Abstract
Background and Aims
One of the special properties of clonal plants is the capacity for physiological integration, which can increase plant performance through mechanisms such as resource sharing and co-ordinated phenotypic plasticity when plants grow in microsites with contrasting resource availabilities. However, many clonal plants are colonized by arbuscular mycorrhizal fungi (AMF). Since AMF are likely to reduce contrasts in effective resource levels, they could also reduce these effects of clonal integration on plasticity and performance in heterogeneous environments.
Methods
To test this hypothesis, pairs of connected and disconnected ramets of the stoloniferous herb Trifolium repens were grown. One ramet in a pair was given high light and low nutrients while the other ramet was given high nutrients and low light. The pairs were inoculated with zero, one or five species of AMF.
Key Results
Pairs of ramets grown without AMF developed division of labour and benefited from resource sharing, as indicated by effects of connection on allocation to roots, accumulation of mass, and ramet production. Inoculation with five species of AMF significantly reduced these effects of connection, both by inhibiting them in ramets given high nutrients and inducing them in ramets given high light. Inoculation with one species of AMF also reduced some effects of connection, but generally to a lesser degree.
Conclusions
The results show that AMF can significantly modify the effects of clonal integration on the plasticity and performance of clonal plants in heterogeneous environments. In particular, AMF may partly replace the effects and benefits of clonal integration in low-nutrient habitats, possibly more so where species richness of AMF is high. This provides the first test of interaction between colonization by AMF and physiological integration in a clonal plant, and a new example of how biotic and abiotic factors could interact to determine the ecological importance of clonal growth.
Key words: Arbuscular mycorrhizal fungi, biomass allocation, clonal plant, division of labour, environmental heterogeneity, light availability, nutrients, white clover
INTRODUCTION
Small-scale spatial heterogeneity in resource availability is common in natural habitats (e.g. Jackson and Caldwell, 1993; Wiens, 2000; Hodge, 2004, 2006). Among plants, species with clonal growth have a unique potential to improve their performance in such heterogeneous environments through physiological integration within a clone (i.e. a group of ramets with the same genotype), such as via the sharing of resources and the co-ordination of phenotypic plasticity between connected ramets (e.g. Chapman et al., 1992; Hutchings and de Kroon, 1994; de Kroon et al., 2005).
One of the most striking co-ordinated plastic responses of clonal plants is division of labour between connected ramets in heterogeneous habitats where microsites low in one resource are high in another (Stuefer et al., 1996; Alpert and Stuefer, 1997; van Kleunen and Stuefer, 1999). For example, in habitats consisting of patches with high availability of light but low availability of nutrients, and patches with high nutrients but low light, a ramet growing in high light and low nutrients will allocate a relatively high proportion of biomass, and thus of carbon, to shoots if it is connected to a ramet growing in low light and high nutrients, and that ramet in high nutrients will allocate a high proportion of biomass to roots (e.g. Alpert et al., 2003; Roiloa et al., 2007). Each ramet thus specializes to acquire the resource that is abundantly available to it, a plastic response opposite to the specialization to acquire scarce resources that is typical of non-clonal plants and of single or experimentally disconnected ramets of clonal plants (Alpert and Stuefer, 1997).
Division of labour has been generally thought to be likely to increase fitness in clonal plants (Alpert and Stuefer, 1997; Hutchings and Wijesinghe, 1997; Roiloa et al., 2007). However, little is known about the extent of division of labour in clonal plants under natural conditions (Stuefer et al., 2002).
One important factor that might reduce the effects of physiological integration in clonal plants in natural habitats is the presence of arbuscular mycorrhizal fungi (AMF). AMF are one of the most abundant groups of terrestrial fungi and form symbiotic associations with the roots of most herbaceous species of plants (e.g. Smith and Read, 1997). AMF often supply nutrients to the host plant, increasing the effective availability of relatively immobile elements such as phosphorus (Maiquetía et al., 2009). On the other hand, the fungi typically import carbohydrates from the plant (e.g. Wright et al., 1998; Miller et al., 2002), and colonization by AMF can either increase or decrease plant growth depending on nutrient and light availability (e.g. Hetrick et al., 1992; Koide and Schreiner, 1992; Hartnett et al., 1993; Johnson et al., 1997). AMF can also alter the morphology and phenology of plants (Maiquetía et al., 2009), and abundance and species composition of AMF can vary on small scales (Wolfe et al., 2007).
At least four studies have specifically examined colonization of clonal plants by AMF. Different AMF can affect stolon length and branching differently (Streitwolf-Engel et al., 1997); AMF can influence vegetative reproduction (Streitwolf-Engel et al., 2001); colonization by AMF can depend upon ramet age (Watson et al., 2001); and AMF can reduce the growth of both colonized and uncolonized but connected ramets (Sudová and Vosátka, 2008). However, no previous study appears to have tested the hypothesis that AMF can reduce the effects of clonal integration in heterogeneous environments.
Because AMF could compensate for low nutrient availability by exporting nutrients to ramets, and diminish effective light availability by importing photosynthates from ramets, it seems likely that AMF could particularly reduce effects of integration in habitats where nutrients and light tend not to occur together, by reducing effective differences in resource availabilities to connected ramets. We therefore predicted that colonization by AMF would reduce the effect of connection between ramets in microsites with high light and low nutrients and ramets in microsites with high nutrients and low light on phenotypic plasticity such as division of labour and on plant performance as measured by accumulation of mass and production of new ramets.
Different species of AMF often co-occur in nature and colonize the same host plant (Maherali and Klironomos, 2007). Colonization by a greater number of species of AMF can more strongly affect the growth of individual plants (Streitwolf-Engel et al., 2001), the species diversity and composition of plant communities (van der Heijden et al., 1998a) and the stability and productivity of ecosystems (van der Heijden et al., 1998b; van der Heijden and Cornelissen, 2002). In view of these added effects of a greater number of species of AMF at a variety of levels, it was therefore predicted that colonization by several species of AMF would reduce effects of connection between ramets more than colonization by a single species.
To test these predictions, pairs of connected or disconnected ramets of the clonal plant Trifolium repens were grown in environments consisting of patches of high light and low nutrients (sometimes referred to, for brevity, as ‘high light’) and of high nutrients and low light (‘high nutrients’) without AMF or following inoculation with one species of AMF or with a mixture of this species and four others.
