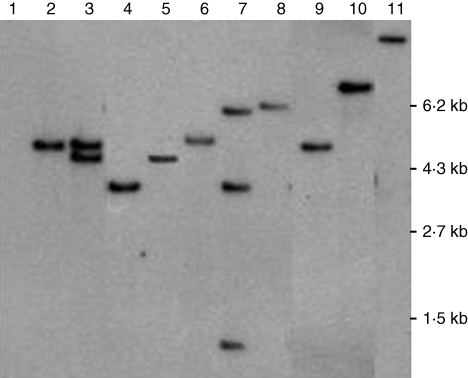
Seven of the eight lines contained a single T-DNA insertion, and one (4-6), characterized by maximum coiling of hypocotyls, contained three insertions (Fig. 1). Lines 50-7 and 50-4 were independent T-DNA segregants of the same T1 line 50, which had two T-DNA insertions (labelled 50-6 in Fig. 1). Southern blot analyses were conclusive, revealing independent T-DNA insertions but failed to detect the endogenous L. japonicus ETR1 homologue (about 84 % identical).
Fig. 1.
Atetr1-1 transgene copy number in transgenic Lotus japonicus. Approximately 8 µg genomic DNA was blotted on Nylon membrane after digestion with EcoRI, and probed by Atetr1-1 cDNA sequence. Lane 1 = wild-type Gifu, 2 = 50-7, 3 = 50-6, 4 = 23-3, 5 = 50-4, 6 = 15-1, 7 = 4-6, 8 = 129-3 (=LjETR1-1), 9 = 120-1, 10 = 125-3, 11 = transformation vector as positive control.
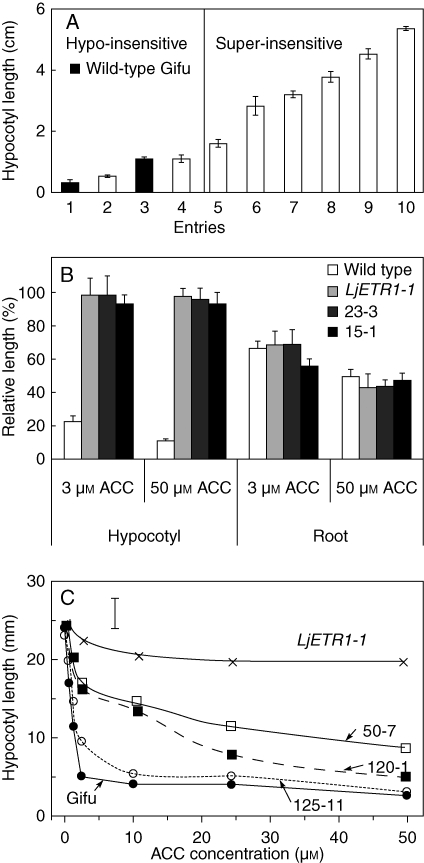
Transgenic lines differed in ethylene insensitivity, as evidenced by the difference in hypocotyl length (Fig. 2A), but not root length (Fig. 2B), after germination in the dark in the presence of ACC.
Fig. 2.
Differential ethylene insensitivity of L. japonicus lines transgenic for Atetr1-1. (A) Hypocotyl length of seedlings germinated on 25 µm ACC for 7 d in the dark. 1 = Wild-type Gifu, 2 = 120-1, 3 = wild-type Gifu germinated without ACC, 4 = 50-7, 5 = 125-3, 6 = 4-6, 7 = 50-4, 8 = 129–13, 9 = 23-3, 10 = 15-1. Transgenic lines with hypocotyl length significantly higher than the wild type grown on ACC but lower or the same as the wild-type grown without ACC are grouped as ‘hypo-insensitive’. Lines with hypocotyls significantly longer than the wild type grown without ACC are grouped as ‘hyper-insensitive’ lines. (B) Hypocotyl and root growth sensitivity of the wild type and three independent transgenic lines at 3 and 50 µm ACC. The relative hypocotyl and root length are shown as percentage of the wild type grown in the absence of ACC. (C) Hypocotyl length of different transgenic lines compared with wild-type Gifu at different ACC concentrations. In all experiments, measurement was made on at least 15 seedlings for each treatment; error bars indicate s.e. in (A, B), and LDS in (C).