MATERIALS AND METHODS
Plant and fungal materials
Trifolium repens L. is a perennial herb in the Fabaceae that is distributed over a wide range, from the Arctic to the subtropics (Williams, 1987), and shows high plasticity in response to both abiotic and biotic environmental factors (Gautier et al., 1998; Weijschedé et al., 2006). The species reproduces vegetatively by means of stolons that can root at each node and produce a new ramet consisting of a leaf, a set of roots, and an axillary meristem that can remain dormant or develop into a new stolon or a terminal inflorescence. Clones show a high degree of physiological integration, including both acropetal and basipetal translocation of resources between ramets along a stolon (Chapman et al., 1992; Lotscher and Hay, 1996). Roots of T. repens are commonly associated with AMF under natural conditions: colonization rates, measured as proportion of 1-cm root lengths with AMF, often exceed 60 % (Li et al., 2005).
Ramets of T. repens were collected from the Botanical Garden of the Institute of Botany of the Chinese Academy of Sciences (IBCAS) in Beijing and vegetatively propagated in a glasshouse at IBCAS. To obtain AMF-free plants, new ramets produced along stolons were rooted in plastic pots (18 cm in diameter and 16 cm in height) containing coarse sand sterilized by γ-ray irradiation (10 kGy; Jakobsen and Andersen, 1982; McNamara et al., 2003). Ramets were watered with deionized water and propagated under AMF-free conditions through at least three vegetative generations to minimize potential carry-over effects of their previous environments. Based on leaf markings, ramets probably came from a number of genetic individuals, but no attempt was made to test for differences between genotypes. Lack of control of genotype may have introduced additional unexplained variation into the results and caused us to fail to detect some effects of AMF, but this should not invalidate those effects that were found to be significant.
For the experiment, pairs of new, similarly sized ramets that were adjacent to each other along a stolon were rooted in black plastic trays (32 cm long × 15 cm wide × 9 cm high) filled with sterilized sand, with each ramet in a pair rooted in a separate tray. The younger ramet in a pair was given high light (ambient light, about 1100 µmol m−2 s−1 at plant level at noon on a clear day) and low nutrients (a modified Hoagland solution diluted to 5 % of full strength, containing KH2PO4 0·025 mmol L−1). The older ramet was given low light (25 % of ambient, reduced with neutral shading cloth) and high nutrients (nutrient solution diluted to 50 %, containing KH2PO4 0·25 mmol L−1). The nutrient levels, especially the levels of P, were chosen to ensure a high degree of AMF colonization on ramets in high light and in high nutrients while still providing a large contrast between levels (Menge et al., 1978). Trays were watered once every other week with 300 mL of nutrient solution; deionized water was applied between waterings as needed to prevent wilting, and more water was adding in high light than in high nutrient to compensate for differences in evapotranspiration.
Two inocula consisting of AMF spores, root fragments and substrata were obtained from the Bank of Glomales of the Institute of Plant Nutrition and Resources at the Beijing Academy of Agriculture and Forestry Sciences. One inoculum contained only Glomus mosseae (Nicol. and Gerdemann) Gerdemann and Trappe, one of the most common AMF in soils. Glomus mosseae is associated with many plant species worldwide (Mosse and Bowen, 1968; Gerdemann and Trappe, 1974). In T. repens, G. mosseae has been shown to increase uptake of phosphorus (Li et al., 1991) and rate of photosynthesis (Wright et al., 1998). The other inoculum contained G. mosseae plus G. etunicatum Becker and Gerdemann, G. coronatum Giovannetti, G. aggregatum Schenck and Smithand and Acaulospora delicata Walker, Pfeiffer and Bloss. These additional four species are either known to be naturally associated with T. repens or have been used to study effects of AMF on T. repens in laboratory or greenhouse experiments (Habte et al., 1999; Liu and Wang, 2003; Calvet et al., 2004; Cornejo et al., 2004). All the AMF species were separately cultivated in pots filled with sterilized zeolite and river sand and planted with sorghum (Sorghum vulgare Pers.).
Experimental design
Pairs of ramets were randomly assigned to one of six treatments, two connection treatments (connected, ramets in a pair left connected by the stolon between them; severed, ramets disconnected by cutting the stolon) crossed with three AMF treatments (AMF0, sterilized inoculum added to the soil; AMF1, the inoculum containing only one species of AMF added to the soil; AMF5, the inoculum containing five species of AMF added). The sterilized inoculum was prepared by autoclaving single-species inoculum at 121 °C for 2 h. In each of the AMF treatments, 40 g of inoculum was mixed into the top 3 cm of sand in each tray. To reduce differences between treatments in soil flora other than AMF (Koide and Li, 1989), each tray was also given 200 mL of a suspension of the AMF1 inoculum, filtered to remove AMF but not smaller soil microorganisms such as bacteria. Endosymbiotic N-fixing bacteria were not purposely added to any treatments, and ramets did not become nodulated during the experiment. This was done to isolate the effects of AMF better, since rhizobia can interact both positively and negatively with AMF (Vejsadová et al., 1992; Catford et al., 2003). On the other hand, this meant that the experimental results might overstate the effects of AMF in nodulated plants and so should be interpreted with caution. There were 20 replicates for each connection × AMF0 or AMF1 treatment, and 10 replicates for each connection × AMF5 treatment.
The experiment started on 18 September 2007 and ended 12 weeks later on 12 December. Mean temperature and relative humidity in the greenhouse during treatments were, respectively, 18·6 °C and 68·7 %, as measured hourly by two Hygrochron temperature/humidity dataloggers (iButton DS 1923; Maxim Integrated Products). The connecting stolon or one of the ramets died in two replicates of the connected, AMF0 treatment and in three replicates of the connected, AMF1 treatment during the experiment; these replicates were excluded from analyses.
Measurements
At the end of the experiment, the number of new, offspring ramets produced by each original ramet was counted and the lengths of all stolons and petioles were measured. To measure total leaf area and total root length of each original ramet plus its new offspring, their leaf laminas and roots were scanned (Epson Perfection 4990 Photo: 150 dpi for leaves and 400 dpi for roots) and the images analysed using WinFOLIA Pro 2004a for leaves and WinRHIZO Pro 2004a for roots (Regent Instruments, Inc.).
To measure degree of colonization by AMF, a subsample of roots from each tray was cut into 1-cm lengths, cleared with 10 % KOH, and stained with trypan blue (Phillips and Hayman, 1970). The percentage of the root pieces colonized by AMF was estimated by the gridline intersection method (Giovannetti and Mosse, 1980); at least 100 line intersections per root sample were inspected, except for 12 samples where 26–92 intersections per sample were scored because of small sample size.
Finally, the laminas, petioles, stolons and roots from each tray were dried separately at 70 °C for 48 h and weighed. Specific leaf area was calculated as total leaf area divided by total dry mass of leaf laminas, and specific root length as total root length divided by total mass of roots.
Statistical analyses
Effects of treatments on degree of colonization of roots by AMF were tested separately for original plus new ramets in high nutrients and original plus new ramets in high light, using two-way ANOVAs, with connection and AMF (AMF1 or AMF5) as fixed effects. The AMF0 treatments were not included because they had zero colonization (see Results).
Effects of connection and AMF on measures of allocation, morphology and performance of plants were tested with similar two-way ANOVAs, but including all three AMF treatments and with planned comparisons between AMF0 and AMF1 and between AMF1 and AMF5, to test effects of colonization by a single species of AMF and of AMF species richness, respectively. The key effects used to test whether presence or richness of AMF modified the effects of physiological integration on plant growth were AMF0 versus AMF1 × connection, and AMF1 versus AMF5 × connection.
Separate ANOVAs were conducted for the ramets given high light and low nutrients and for the ramets given high nutrients and low light. Since the primary indication of division of labour was a shift between ramets given high light and ramets given high nutrients in their relative pattern of allocation to roots, an ANOVA was also performed on the difference between ramets within pairs in root mass ratio. To test effects on performance at the level of clones, ANOVAs of measures of performance were conducted for the pairs of original ramets plus their new ramets, i.e. clonal fragments. To increase normality and homogeneity of variance, total leaf area, total root length, specific root length, total biomass and ramet number were transformed to the natural log before analysis. Analyses were conducted with SAS 8·2 (SAS Institute, 1999).
RESULTS
Colonization of plants by AMF
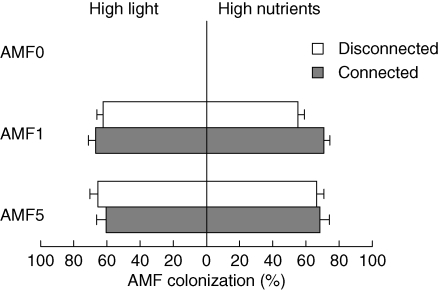
None of the roots of the plants in the AMF0 treatments were colonized by AMF, indicating that sterilization of the inoculum was effective and that only purposely added AMF were present in the treatments (Fig. 1). In contrast, colonization was extensive in each of the AMF1 and AMF5 treatments (Fig. 1, mean percentage of root segments colonized in each treatment 50–70 %), and was not strongly affected by either AMF species richness (AMF1 versus AMF5) or connection between ramets. This suggested that degree of colonization was not an important factor underlying any effects of connection or AMF richness on plants.
Fig. 1.
Colonization of roots of Trifolium repens by AMF (percentage of 1-cm root segments with AMF; mean + s.e.): effects of resource levels (High light, high light and low nutrients; High nutrients, high nutrients and low light), AMF treatment (AMF0, sterilized inoculum; AMF1, inoculated with one species of AMF; AMF5, inoculated with five species), and connection between ramets (ramets disconnected or left connected, as indicated). Values for AMF0 were zero. ANOVA for ramets in high light: AMF richness (AMF1 vs. AMF5), F1,53 = 0·10, P = 0·75; connection, F1,53 < 0·01, P > 0·9; richness × connection, F1,53 = 1·01, P = 0·32; ANOVA for ramets in high nutrients: richness, F1,53 = 1·00, P = 0·32; connection, F1,53 = 3·94, P = 0·053; richness × connection, F1,53 = 2·36, P = 0·13.
Allocation of mass between roots and shoots
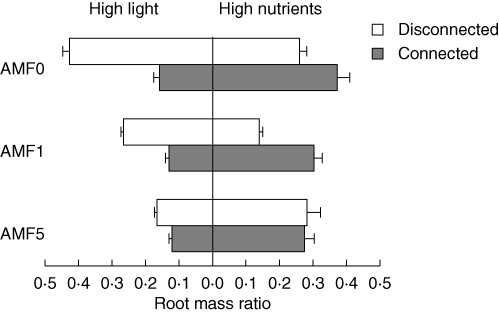
Connection between ramets given contrasting, complementary levels of light and nutrients induced division of labour in the absence of AMF (Fig. 2, AMF0). Disconnected ramets in the AMF0 treatment allocated a high proportion of biomass to roots in the high light and low nutrient treatment and a low proportion in the low light and high nutrient treatment, indicating morphological specialization to acquire scarce resources, typical of non-clonal plants and of single ramets of clonal plants. Connected ramets in the AMF0 treatment allocated a low proportion of biomass to roots in the high light and low nutrient treatment and a high proportion in the low light and high nutrient treatment, indicating specialization to acquire abundant resources, a main criterion for division of labour between connected ramets in clonal plants.
Fig. 2.
Allocation to roots (root mass ratio; mean + s.e.) in Trifolium repens: effects of resource levels (high light and low nutrients or high nutrients and low light), inoculation with AMF (zero, one or five species), and connection between ramets (ramets disconnected or ramets left connected, as indicated). See Table 1 for ANOVAs.