Stable ethylene-insensitive lines were tested for altered sensitivity to varying concentrations of ACC in etiolated seedling growth assays (Fig. 2C). Lines with longer hypocotyls were generally also characterized by reduced hypocotyl thickness. Additionally, lines 50-4, 4-6, LjETR1-1, 23-3, and 15-1 had twisted and coiled hypocotyls independent of the presence of ACC (Fig. 3E). By comparing the degree of hypocotyl extension of dark-grown seedlings of transgenic lines germinated in the presence of 25 µm ACC with Gifu seedlings germinated in the absence of ACC, lines could be divided into two groups. Lines 120-1 and 50-7, which had hypocotyl length less than Gifu without ACC, were considered ‘hypo-insensitive’. Lines LjETR1-1, 50-4, 4-6, 125-3 (non-coiled hypocotyl), 23-3 and 15-1 (coiled hypocotyls) with extension larger than Gifu in the absence of ACC, were termed ‘super-insensitive’.
Fig. 3.
Growth and nodulation phenotypes of ethylene-insensitive transgenic lines of L. japonicus compared with the wild type Gifu. Floral and pod phenotypes of wild-type Gifu (A, C) and ethylene-insensitive L. japonicus (B, D) compared at the same developmental stage. Note the persistent petals and curved pods in ethylene-insensitive line. (E) Twisting of hypocotyl of transgenic line LjETR1-1 (left) compared with the hypocotyls of wild-type Gifu (right). Seedlings were grown without ACC for 1 week in the dark. (F) A nodulation zone in wild-type Gifu. (G) Increased nodulation in the nodulation zone of insensitive line LjETR1-1; note the sickle shape of the nodulation zone. Images were taken 2 weeks after inoculation with Mesorhizobium loti strain NZP2235.
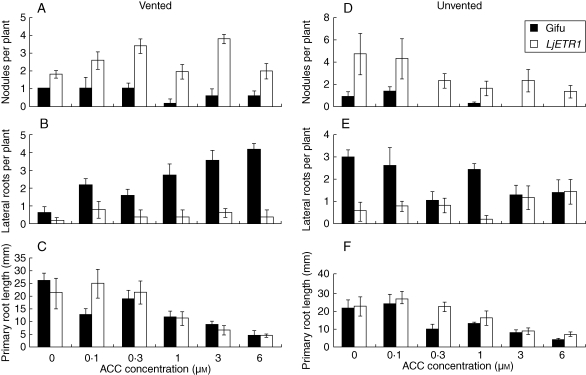
Line LjETR1-1, with high seedling insensitivity to ACC, maintained the ability to nodulate on either 3 or 6 µm ACC (Fig. 4A, D) in agar plate assays, whereas Gifu nodulation was severely inhibited. However, primary root growth was inhibited by ACC to the same degree as in Gifu whether plates were vented or sealed (Fig. 4C, F). Ethylene-insensitive lines developed significantly fewer lateral roots (Fig. 4B, E). Venting agar plates lowered ACC toxicity. Nodule inhibition was more pronounced in sealed plates and lateral root numbers were more stimulated in vented plates of Gifu than LjETR1-1.
Fig. 4.
Nodulation and root growth characteristics of etr1-1 transgenics (line LjETR1-1). Seedlings were inoculated with Mesorhizobium loti NZP2235 and grown for 36 d at different ACC concentrations. (A–C) Plants underwent ‘vented’ treatment (plates opened for 10 s in laminar flow every second day). The ‘unvented’ plates (D–F) were kept sealed until the end of the experiment. All error bars are s.e. (A, D) Nodule numbers per plant on day 36; (B, E) lateral root number per plant on day 36; (C, F) primary root growth (mm).