Effect of connection on the difference between ramets within pairs in allocation to roots did not differ between pairs uncolonized by AMF and pairs colonized by G. mosseae (Fig. 2, AMF0 versus AMF1; Table 1, effect of AMF0 versus AMF1 × connection), indicating that this single species did not affect induction of division of labour. However, effect of connection on difference in allocation was less in pairs of ramets colonized by five species of AMF than in those colonized by G. mosseae alone (Fig. 2, AMF1 versus AMF5; Table 1, effect of AMF1 versus AMF5 × connection), indicating that another species of AMF or a mix of species did reduce induction of division of labour. AMF reduced this effect by decreasing specialization to acquire scarce resources in disconnected ramets (Fig. 2 and Table 1). The increase in allocation to roots in ramets given high nutrients that was induced by connection in the AMF0 treatment was completely inhibited in the AMF5 treatment. In ramets given high light, AMF reduced the effect of connection, not by preventing a decrease in allocation to roots in connected ramets, but by causing a decrease in allocation to roots in disconnected ramets, and replacing rather than inhibiting the effect of connection.
Table 1.
ANOVAs of difference in allocation to roots (root mass ratio) between ramets of Trifolium repens given high light and ramets given high nutrients, and of allocation to roots in each type of ramets separately, as affected by AMF and connection treatments, with planned comparisons between individual AMF treatments
| Effect | Difference | High light | High nutrients |
|---|---|---|---|
| AMF | 6·65** | 49·29*** | 7·26** |
| AMF 0 vs. 1 | <0·001ns | 49·62*** | 14·43*** |
| AMF 1 vs. 5 | 10·91** | 11·30** | 3·58† |
| Connection (C) | 84·93*** | 143·95*** | 14·91*** |
| AMF × C | 13·51*** | 26·85*** | 4·11* |
| AMF 0 vs. 1 × C | 2·11ns | 24·32*** | 1·07ns |
| AMF 1 vs. 5 × C | 15·28*** | 7·98** | 8·18** |
The values are F; symbols show P, *** < 0·001; ** < 0·01; * <0·05; † <0·1; ns ≥0·1. d.f. = 2,89 for AMF and AMF × connection, 1,89 for other effects.
Other morphological traits
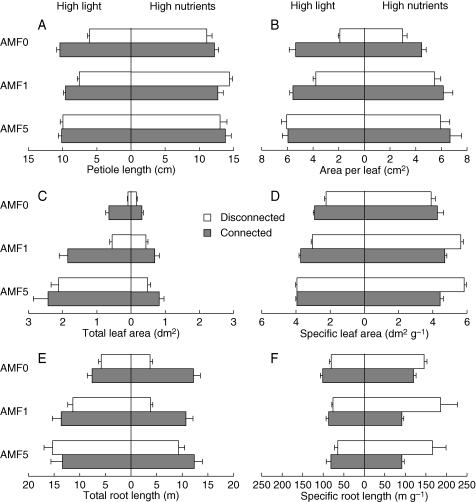
Effects of connection on the six other measured traits of leaf and root morphology were also generally consistent with induction of division of labour in the absence of AMF (Fig. 3, AMF0). For example (Fig. 3A–D), disconnected ramets in the AMF0 treatments had longer petioles, larger leaves, greater total leaf area and higher specific leaf area if given high nutrients and low light than if given high light and low nutrients, suggesting specialization for acquiring light when light was scarce. In contrast, connected ramets in the AMF0 treatments had larger leaves and greater total leaf area if given high light, suggesting specialization for acquiring light when light was abundant. Connected ramets showed less difference than disconnected ramets in petiole length and specific leaf area between resource treatments, suggesting reduced specialization to acquire scarce resources, if not specialization to acquire abundant resources.
Fig. 3.
Effects of inoculation with AMF (zero, one or five species) and connection between ramets (ramets disconnected or left connected, as indicated) on six morphological traits in ramets of Trifolium repens given either high light and low nutrients or high nutrients and low light: (A) petiole length, (B) area per leaf, (C) total leaf area, (D) specific leaf area, (E) total root length and (F) specific root length. Values are means + s.e. See Table 2 for ANOVAs.
Effect of connection on root length in the absence of AMF (Fig. 3E, AMF0) similarly suggested specialization to acquire nutrients when they were abundant. Connection reduced differences in specific root length between ramets given high light and those given high nutrients (Fig. 3F). For each of these six traits, effect of connection was highly significant for ramets in high light, ramets in high nutrients, or both (Table 2).
Table 2.
ANOVAs of morphological traits in Trifolium repens as affected by AMF and connection treatments, with planned comparisons between individual AMF treatments
| Effect | Petiole length | Area per leaf | Total leaf area | Specific leaf area | Total root length | Specific root length |
|---|---|---|---|---|---|---|
| Ramets given high light and low nutrients | ||||||
| AMF | 10·56*** | 22·11*** | 92·82*** | 137·76*** | 14·48*** | 4·86** |
| AMF 0 vs. 1 | 1·01ns | 11·98*** | 95·38*** | 124·30*** | 20·49*** | 1·35ns |
| AMF 1 vs. 5 | 13·24*** | 13·55*** | 20·06*** | 41·51*** | 0·52ns | 4·54* |
| Connection (C) | 47·81*** | 37·39*** | 47·45*** | 46·39*** | 0·03ns | 6·92* |
| AMF × C | 13·83*** | 12·47*** | 12·91*** | 9·46*** | 0·85ns | 0·48ns |
| AMF 0 vs. 1 × C | 11·35** | 7·65** | 4·97* | <0·01ns | 0·23ns | 0·75ns |
| AMF 1 vs. 5 × C | 5·00** | 6·75** | 10·21** | 15·77*** | 0·80ns | 0·61ns |
| Ramets given high nutrients and low light | ||||||
| AMF | 4·67* | 11·78*** | 14·38*** | 14·37*** | 5·59** | 1·23ns |
| AMF 0 vs. 1 | 8·04** | 11·78*** | 18·54*** | 24·08*** | 0·32ns | 1·44ns |
| AMF 1 vs. 5 | 0·03ns | 0·64ns | 1·16ns | 0·01ns | 10·41** | 0·16ns |
| Connection (C) | 0·01ns | 4·01* | 7·77** | 10·53** | 36·32*** | 38·08*** |
| AMF × C | 2·45† | 0·34ns | 0·89ns | 7·24** | 3·76* | 3·32* |
| AMF 0 vs. 1 × C | 4·38* | 0·59ns | 1·55ns | 8·84** | 0·68ns | 6·03* |
| AMF 1 vs. 5 × C | 2·36ns | 0·00ns | 0·01ns | 0·80ns | 4·08* | 0·11ns |
The values are F; symbols show P: *** < 0·001; ** < 0·01; * < 0·05; † < 0·1; ns ≥ 0·1. d.f. = 2,89 for AMF and AMF × connection, 1,89 for other effects.