Floral petals of super-insensitive plants persisted even after ripening of the pod whereas those on Gifu and hypo-insensitive plants generally dried and abscised (Fig. 3A, B). Petals of super-insensitive plants occasionally adhered to both the base and the tip of the pod, resulting in a pod curvature (Fig. 3D). In contrast, petals in the wild type were carried on the tip of the pod, or lost altogether without any curvature (Fig. 3C). Lotus japonicus ethylene-insensitive plants took longer to flower and the pods took longer to ripen than the wild type (30–50 % longer). For example, lines LjETR1-1, 4-6, 23-3 and 50-7 flowered by 64 ± 5 DAP, while Gifu flowered at 52 ± 4 DAP (days after planting, with long day period).
Transgene RNA expression level is correlated with ethylene insensitivity
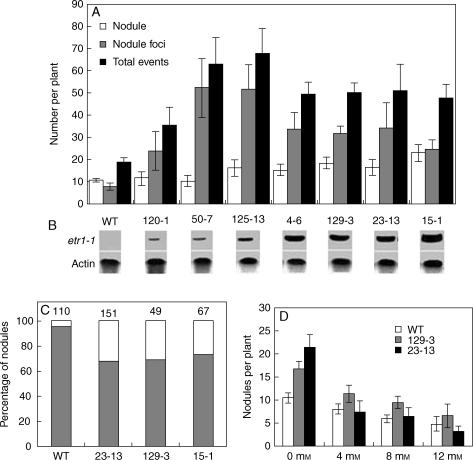
Transgene RNAs were measured semi-quantitatively in stable transgenic lines of L. japonicus by northern hybridization using an Atetr1-1-specific probe. Lines with higher insensitivity in the triple response assay (e.g. LjETR1-1, 15-1, 23-3, 4-6) tended to have the highest Atetr1-1 transcript level (Fig. 5B) while lines with marginal insensitivity, like 120-1, showed the lowest level. The ranking order for transgene expression correlated positively (r2 = 0·96) with the phenotypic ranking based on seedling ACC responses and nodulation tests (r2 = 0·58).
Fig. 5.
Nodulation characteristics of ethylene-insensitive transgenic Lotus japonicus lines. (A) Nodule foci, nodules and total events (nodule foci + nodules) per plant. Counting was done 2 weeks after inoculation. Error bars represent 95 % confidence intervals of the means. (B) Atetr1-1 transcript levels in different lines. Northern blot with 20 µg total RNA for each line was probed with Atetr1-1 sequence. The level of actin expression shows the loading control. (C) Positional control of nodule focus development in ethylene-insensitive Lotus japonicus. The number at top of a bar is the number of total nodules observed for that particular line. The dotted portion of the bar represents the proportion of nodules originating between protoxylem poles and the brick-pattern portion that originating opposite protoxylem poles. (D) Nitrate inhibition of nodulation in ethylene-insensitive L. japonicus lines. Nodules were counted 2 weeks after inoculation. Nitrate was supplied as KNO3. Error bars represent 95 % confidence intervals of the means. WT = wild-type Gifu.
AtETR1-1 confers hyperinfection and increased nodulation, but not classical hypernodulation in L. japonicus
Nodules and nodule foci were counted in the seven independent insensitive lines and the wild-type control grown in the absence of exogenous ACC. In hypo-insensitive lines 120-1 and 50-7, nodule number was statistically the same as in the wild type (Fig. 5A). Larger numbers of root nodules compared with Gifu developed on plants with higher ethylene insensitivity such as lines LjETR1-1, 125-3, 4-6, 23-3 and 15-1 (Fig. 5A). Nodule number per plant was positively correlated with the degree of hypocotyl extension (Fig. 2) and the Atetr1-1 mRNA levels (Fig. 5B) detected in transgenic plants. The number of nodule foci was higher in all insensitive lines compared with the wild type, while the total number of nodulation events (i.e. nodule foci plus nodules) was statistically the same in all super-insensitive lines but was significantly higher than in the wild type and hypo-insensitive lines (Fig. 5A).
The nodule number per nodulation zone (a stretch of root with contiguous nodules usually corresponding to the 2–3 cm behind the root tip at the time of inoculation) was counted in five ethylene-insensitive lines (120-1, 125-3, 4-6, 50-4 and LjETR1-1) and Gifu. Ethylene-insensitive lines possessed more nodules per nodulation zone (120-1 = 2·9 ± 0·35, 125-3 = 4·0 ± 0·27, 4-6 = 5·2 ± 0·51, 50-4 = 6·3 ± 0·78, LjETR1-1 = 6·8 ± 0·77) than the wild type (1·9 ± 0·21). Nodule number in this zone positively correlated with the hypocotyl lengths (Fig. 2) of lines (r2 = 0·99), suggesting that most of the nodule increase stemmed from this region and not an extension of the nodulation zone as seen in hypernodulation mutants (Carroll et al., 1985a; Wopereis et al., 2000). However, nodule size (mg per nodule; data not shown) decreased with increased ethylene insensitivity.
Ethylene also acts at later stages of nodule symbiosis
The morphology of bacteroids observed by transmission electron microscopy in the mature zone of two independent insensitive lines (125-3 and 50-4) and the number of uninfected cells in the central zone was similar to those of Gifu, but the number of bacteroids per symbiosome in insensitive lines was significantly higher than in Gifu. For example, line 125-3 had an average bacteroid number per symbiosome of 2·1 ± 0·2 (n = 461) with a range of 1 to 6. Similarly, in line 50-4, the average was 2·4 ± 0·2 (n = 188) with a range of 1 to 6, compared with the wild-type value of 1·4 ± 0·1 with a range of 1 to 4 (n = 107).
ACC and ethylene are positional signals for nodule initiation
Paraffin-embedded sections (7 µm thickness) of the nodulated roots of the wild type and three super-insensitive lines (LjETR1-1, 23-3 and 15-1) were scored by light microscopy for the percentage of nodules originating opposite as well as between protoxylem poles (Fig. 5C). In insensitive lines, 27–32 % of the nodules formed between protoxylem poles compared with only 4·5 % in the wild type, representing a seven-fold increase in the zone of permitted cell division response.
Nitrate sensitivity of nodulation in ethylene-insensitive L. japonicus lines
Nodulation of wild type Gifu has a high tolerance to inhibitory levels of nitrate whether tested in soil (Hussain et al., 1999) or on agar plates (present study), as 12 mm nitrate, which normally would limit soybean nodulation by 90–95 %, still permitted nodule initiation (though smaller in size and unable to fix nitrogen) (Fig. 5D).
The effect of nitrate addition on nodule number was examined in two super-insensitive lines (LjETR1-1 and 23-3) and Gifu. Addition of 4 mm nitrate (supplied as potassium nitrate) partially inhibited nodule formation in both ethylene super-insensitive lines and the wild type. At 8 and 12 mm the proportional inhibition of nodule number was indistinguishable for the ethylene-insensitive transgenics and Gifu.
DISCUSSION
The well-characterized Arabidopsis ethylene insensitivity receptor gene Atetr1-1 under the control of the constitutive CaMV 35S promoter was expressed in the legume Lotus japonicus to demonstrate that ethylene (or ACC) is a negative regulator of both early and late stages of the nodulation symbiosis. Both nitrate control and autoregulation of nodulation (AON; Gresshoff, 1993) were not directly associated with ethylene insensitivity. Significantly, ethylene was needed for lateral root formation, possibly reflecting a nexus between auxin and ethylene signalling (Ferguson and Mathesius, 2003). The ethylene-insensitive transgenics of L. japonicus described here were similar in phenotype to the chemically induced ‘sickle’ mutant of Medicago truncatula (Penmetsa and Cook, 1997), even producing the ‘sickle’-shaped nodulation and root curvature patterns. Significantly, sickle is altered in the EIN2 gene, functioning after the ETR1 receptor.
Parallel work in L. japonicus using a less well-characterized ethylene receptor gene (of the ERS1 type distinct from the two-component histidine kinase type ETR1 receptor) from melon demonstrated that transgenic alteration of ethylene perception altered the infection and nodulation phenotype (Nukui et al., 2004). We took advantage of the well-characterized etr1-1 allele from Arabidopsis to construct L. japonicus transgenic plants (in the same parent cultivar as Nukui et al., 2004) insensitive to ethylene. Stable seed material is available from the corresponding author upon request.