AMF dampened some of these effects of connection on each of the six measured traits except specific root length, on which AMF enhanced effect of connection (Fig. 3 and Table 2). This was generally consistent with results for allocation to roots, with the difference that interaction between effect of connection and effect of AMF was seen in some cases, not just for the AMF5 treatment, but also for the AMF1 treatment. For example, effects of connection on petiole length, area per leaf, and total leaf area in ramets given high light were less in the AMF1 than in the AMF0 treatment (Fig. 3A–C and Table 2, effect of AMF0 vs. AMF1 × connection). For these three traits, effect of connection in ramets given high light was again less in the AMF5 than in the AMF1 treatment (Table 2, effect of AMF1 vs. AMF5 × connection), consistent with the prediction that greater richness of AMF would inhibit division of labour more.
The other two traits in which AMF dampened effect of connection were: (1) specific leaf area for ramets given high light (Fig. 3D) and (2) total root length for ramets given high nutrients (Fig. 3E). In both of these cases, effect of connection was less in the AMF5 than in the AMF1 treatment but did not differ between the AMF1 and the AMF0 treatment (Table 2). All these interactions between effects of AMF and connection were caused by increased specialization of disconnected ramets to acquire abundant resources, rather than decreased specialization of connected ramets to do so.
At least two effects of AMF and connection on morphological traits were synergistic. AMF enhanced rather than dampened effects of connection on specific leaf area and specific root length in ramets given high nutrients (Fig. 3D, F and Table 2). The enhanced effect of connection on specific leaf area was due to higher specific leaf area in disconnected ramets in the AMF1 and AMF5 than in the AMF0 treatment, suggesting greater specialization to acquire scarce resources in disconnected ramets with AMF than in those without AMF. The enhanced effect of connection on specific root length in the presence of AMF was due to effects of AMF on both disconnected and connected ramets.
Plant performance
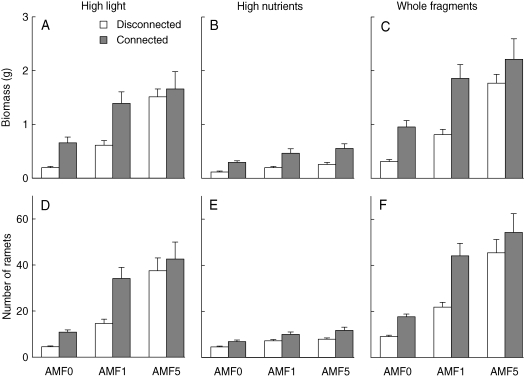
Both connection and AMF independently increased performance of fragments and of their component portions in the each of the two resource treatments (Fig. 4 and Table 3), as measured both by net accumulation of mass and net production of ramets.
Fig. 4.
Effects of inoculation with AMF (zero, one or five species) and connection between ramets (ramets disconnected or left connected, as indicated) on two measures of performance, dry biomass and number of new ramets produced, in pairs of ramets (fragments) of Trifolium repens, and in the ramets within each pair given high light and low nutrients, and the ramets within pairs given high nutrients and low light. Values are means + standard error. See Table 3 for ANOVAs.
Table 3.
ANOVAs of performance traits in Trifolium repens as affected by AMF and connection treatments, with planned comparisons between individual AMF treatments
| Ramets in high light |
Ramets in high nutrients |
Clonal fragments |
||||
|---|---|---|---|---|---|---|
| Effect | Biomass | Ramets | Biomass | Ramets | Biomass | Ramets |
| AMF | 37·62*** | 72·95*** | 6·95** | 14·31*** | 36·82*** | 65·44*** |
| AMF 0 vs. 1 | 38·16*** | 78·11*** | 5·46* | 17·40*** | 35·77*** | 67·86*** |
| AMF 1 vs. 5 | 8·44** | 13·86*** | 2·70ns | 1·62ns | 9·29** | 13·75*** |
| Connection (C) | 13·10*** | 21·28*** | 29·38*** | 10·73** | 24·94*** | 23·55*** |
| AMF × C | 4·53* | 4·66* | 0·75ns | 0·02ns | 5·15** | 3·24* |
| AMF 0 vs. 1 × C | 0·67ns | 0·21ns | 1·07ns | 0·04ns | 1·41ns | 0·07ns |
| AMF 1 vs. 5 × C | 5·19* | 6·45* | 0·02ns | 0·01ns | 4·83** | 4·76* |
The values are F; symbols show P: *** < 0·001; ** < 0·01; * < 0·05; † < 0·1; ns≥ 0·1. d.f. = 2,89 for AMF and AMF × connection, 1,89 for other effects.
Inoculation with five, but not with one, species of AMF reduced the positive effects of connection on the performance of clones (Fig. 4 and Table 3, effect of AMF × connection) by disproportionately increasing the performance of the disconnected ramets in high light.
For example, the dry mass of clonal fragments (i.e. pairs of original ramets plus their new ramets and stolons) was not differently affected by connection in the AMF0 and AMF1 treatments (Fig 4C and Table 3, effect of AMF0 vs. AMF1 × connection). However, effect of connection on the mass of fragments was less in the AMF5 than in the AMF1 treatment (Fig. 4C and Table 3, effect of AMF1 vs. AMF5 × connection), because the mass of fragments with disconnected ramets was disproportionately higher in the AMF5 than in the AMF1 treatment. This disproportionate, positive effect of the AMF5 treatment en masse was seen only in the portion of the fragment given high light and low nutrients (Fig. 4A) and not in the portion given high nutrients and low light (Fig. 4C). Results were similar for performance as measured by number of ramets produced (Fig. 4D–F and Table 3).
DISCUSSION
Results supported the hypothesis that colonization by AMF can reduce effects of physiological integration between connected ramets in heterogeneous environments. In Trifolium repens, AMF reduced effect of connection on allocation to roots, a key indicator of division of labour in clonal plants (Stuefer et al., 1996; Alpert and Stuefer, 1997; Hutchings and Wijesinghe, 1997; van Kleunen and Stuefer, 1999; Alpert et al., 2003; Roiloa et al., 2007). Inoculation with five species of AMF reduced the effect of connection more than inoculation with just one species. However, much of the reduction in effect of connection by AMF was due to apparent replacement rather than to inhibition of the effect of connection. For example, in the high light treatment, inoculation with five species of AMF caused disconnected ramets to have low levels of allocation to roots, similar to the effect of connection on ramets without AMF. Inoculation with AMF did inhibit the induction by connection of high allocation to roots in ramets in high nutrients and low light, though only in the case of inoculation with five species. It thus appears that AMF can reduce effects of clonal integration on plasticity both by replacing and inhibiting effects.
Many of these results can reasonably be interpreted in terms of transfers of resources between plants and fungi. For instance, the reduced allocation to roots caused by AMF in disconnected ramets given high light and low nutrients could be due to export of nutrients and import of photosynthates by the AMF (e.g. Wright et al., 1998; Miller et al., 2002; Landis and Fraser, 2008; Shishkova et al., 2008). A ramet given high nutrients and low light would also be expected to export nutrients and import photosynthates from a ramet given high light and low nutrients (Alpert and Stuefer, 1997). If some effects of connection were due to resource transfers between ramets, then colonization by AMF might well produce similar effects and partly supersede connection and physiological integration between ramets in this case. The more pronounced influence of AMF on effect of connection on allocation to roots in ramets given high light than in ramets given high nutrients could be due to increase in the activity of AMF by greater supply of photosynthates from the ramets in high light (Son and Smith, 1988; Marschner and Dell, 1994). This does not, of course, mean that AMF are likely to functionally replace all aspects of clonal integration, nor to reproduce effects of connection between ramets under all environmental conditions.
The greater reduction of effects of connection by the inoculum with five AMF than by the inoculum with just one AMF may mean that more diverse communities of AMF are likely to more strongly reduce the importance of clonal integration between established ramets in heterogeneous environments. However, apparent effects of diversity can reflect sampling effects rather than functional complementarity (van der Heijden et al., 1998a, b; Koide, 2000; Smith et al., 2000; Loreau and Hector, 2001; van der Heijden and Cornelissen, 2002). For example, there are no data on which species of AMF colonized roots in the AMF5 treatment, and the possibility cannot be ruled out that the greater effects of inoculation with five rather than with one species of AMF were due to differences between species in their individual effects. This first study of the effects of AMF on effects of clonal integration was not designed to distinguish between sampling and complementarity, and our inferences about the importance of diversity of AMF can only be tentative.
Inoculation with AMF generally but not invariably reduced the effects of connection on plasticity in morphological traits likely to be associated with acquisition of light and nutrients by plants. In the case of leaf traits of ramets in high light, AMF tended to replace those effects of connection that were measured, consistent with results for allocation of mass to roots. However, there was little indication that AMF inhibited effect of connection on ramets in high nutrients, except on total root length. AMF enhanced the effect of connection on plasticity in specific leaf area of ramets given high nutrients, by disproportionately increasing the specific leaf area of disconnected ramets.
Inoculation with AMF and connection between ramets each independently increased the performance of ramets in high light, ramets in high nutrients, and clonal fragments, as measured both by accumulation of mass and production of new ramets. Connection between ramets given contrasting availabilities of light and nutrients has commonly been found to increase their combined performance, presumably due to clonal integration (e.g. Alpert and Stuefer, 1997; Alpert et al., 2003; Roiloa et al., 2007). AMF have sometimes been found to increase the performance of clonal plants (Streitwolf-Engel et al., 1997, 2001) and sometimes not (Sudová and Vosátka, 2008). This could be due to differences in the levels of nutrients supplied, since AMF may not promote plant growth when soil nutrient availability is high (Koide and Schreiner, 1992; DeLucia et al., 1997). It could also be due to differences in light levels, since carbon import by AMF may decrease growth of clonal hosts (Wright et al., 1998). In the present experiment, AMF increased the mass and number of ramets produced by T. repens much less in ramets given high nutrients and low light than in those given low nutrients and high light, consistent with both possibilities.
Inoculation with five species of AMF decreased the effect of connection on the performance of clonal fragments by disproportionately increasing the performance of disconnected ramets in high light and low nutrients. Inoculation with one species of AMF did not change effect of connection on performance, and AMF did not change effect of connection on the performance of ramets in high nutrients. These results provide an initial suggestion that AMF may to some extent substitute for clonal integration in habitats where nutrients are strongly limiting and richness of AMF is high. If so, physiological integration between established ramets might be less advantageous and less strongly selected for in habitats where nutrients are scarce and ramets are colonized by AMF.
These results provide the first evidence for interactions between colonization by AMF and effects of physiological integration in a clonal plant. Given the wide distribution of both AMF and clonal plants, the ubiquitous nature of heterogeneity in resource supply, and the large variation between plant species in degree of association with AMF (e.g. Johnson et al., 1997; Klironomos, 2003; Reynolds et al., 2006), such interactions could be significant factors in the spatial and temporal composition of natural plant communities.
ACKNOWLEDGEMENTS
We thank Prof. You-Shan Wang for providing inocula, Dr Tao Guo for instruction in techniques, and Shu-Qin Gao, Yuan Sui, Jian-Jiang Qiao, Qing-Guo Cui and Guo-Fang Liu for assistance with the experiment. Research was supported by grants from the National Science Foundation of China (30521005, 30770357) and from the Chinese Academy of Sciences (KZCX2-XB2-01).