The level of ethylene insensitivity in Atetr1-1 transgenic plants varied in independent Lotus lines. A quantitative difference for ethylene insensitivity in etr1-1 transformants ranging from no to extreme ethylene insensitivity was also reported in Arabidopsis by Chang et al. (1993), who suggested that partial ethylene insensitivity of transformants was caused by low expression of etr1-1. Positional effects, DNA methylation or incomplete transfection could cause such differences. As Lotus transgenics in our studies showed an excellent correspondence between ethylene insensitivity of seedling growth, nodule primordium induction and Atetr1-1 transcript levels, the level of transgene expression itself most likely explains the variation in ethylene insensitivity.
Several transgenic lines showed super-insensitivity to ethylene as evidenced by a decreased hypocotyl inhibition of seedlings in the presence of ACC than the wild type without ACC. Ethylene-insensitive mutants with similarly high levels of insensitivity have not previously been reported. The super-insensitive L. japonicus plants had twisted and coiled hypocotyls resembling the epinastic response of leaf petioles to exogenous ethylene (Lanahan et al., 1994).
Increased delay of abscission and senescence of petals in ethylene-insensitive L. japonicus lines mirrored other ethylene-insensitive plants such as tomato, petunia and Medicago truncatula (Wilkinson et al., 1997; Penmetsa et al., 2003). etr1-1 Arabidopsis mutants exhibited delayed flowering (Bleecker et al., 1988) similar observations here in ethylene-insensitive L. japonicus.
As the number of bacteroids per symbiosome was twice as high in ethylene-insensitive plants as in the wild type, ethylene may regulate terminal rhizobial cell division directly, or slow plant stress responses (Boller, 1991; Goormachtig et al., 2004). According to Szczyglowski et al. (1998), an L. japonicus symbiosome on average contains 1·2 ± 0·5 (n = 340) endosymbiotic bacteria with a range of 1–4. This value is in good agreement with our estimate (1·4 ± 0·1) for wild-type nodules, although bacteroid numbers per symbiosome were significantly higher (2·1–2·4) in nodules of ethylene-insensitive lines.
A significant increase in the number of nodule foci in ethylene-insensitive plants compared with the wild type was observed. This finding is in agreement with Penmetsa and Cook (1997) and Nukui et al. (2000) who noted increased infection and increased nodulation in a discrete crescent-shaped zone in the chemically induced ‘sickle’ mutant of M. truncatula and ethylene receptor Cm-ERS1 transgenics of L. japonicus. Therefore, we expected that transgenic plants insensitive to both endogenous and exogenous ethylene might form more nodule foci. This indeed was the case. Lotus transgenics with different levels of seedling insensitivity to ethylene, and correlated mRNA expression levels, varied in the number of nodule foci. The total number of nodulation events (proper ‘externally visible’ nodules plus nodule foci) per plant increased with ethylene insensitivity ultimately reaching a plateau.
Super-insensitive lines developed more nodules than the wild type. Nodule number increased proportionally with the increase of insensitivity. As the total number of nodule foci was statistically the same in this group of lines, ethylene insensitivity may be promoting the growth of more nodule foci to nodules at higher insensitivity levels. Thus, ethylene not only controls the initiation of nodule foci, but also their growth into a functional nodule.
Nodule number per plant in hypo-insensitive L. japonicus lines was statistically the same as the wild type, indicating that the level of ethylene insensitivity was not enough to promote increased nodulation. Schmidt et al. (1999) reported soybean nodulation to be independent of ethylene signalling on the grounds that insensitive mutants did not nodulate more profusely. According to our classification of insensitivity, the most insensitive soybean mutant reported should be classified as ‘hypo-insensitive’, as its hypocotyl length in the presence of ethylene was smaller than that of the wild type without ethylene when germinated in the dark (12·8 cm in mutant vs. 12–15 cm in the wild type). Therefore, a soybean mutant with a much higher level of ethylene insensitivity such as ‘sickle’ or the Lotus transgenics reported here is required to determine whether soybean nodulation indeed is ethylene insensitive.
Ethylene restricted the position of nodule foci initiation, lending support to histochemical observations (Heidstra et al., 1997). This was supported by the large increase in nodule foci forming in between protoxylem poles in ethylene-insensitive plants compared with the wild type. However, the availability of these new sites for nodule initiation in the transgenic plants did not explain the overall increase in the number of nodule foci. Therefore, ethylene most likely regulates the number of developing nodules not only by controlling the position of nodule initiation but also by controlling the ability of plant cells to initiate nodule foci. One possible explanation is enhanced rhizobial infection/invasion of ethylene-insensitive plants as noted here and elsewhere for Lotus (Nukui et al., 2000) and ‘sickle’ (Penmetsa et al., 2003).
It has been reported that the nodules formed early during the nodulation process inhibit the formation of new nodules (AON of nodulation or feedback inhibition of nodulation). However, the Lotus transgenic lines described here were not super- or hypernodulated over a large portion of the root, and did not exhibit nitrate tolerance in nodulation, demonstrating that ethylene regulates nodulation at other sites and is not directly functional in AON and nitrate inhibition of nodulation.
ACKNOWLEDGEMENTS
We thank the Australian Research Council, the Queensland Government, the BACS Faculty and the University of Queensland Strategic Funds for providing funding for the Centre of Excellence. Sem Habamunga, Qunyi Jiang and Dongxu Li are thanked for technical assistance. John Dunlap provided support for histology. Dr C. Nessler provided the binary vector carrying the Atetr1-1 cDNA. This project was in part funded by NSF grant BIOL-99-234 and the Australian Research Council [No. DP0210101 (to J.S.) and CEO348212 (to P.M.G.)).
LITERATURE CITED
- Ané J-M, Kiss GB, Riely BK, et al. Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science. 2004;303:1364–1367. doi: 10.1126/science.1092986. [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Gresshoff PM, Rameau C, Turnbull CGN. Additional signalling compounds are required to orchestrate plant development. Journal of Plant Growth Regulators. 2003;22:15–24. [Google Scholar]
- Beveridge CA, Mathesius U, Rose R, Gresshoff PM. Common threads of meristem development and homeostasis: branching, nodules and lateral roots. Current Opinions in Plant Biology. 2007;10:44–51. doi: 10.1016/j.pbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Biswas B, Chan PK, Gresshoff PM. Molecular Plant. 2009. A novel ABA insensitive mutant of Lotus japonicus with a wilty phenotype but unaltered nodulation regulation. (in press). doi:10.1093/mp/ssp009. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Boivin C, Camut S, Malpica CA, Truchèt G, Rosenberg C. Rhizobium meliloti genes encoding catabolism of trigonelline are induced under symbiotic conditions. Plant Cell. 1990;2:1157–1170. doi: 10.1105/tpc.2.12.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T. Ethylene in pathogens and disease resistance. In: Mattoo AK, Suttle JC., editors. The plant hormone ethylene. London: CRC Press; 1991. pp. 293–314. [Google Scholar]
- Caba JM, Poveda JL, Gresshoff PM, Ligero F. Differential sensitivity of nodulation to ethylene in soybean cv. Bragg and a supernodulating mutant. New Phytologist. 1999;142:233–242. [Google Scholar]
- Caetano-Anollés G, Gresshoff PM. Plant genetic control of nodulation in legumes. Annual Review of Microbiology. 1991;45:345–382. doi: 10.1146/annurev.mi.45.100191.002021. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, McNeil DL, Gresshoff PM. A supernodulation and nitrate-tolerant symbiotic (nts) soybean mutant. Plant Physiology. 1985;a 78:34–40. doi: 10.1104/pp.78.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, McNeil DL, Gresshoff PM. Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proceeding of the National Academy of Sciences USA. 1985;b 82:4162–4166. doi: 10.1073/pnas.82.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Shockey JA. The ethylene-response pathway: signal perception to gene regulation. Current Opinion in Plant Biology. 1999;5:352–358. doi: 10.1016/s1369-5266(99)00004-7. [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–545. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Molecular Biology Reports. 1983;4:19–21. [Google Scholar]