LITERATURE CITED
- Alpert P, Stuefer JF. Division of labour in clonal plants. In: de Kroon H, van Groenendael J., editors. The ecology and evolution of clonal plants. Leiden: Backbuys Publishers; 1997. pp. 137–154. [Google Scholar]
- Alpert P, Holzapfel C, Slominski C. Differences in performance between genotypes of Fragaria chiloensis with different degrees of resource sharing. Journal of Ecology. 2003;91:27–35. [Google Scholar]
- Calvet C, Estaún V, Camprubí A, Hernández-Dorrego A, Pinochet J, Moreno MA. Aptitude for mycorrhizal root colonization in Prunus rootstocks. Scientia Horticulturae. 2004;100:39–49. [Google Scholar]
- Catford JG, Staehelin C, Lerat S, Piche Y, Vierheilig H. Suppression of arbuscular mycorrhizal colonization and nodulation in split-root systems of alfalfa after pre-inoculation and treatment with Nod factors. Journal of Experimental Botany. 2003;54:1481–1487. doi: 10.1093/jxb/erg156. [DOI] [PubMed] [Google Scholar]
- Chapman DF, Robson MJ, Snaydon RW. Physiological integration in the clonal perennial herb Trifolium repens L. Oecologia. 1992;89:338–347. doi: 10.1007/BF00317411. [DOI] [PubMed] [Google Scholar]
- Cornejo P, Azcón-Aguilar C, Barea JM, Ferrol N. Temporal temperature gradient gel electrophoresis TTGE as a tool for the characterization of arbuscular mycorrhizal fungi. FEMS Microbiology Letters. 2004;241:265–270. doi: 10.1016/j.femsle.2004.10.030. [DOI] [PubMed] [Google Scholar]
- DeLucia EH, Callaway RM, Thomas EM, Schlesinger WH. Mechanisms of phosphorus acquisition for ponderosa pine under different climatic regimes. Annals of Botany. 1997;79:111–120. [Google Scholar]
- Gautier H, Varlet-Grancher C, Baudry N. Comparison of horizontal spread of white clover Trifolium repens L. grown under two artificial light sources differing in their content of blue light. Annals of Botany. 1998;82:41–48. [Google Scholar]
- Gerdemann JW, Trappe JM. The Endogonaceae in the Pacific Northwest. Mycologia Memoir. 1974;5:1–76. [Google Scholar]
- Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist. 1980;84:489–500. [Google Scholar]
- Habte M, Zhang Y-C, Schmitt DP. Effectiveness of Glomus species in protecting white clover against nematode damage. Canadian Journal of Botany. 1999;77:135–139. [Google Scholar]
- Hartnett DC, Hetrick BAD, Wilson GWT, Gibson DJ. Mycorrhizal influence of intra- and interspecific neighbour interactions among co-occurring prairie grasses. Journal of Ecology. 1993;81:787–795. [Google Scholar]
- van der Heijden MGA, Boller T, Wiemken A, Sanders IR. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology. 1998;a 79:2082–2091. [Google Scholar]
- van der Heijden MGA, Klironomos JN, Ursic M, et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature. 1998;b 396:69–72. [Google Scholar]
- van der Heijden MGA, Cornelissen JHC. The critical role of plant-microbe interactions on biodiversity and ecosystem functioning: arbuscular mycorrhizal associations as an example. In: Loreau M, Naeem S, Inchausti P, editors. Biodiversity and ecosystem functioning: synthesis and perspectives. New York, NY: Oxford University Press; 2002. pp. 181–192. [Google Scholar]
- Hetrick BAD, Wilson GWT, Todd TC. Relationships of mycorrhizal symbiosis, rooting strategy, and phenology among tallgrass prairie forbs. Canadian Journal of Botany. 1992;70:1521–1528. [Google Scholar]
- Hodge A. Plastic plants and patchy soils. Journal of Experimental Botany. 2004;57:401–411. doi: 10.1093/jxb/eri280. [DOI] [PubMed] [Google Scholar]
- Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist. 2006;162:9–24. [Google Scholar]
- Hutchings MJ, de Kroon H. Foraging in plants: the role of morphological plasticity in resource acquisition. Advances in Ecological Research. 1994;25:159–238. [Google Scholar]
- Hutchings MJ, Wijesinghe DK. Patchy habitats, division of labour and growth dividends in clonal plants. Trends in Ecology and Evolution. 1997;12:390–394. doi: 10.1016/s0169-5347(97)87382-x. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Caldwell MM. The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology. 1993;74:612–614. [Google Scholar]
- Jakobsen I, Andersen AJ. Vesicular-arbuscular mycorrhiza and growth in barley: effects of irradiation and heating of soil. Soil Biology and Biochemistry. 1982;14:171–178. [Google Scholar]
- Johnson NC, Graham JH, Smith FA. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytologist. 1997;135:575–585. [Google Scholar]
- van Kleunen M, Stuefer JF. Quantifying the effects of reciprocal assimilate and water translocation in a clonal plant by the use of steam-girdling. Oikos. 1999;85:135–145. [Google Scholar]
- Klironomos JN. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology. 2003;84:2292–2301. [Google Scholar]
- Koide RT. Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytologist. 2000;147:233–235. [Google Scholar]
- Koide RT, Li M-G. Appropriate controls for vesicular-arbuscular mycorrhiza research. New Phytologist. 1989;111:35–44. [Google Scholar]
- Koide RT, Schreiner RP. Regulation of the vesicular-arbuscular mycorrhizal symbiosis. Annual Review of Plant Physiology and Plant Molecular Biology. 1992;43:557–581. [Google Scholar]
- de Kroon H, Huber H, Stuefer JF, van Groenendael JM. A modular concept of phenotypic plasticity in plants. New Phytologist. 2005;166:73–82. doi: 10.1111/j.1469-8137.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- Landis FC, Fraser LH. A new model of carbon and phosphorus transfers in arbuscular mycorrhizas. New Phytologist. 2008;177:466–479. doi: 10.1111/j.1469-8137.2007.02268.x. [DOI] [PubMed] [Google Scholar]
- Li L-F, Yang A, Zhao Z-W. Seasonality of arbuscular mycorrhizal symbiosis and dark septate endophytes in a grassland site in southwest China. FEMS Microbiology Ecology. 2005;54:367–373. doi: 10.1016/j.femsec.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Li X-L, Marschner H, George E. Acquisition of phosphorus and copper by VA-mycorrhizal hyphae and root-to-shoot transport in white clover. Plant and Soil. 1991;136:49–57. [Google Scholar]