- Ferguson BJ, Mathesius U. Signaling interactions during nodule development. Journal of Plant Growth Regulators. 2003;22:47–72. [Google Scholar]
- Gamble RL, Qu X, Schaller EC. Mutational analysis of the ethylene receptor ETR1. Role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiology. 2002;128:1428–1438. doi: 10.1104/pp.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg OL. The effects of amino acids and ammonium on the growth of plant cells in suspension culture. Plant Physiology. 1970;45:372–375. doi: 10.1104/pp.45.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goormachtig S, Capoen W, James EK, Holsters M. Switch from intracellular to intercellular invasion during water-stress-tolerant legume nodulation. Proceedings of the National Academy of Sciences USA. 2004;101:6303–6308. doi: 10.1073/pnas.0401540101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ET, Joshi PA. Novel fixation of plant tissue, staining through paraffin with Alcian Blue and Hematoxylin, and improved slide preparation. Biotechnic & Histochemistry. 1995;70:263–266. doi: 10.3109/10520299509108204. [DOI] [PubMed] [Google Scholar]
- Gresshoff PM. Molecular genetic analysis of nodulation genes in soybean. Plant Breeding Reviews. 1993;11:275–318. [Google Scholar]
- Gresshoff PM. Post-genomic insights into nodulation. Genome Biology. 2003;4:201. doi: 10.1186/gb-2003-4-1-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinel FC, LaRue TA. Ethylene inhibitors partly restore nodulation to pea mutant E107 (brz) Plant Physiology. 1992;99:515–518. doi: 10.1104/pp.99.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handberg K, Stougaard J. Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant Journal. 1992;2:487–496. [Google Scholar]
- Heidstra R, Yang WC, Yalcin Y, et al. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development. 1997;124:1781–1787. doi: 10.1242/dev.124.9.1781. [DOI] [PubMed] [Google Scholar]
- Hirsch AM. Developmental biology of legume nodulation. Plant Physiology. 1992;122:211–237. doi: 10.1111/j.1469-8137.1992.tb04227.x. [DOI] [PubMed] [Google Scholar]
- Hussain AKM, Jiang Q, Broughton WJ, Gresshoff PM. Lotus japonicus nodulates and fixes nitrogen with the broad host range Rhizobium sp. NGR234. Plant Cell Physiology. 1999;40:894–899. [Google Scholar]
- Jiang Q, Gresshoff PM. Classical and molecular genetics of the model legume Lotus japonicus. Molecular Plant–Microbe Interactions. 1997;10:559–568. doi: 10.1094/MPMI.1997.10.1.59. [DOI] [PubMed] [Google Scholar]
- Kinkema M, Scott P, Gresshoff PM. Legume nodulation: successful symbiosis through short and long-distance signalling. Functional Plant Biology. 2006;33:770–785. doi: 10.1071/FP06056. [DOI] [PubMed] [Google Scholar]
- Klee HJ. Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiology. 2004;135:660–667. doi: 10.1104/pp.104.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusell L, Madsen LH, Sato S, et al. Shoot control of root development is mediated by a receptor-like kinase. Nature. 2002;420:422–426. doi: 10.1038/nature01207. [DOI] [PubMed] [Google Scholar]
- Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. The NEVER RIPE mutation blocks ethylene perception in tomato. Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohar D, Schuller KA, Buzas DM, Gresshoff PM, Stiller J. Transformation of Lotus japonicus using the herbicide resistance bar gene as a selectable marker. Journal of Experimental Botany. 2001;52:1697–1702. [PubMed] [Google Scholar]
- Ma W, Penrose DM, Glick BR. Strategies used by rhizobial to lower plant ethylene levels and increase nodulation. Canadian Journal of. Microbiology. 2002;48:947–954. doi: 10.1139/w02-100. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu G-J, et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- Nukui N, Ezura H, Yuhashi K-I, Yasuta T, Minamisawa K. Effects of ethylene precursor and inhibitors for ethylene biosynthesis and perception on nodulation in Lotus japonicus and Macroptilium atropurpureum. Plant Cell Physiology. 2000;41:893–897. doi: 10.1093/pcp/pcd011. [DOI] [PubMed] [Google Scholar]
- Nukui N, Ezura H, Minamisawa K. Transgenic Lotus japonicus with an ethylene receptor gene Cm-ERS1/H70A enhances formation of infection threads and nodule primordia. Plant Cell Physiology. 2004;45:427–435. doi: 10.1093/pcp/pch046. [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Engstrom EM, Long SL. Ethylene inhibits the nod factor signal transduction pathway of Medicago truncatula. Plant Cell. 2001;13:1835–1849. doi: 10.1105/TPC.010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Frugoli JA, Smith LS, Long SR, Cook DR. A dual mechanism of nodule control in Medicago truncatula. Plant Physiology. 2003;131:1–11. doi: 10.1104/pp.015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa RV, Uribe P, Anderson J, et al. The Medicago truncatula of the Arabidopsis EIN2 gene, sickle, is a negative regulator of symbiotic and pathogenic microbial interactions. Plant Journal. 2008;55:580–595. doi: 10.1111/j.1365-313X.2008.03531.x. [DOI] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature. 2003;425:585–592. doi: 10.1038/nature02039. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- Schmidt JS, Harper JE, Hoffman TK, Bent AF. Regulation of soybean nodulation independent of ethylene signaling. Plant Physiology. 1999;119:951–959. doi: 10.1104/pp.119.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle IR, Men AM, Laniya TS, et al. Long distance signalling for nodulation control in legumes requires a CLAVATA1-like receptor kinase. Science. 2003;299:108–112. doi: 10.1126/science.1077937. [DOI] [PubMed] [Google Scholar]
- Stacey G, Libault M, Brechenmacher L, Wan J, May GD. Genetics and functional genomics of legume nodulation. Current Opinions in Plant Biology. 2006;9:1–12. doi: 10.1016/j.pbi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Stiller J, Martirani L, Tuppale S, Chian R-J, Chiurazzi M, Gresshoff PM. High frequency transformation and regeneration of transgenic plants in the model legume Lotus japonicus. Journal of Experimental Botany. 1997;48:1357–1365. [Google Scholar]
- Suganuma N, Yamauchi H, Yamamoto K. Enhanced production of ethylene by soybean roots after inoculation with Bradyrhizobium japonicum. Plant Science. 1995;111:163–168. [Google Scholar]
- Sugawara M, Okazaki S, Nukui N, Ezura H, Mitsui H, Minamisawa K. Rhizotoxine modulates plant–microbe interactions by ethylene inhibition. Biotechnology Advances. 2006;24:382–388. doi: 10.1016/j.biotechadv.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Szczyglowski K, Shaw RS, Wopereis J, et al. Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Molecular Plant–Microbe Interactions. 1998;11:684–697. [Google Scholar]
- Verwoerd TC, Dekker MM, Hoeckema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acid Research. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Schuerman P, Woeste K, Brandstatter I, Kieber JJ. Isolation and characterization of Arabidopsis mutants defective in the induction of ethylene biosynthesis by cytokinin. Genetics. 1998;149:417–427. doi: 10.1093/genetics/149.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, et al. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nature Biotechnology. 1997;15:444–447. doi: 10.1038/nbt0597-444. [DOI] [PubMed] [Google Scholar]
- Wopereis J, Pajuelo E, Dazzo FB, et al. Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant Journal. 2000;23:97–114. doi: 10.1046/j.1365-313x.2000.00799.x. [DOI] [PubMed] [Google Scholar]
- Zaat SAJ, Van Brussel AAN, Tak T, Lugtenberg BJJ, Kijne JW. The ethylene-inhibitor aminoethoxyvinylglycine restores normal nodulation by Rhizobium leguminosarum biovar. vicieae on Vicia sativa subsp. nigra by suppressing the ‘thick and short roots’ phenotype. Planta. 1989;177:141–150. doi: 10.1007/BF00392802. [DOI] [PubMed] [Google Scholar]