- Liu R-J, Wang F-Y. Selection of appropriate host plants used in trap culture of arbuscular mycorrhizal fungi. Mycorrhiza. 2003;13:123–127. doi: 10.1007/s00572-002-0207-4. [DOI] [PubMed] [Google Scholar]
- Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- Lotscher M, Hay MJM. Distribution of phosphorus and calcium from nodal roots of Trifolium repens: the relative importance of transport via xylem or phloem. New Phytologist. 1996;133:445–452. [Google Scholar]
- McNamara NP, Black HIJ, Beresford NA, Parekh NR. Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Applied Soil Ecology. 2003;24:117–132. [Google Scholar]
- Maherali H, Klironomos JN. Influence of phylogeny on fungal community assembly and ecosystem functioning. Science. 2007;316:1746–1748. doi: 10.1126/science.1143082. [DOI] [PubMed] [Google Scholar]
- Maiquetía M, Cáceres A, Herrera A. Mycorrhization and phosphorus nutrition affect water relations and CAM induction by drought in seedlings of Clusia minor. Annals of Botany. 2009;103:525–532. doi: 10.1093/aob/mcn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H, Dell B. Nutrient uptake in mycorrhizal symbiosis. Plant and Soil. 1994;159:89–102. [Google Scholar]
- Menge JA, Steirle D, Bagyaraj DJ, Johnson ELV, Leonard RT. Phosphorus concentrations in plants responsible for inhibition of mycorrhizal infection. New Phytologist. 1978;80:575–578. [Google Scholar]
- Miller RM, Miller SP, Jastrow JD, Rivetta CB. Mycorrhizal mediated feedbacks influence net carbon gain and nutrient uptake in Andropogon gerardii. New Phytologist. 2002;155:149–162. doi: 10.1046/j.1469-8137.2002.00429.x. [DOI] [PubMed] [Google Scholar]
- Mosse B, Bowen GD. The distribution of Endogone spores in some Australian and New Zealand soils and in an experimental soil at Rothamsted. Transactions of the British Mycological Society. 1968;51:485–492. [Google Scholar]
- Phillips JM, Hayman DS. Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society. 1970;55:158–161. [Google Scholar]
- Reynolds HL, Vogelsang KM, Hartley AE, Bever JD, Schultz PA. Variable response of old-field perennials to arbuscular mycorrhizal fungi and phosphorus sources. Oecologia. 2006;147:348–358. doi: 10.1007/s00442-005-0270-6. [DOI] [PubMed] [Google Scholar]
- Roiloa SR, Alpert P, Tharayil N, Hancock G, Bhowmik PC. Greater capacity for division of labour in clones of Fragaria chiloensis from patchier habitats. Journal of Ecology. 2007;95:397–405. [Google Scholar]
- SAS Institute. SAS/STAT user's guide. Cary, NC: SAS Institute, Inc.; 1999. Version 8. [Google Scholar]
- Shishkova S, Rost TL, Dubrovsky JG. Determinate root growth and meristem maintenance in angiosperms. Annals of Botany. 2008;101:319–340. doi: 10.1093/aob/mcm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FA, Jakobsen I, Smith SE. Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago truncatula. New Phytologist. 2000;147:357–366. [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. 2nd edn. London: Academic Press; 1997. [Google Scholar]
- Son CL, Smith SE. Mycorrhizal growth responses: interactions between photon irradiance and phosphorus nutrition. New Phytologist. 1988;108:305–314. doi: 10.1111/j.1469-8137.1988.tb04167.x. [DOI] [PubMed] [Google Scholar]
- Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR. Clonal growth traits of two Prunella species are determined by co-occuring arbuscular mycorrhizal fungi from a calcareous grassland. Journal of Ecology. 1997;85:181–191. [Google Scholar]
- Streitwolf-Engel R, van der Heijden MGA, Wiemken A, Sanders IR. The ecological significance of arbuscular mycorrhizal fungal effects on clonal reproduction in plants. Ecology. 2001;82:2846–2859. [Google Scholar]
- Stuefer JF, de Kroon H, During HJ. Exploitation of environmental heterogeneity by spatial division of labour in a clonal plant. Functional Ecology. 1996;10:328–334. [Google Scholar]
- Stuefer JF, Erschamber B, Huber H, Suzuki J-I. The ecology and evolutionary biology of clonal plants: an introduction to the proceedings of Clone-2000. Evolutionary Ecology. 2002;15:223–230. [Google Scholar]
- Sudová R, Vosátka M. Effects of inoculation with native arbuscular mycorrhizal fungi on clonal growth of Potentilla reptans and Fragaria moschata Rosaceae. Plant and Soil. 2008;308:55–67. [Google Scholar]
- Vejsadová H, Siblíková D, Hršelová H, Vančura V. Effect of the VAM fungus Glomus sp. on the growth and yield of soybean inoculated with Bradyrhizobium japonicum. Plant and Soil. 1992;140:121–125. [Google Scholar]
- Watson MA, Scott K, Griffith J, Dieter S, Jones CS, Nanda S. The developmental ecology of mycorrhizal associations in mayapple, Podophyllum peltatum, Berberidaceae. Evolutionary Ecology. 2001;15:425–442. [Google Scholar]
- Weijschedé J, Martínková J, de Kroon H, Huber H. Shade avoidance in Trifolium repens: costs and benefits of plasticity in petiole length and leaf size. New Phytologist. 2006;172:655–666. doi: 10.1111/j.1469-8137.2006.01885.x. [DOI] [PubMed] [Google Scholar]
- Wiens JA. Ecological heterogeneity: an ontogeny of concepts and approaches. In: Hutchings MJ, John E, Stewart AJ, editors. The ecological consequences of environmental heterogeneity. Oxford: Blackwell Science; 2000. pp. 9–31. [Google Scholar]
- Williams WM. Adaptive variation. In: Baker MJ, Williams WM, editors. White clover. Wallingford: CAB International; 1987. pp. 299–321. [Google Scholar]
- Wolfe BE, Mummey DL, Rillig MC, Klironomos JN. Small-scale spatial heterogeneity of arbuscular mycorrhizal fungal abundance and community composition in a wetland plant community. Mycorrhiza. 2007;17:175–183. doi: 10.1007/s00572-006-0089-y. [DOI] [PubMed] [Google Scholar]
- Wright DP, Scholes JD, Read DJ. Effects of VA mycorrhizal colonization on photosynthesis and biomass production of Trifolium repens L. Plant, Cell & Environment. 1998;21:209–216. [Google Scholar]